Abstract
An efficient screening method following UV mutagenesis yielded a high frequency of improved mutants of Trichosporon brassicae CGMCC 0574, a wild-type esterase-producer capable of enantioselectively hydrolyzing the ethyl ester of ketoprofen [2-(3-benzoylphenyl) propionic acid]. The mutant had an activity 1.8-fold higher than the wild type and was stable in its enzyme production for ten serial transfers. As the best single carbon source, isopropanol improved the specific activity of the enzyme 5-fold; and this did not result from the effect of cell permeabilization. An 18-h culture grown on a medium containing 0.5% glucose plus 0.5% isopropanol produced 3-fold as much esterase as a culture grown on 1% glucose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a member of the 2-arylpropionic acids that represent an important group of non-steroidal anti-inflammatory drugs, ketoprofen [2-(3-benzoylphenyl) propionic acid] exhibits a pharmaceutical activity which reduces inflammation and relieves pain mainly with its (S)-enantiomer, while its (R)-enantiomer can be used as a toothpaste additive to prevent periodontal diseases [4]. Therefore, it is desirable to obtain optically pure (S)- or (R)-ketoprofen from the chemically synthesized racemic mixture, using kinetic resolution [13] or chiral inversion [15].
Among many of the efforts made in biocatalytic resolution to obtain optically pure ketoprofen, commercial enzymes were frequently used as biocatalysts. Although different strategies have been proposed to enhance the activity and enantioselectivity of existing commercial enzymes, including optimization of reaction conditions [11, 12], modification of the substrate and/or enzyme [3, 10], improvement of the mass transfer in the reaction [18] and purification of enzyme preparations [14], these cumbersome processes may increase the operational difficulty and process cost. Additionally, in many cases, it is very difficult to find a commercial enzyme with both satisfactory enantioselectivity and activity for the kinetic resolution of some unnatural substrates, such as ketoprofen. Therefore, it is an effective alternative to isolate from a broader diversity of microorganisms in soil samples, using the target substrate or its derivatives as sole carbon source and to catalyze the reaction directly using whole cells.
In our previous report [16], a yeast strain, Trichosporon brassicae CGMCC 0574, was successfully isolated for the enantioselective hydrolysis of ketoprofen ethyl ester to prepare the optically pure (S)-ketoprofen. However, the activity of T. brassicae CGMCC 0574, like most other wild-type strains, was not satisfactory in meeting the needs of industrial application. Aside from the strategies of cell permeabilization and fermentation optimization, strain improvement by genetic manipulation is also an important means for increasing the production of target enzymes or metabolic products. Random mutagenesis followed by a suitable screening method represents the classic route for this purpose. For instance, a mutant showing 5- to 7-fold higher cellulase activity compared with the wild type of Penicillium occitanis was isolated after seven rounds of mutagenesis with UV light, ethylmethanesulfonate and N-methyl N′-nitro N-nitrosoguanidine [8]. However, to our knowledge, there is no report on improved mutants of the esterase producer for enantioselective hydrolysis of the racemic ketoprofen ester.
In this work, a simple and efficient screening procedure following UV mutagenesis was established to provide a high frequency of positive mutants of the esterase producer, T. brassicae CGMCC 0574. One of the mutants increased its esterase activity on the ethyl ester of (S)-ketoprofen by 1.8-fold, even when the organism was grown on the original medium (10 g glucose/l, 5 g peptone/l, 5 g yeast extract/l). Its genetic stability served as our motivation to optimize the medium to improve enzyme production. As a carbon source for the growth of the mutant, isopropanol led to a pronounced increase (as much as 5-fold) in the specific activity of the ketoprofen ester hydrolase. In the meantime, enzyme production increased 3-fold after 18 h of culture when glucose and isopropanol were jointly used as the carbon source.
Materials and methods
Chemicals
Racemic ketoprofen was kindly donated by Xi'nan Pharmaceutical Factory, Chongqing, China. The ethyl ester of ketoprofen was prepared following the method described by Hernaiz et al. [7]. The structure of the ethyl ester was confirmed by 1H-NMR (500 MHz, CDCl3), showing δppm values of 7.40-7.85 (m, 9H, ArH), 4.08-4.18 (m, 2H, –OCH2–), 3.79 (q, 1H, J=7.18 Hz, –CH(CH3)COOH), 1.50 (d, 3H, J=7.18 Hz, CH3–CH(COOH)–), 1.22 (t, 3H, J=7.12 Hz, CH3–CH2O). All other chemicals were obtained commercially and were of analytical grade.
Microorganism, media and culture conditions
The wild-type T. brassicae CGMCC 0574 was described elsewhere [16]. The strain was identified at the Institute of Microbiology, The Chinese Academy of Sciences (Beijing, China), and is currently deposited in the China General Microbiological Culture Collection Center (Beijing). The strain was grown on GM medium (5 g peptone/l, 5 g yeast extract/l, 10 g glucose/l). Medium for strain enrichment (EM) contained (per liter): 2 g (NH4)2SO4, 2 g K2HPO4, 0.5 g NaCl, 0.5 g MgSO4·7H2O and ketoprofen ethyl ester at a final concentration of 1% (w/v), which was added to the medium as either an ethanol solution or a Tween-80 emulsion. The medium used for optimizing the carbon sources contained (per liter): 5 g peptone, 5 g yeast extract and 10 g each of the carbon sources to be tested. Cultivation was carried out on a rotary shaker at 30 °C and 180 rpm. The initial concentration of substrate in both enrichment culture and bioconversion was 10 mM, unless otherwise specified.
UV mutagenesis and strain selection
The cell suspension of the three best mutant strains obtained from a round of mutagenesis was exposed to UV light (20 W at 20 cm) for 3 min. After being cultured in the dark, the cell suspension subjected to mutagenesis was inoculated into 20 ml of EM. After two rounds of enrichment cultivation, the culture broth was diluted and spread on agar plates of EM supplemented with substrate emulsion (final concentration 10 mM) and agar (2.0%). Colonies with large clear halos were picked and inoculated into test-tubes containing GM for activity assay. Strains showing a high esterase activity were further subjected to a second round of screening. The activities of strains from the first and second screenings were compared with that of the wild-type strain.
Analytical methods
Concentrations of ketoprofen and its ethyl ester were determined by HPLC, using a Lichrosorb RP-18 (200×5.0 mm, 10 μm) reverse phase column. The UV detector was set at 254 nm and the mobile phase was composed of methanol and water (85:15 by volume) at a flow rate of 0.8 ml/min.
The enantiomers of ketoprofen were analyzed by a chiral HPLC column (Chiracel OJ, 250×4.6 mm; Daicel Co., Japan) which was eluted with hexane:2-propanol:acetic acid (90.0:10.0:0.1 by volume) at 1.0 ml/min and detected at 254 nm. The retention times of (S)- and (R)-ketoprofen ethyl esters and (R)- and (S)-ketoprofen were 9.5, 10.0, 13.0 and 17.4 min, respectively. The enantiomeric excess of product (ee p) was defined as the ratio: [(S)−(R)]/([(S)+(R)]×100%, where (R) and (S) are the concentrations of (R)- and (S)-enantiomers, respectively. The enantiomeric ratio (E-value) was calculated using the following equation [2], where x represents the conversion ratio of ester hydrolysis: E=ln[1−x(1+ee p)]/ln[1−x(1−ee p)].
Isopropanol treatment of resting cells
Cells from 10 ml of 15-h culture broth were harvested by centrifugation at 30,000 g for 10 min and washed once with 0.85% NaCl solution. The washed cells were then permeabilized by resuspending them in 2 ml of potassium phosphate buffer (KPB, 50 mM, pH 8.0) containing 2% (v/v) isopropanol. After incubation on a shaker at 120 rpm and 30 °C for 10 h, the culture broth was centrifuged at 30,000 g for 10 min. The cells collected were washed with saline before they were used for enzyme assay.
Enzyme assay
Cells harvested from 10 ml of culture broth were re-suspended in 2 ml of KPB (50 mM, pH 8.0) containing 10 mM ketoprofen ethyl ester and 0.5% (w/v) Tween-80. The mixture was incubated at 30 °C and 120 rpm for 1 h; and then the residual substrate and its hydrolytic product were extracted with the same volume of ethyl acetate. The extract was directly subjected to HPLC analysis. One unit of hydrolytic activity was defined as the amount of enzyme that catalyzed the hydrolysis of ketoprofen ethyl ester to release 1 μmol ketoprofen/min under the conditions above.
Results and discussion
Results of UV mutagenesis
Although genetic methods such as directed evolution have been used to get the desired mutant [1], UV mutagenesis followed by an efficient screening method still plays an important role in improving the production capacity of many industrial microbial strains. First, a duration of 180 s was determined as the mutagenesis time at a certain UV power (20 W) and exposure distance (20 cm) in order to achieve an inactivation of 75–80%, which was considered beneficial for the generation of genetically stable mutants with suitable variant frequency [19].
In fact, a suitable screening method rather than the mutagen is often the key to getting the desired strains. In this work, a fast and efficient screening method was successfully established, in which two rounds of enrichment cultivation with the ketoprofen ethyl ester as the sole carbon source resulted in a significantly increased frequency of positive mutants. Among all the strains tested, the percentage of positive mutants (mutants with over 1.5-fold higher activity than the wild type) was higher than 11%. Since there was no other report about screening mutants of ketoprofen ester hydrolase-producers, we were unable to evaluate the relative effectiveness of this procedure. In a similar case, among thousands of microbial strains tested, 28 were selected to determine their potential for ketoprofen ethyl ester-hydrolyzing activity from 40 strains showing esterase activity [9]. In this work, the high frequency (>11%) of positive mutants was considered to be due to three possible reasons. First, as described in our previous publications [5, 16], the alternative use of Tween-80 and ethanol as additives during the enrichment culture may help to exclude strains which might utilize the additives (ethanol, Tween-80). In addition, before being plated, two rounds of enrichment culture with ketoprofen ester as the sole carbon source may efficiently limit the growth of negative mutants and multiply positive mutants. Therefore, a higher frequency of positive strains was obtained from the culture spread on agar plates containing EM. However, the two rounds of enrichment culture could allow some siblings to be spread on the same plate, which might artificially increase the ratio of positive variants. Finally, several generations of proliferation were actually experienced during the repeated enrichment culture before the strains were picked up. This may contribute to a genetic stability of selected strains higher than that of the ones isolated immediately after UV mutagenesis.
After three rounds of UV mutagenesis, in which the top 3–5 high-producers from the previous round were used as the starting strains for the next round, several strains were selected and subjected to the final screening. Meanwhile, the E values of the strains with the highest activities were also checked using chiral HPLC (Table 1). Strain 4-3-1-3 rather than strain 5-20-1 was finally selected for further investigation, due to its higher enantioselectivity.
Genetic stability of the selected strain
Many mutants face the problem of genetic instability due to reverse mutations. Therefore, the genetic stability of strain 4-3-1-3 in enzyme production was evaluated by assaying its enzyme activities after ten transfers on slants. As a result, the activities measured ranged from 1.73- to 1.96-fold (an average of 1.8-fold) greater than the wide type, which suggests the mutant was genetically stable. The results above indicate that mutation did take place in the mutant, although more direct proof from molecular information on the DNA sequence needs to be provided from future research.
Effects of the carbon source
A suitable growth condition is essential to ensure that the mutant expresses enzyme fully. In this section, optimizing the carbon source was used to improve the growth condition. A variety of carbon sources, including alcohols, monosaccharides, polysaccharides and fatty acid esters, were evaluated for their capacity to support cell growth and enzyme production. As shown in Table 2, glucose was the best carbon source to support cell growth, but it seriously limited enzyme production. The other carbon sources produced similar cell masses, while the short-chain alcohols, ethanol and glycerol, led to the highest specific activities, which were about 1.5-fold higher than that of the glucose-grown culture. No significant enhancement of enzyme productivity was observed in the case of oleic acid, which was previously reported as a good inducer for lipase production by Candida rugosa [6]. In order to clarify the effect of the lower alcohols on enzyme production, n-propanol and isopropanol were tested further. As shown in Table 2, when isopropanol was used as the carbon source, the esterase specific activity of strain 4-3-1-3 was enhanced as much as 5-fold, although cell growth was considerably limited.
To obtain maximal enzyme production, the effects of glucose and isopropanol concentrations on the cell growth and enzyme production were investigated. Table 3 shows that, while the cell mass increased with the concentration of glucose, enzyme productivity decreased significantly, suggesting that enzyme expression was markedly repressed by glucose. Variations in isopropanol concentration did not show significant effects on either cell mass or enzyme production. Therefore, combining glucose with isopropanol as the carbon source was considered favorable for both cell mass and enzyme production. This may enhance the total enzyme production. Thus, different concentrations of glucose plus isopropanol at a 1:1 (w/w) ratio were also investigated (data not shown). The cell mass increased with the increase in the total amount of carbon source, but enzyme production reached its maximum at the combination of 0.5% glucose with 0.5% isopropanol. When the time-course of enzyme production was further investigated (Fig. 1), the highest enzyme production (ca. 8.5 units/l) was observed at 18 h. It was 3-fold higher than that given by cells grown on the original GM.
Mechanism of the isopropanol effect
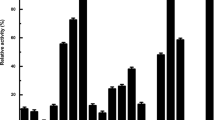
In our previous work, we reported that isopropanol, as an efficient permeabilizer, led to a significant enhancement (310%) in the activity of the esterase of T. brassicae CGMCC 0574 [17]. This result motivated us to find out whether the improved enzyme production with isopropanol as the carbon source was due to the effect of permeabilization. The data in Fig. 2 show that permeabilized resting cells from glucose-grown culture increased the enzyme activity by 20%, compared with untreated cells, while over 3-fold enhancement in the specific activity of enzyme was observed when the culture was grown with isopropanol as the carbon source. Therefore, it is clear that the improvement in enzyme production in the isopropanol-grown culture of mutant strain 4-3-1-3 did not result from the effect of permeabilization. It is worth noting that the effect of isopropanol permeabilization on mutant strain 4-3-1-3 was not as significant as it was on the wild-type T. brassicae CGMCC 0574.
Effect of isopropanol treatment on the specific activity of cultures grown on different carbon sources. Gluc. Glucose (1.0%), Isop. isopropanol (1.0%), Gluc.+Isop. glucose (0.5%) and isopropanol (0.5%) used jointly as the carbon source. Full, 100% productivity corresponded to the specific activity (5 units/g cells) of isopropanol-treated cells collected from the culture grown on medium containing isopropanol. White columns Resting cells, black columns isopropanol-treated cells
References
Bornscheuer UT, Altenbuchner J, Meyer HH (1998) Directed evolution of an esterase for the stereoselective resolution of a key intermediate in the synthesis of epothilones. Biotechnol Bioeng 58:554−559
Chen CS, Fujmito Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolution of enantiomers. J Am Chem Soc 104:7294−7299
Coltonl IJ, Ahmed SN, Kazlauskas RJ (1995) A 2-propanol treatment increases the enantioselectivity of Candida rugosa lipase toward esters of chiral carboxylic acids. J Org Chem 60:212−217
Famaey JP, Paulus HE (1992) Therapeutic applications of NSAIDs. Dekker, New York
Gong PF, Wu HY, Xu JH, Shen D, Liu YY (2002) Biocatalytic preparation of enantiopure (R)-ketoprofen from its racemic ester by a new yeast isolate Citeromyces matriensis CGMCC 0573. Appl Microbiol Biotechnol 58:728−734
Gordillo MA, Obradors N, Montesinos JL, Valero F, Lafuente J, Sola C (1995) Stability studies and effect of the initial oleic acid concentration on lipase production by Candida rugosa. Appl Microbiol Biotechnol 43:34−41
Hernaiz MJ, Sanchez-Montero JM, Sinisterra JV (1995) Hydrolysis of (R,S) 2-arylpropionic esters by pure lipase B from Candida cylindracea. J Mol Catal 96:317−327
Jain S, Parriche M, Durand H, Tiraby G (1990) Production of polysaccharidases by a cellulase-pectinase hyperproducing mutants (Po16) of Penicillium occitanis. Enzyme Microb Technol 12:691−696
Kim GJ, Choi GS, Kim JY, Lee JB, Jo DH, Ryu YW (2002) Screening, production and properties of a stereospecific esterase from Pseudomonas sp. S34 with high selectivity to (S)-ketoprofen ethyl ester. J Mol Catal B Enzym 17:29−38
Lalonde J, Govardhan C, Khalaf N, Martinez AG, Visuri K, Margolin AL (1995) Cross-linking crystals of Candida rugosa lipase: highly efficient catalysts for the resolution of chiral esters. J Am Chem Soc 117:6845−6852
Liu YY, Xu JH, Xu QG, Hu Y (1999) Significant enhancement of lipase enantioselectivity toward (S)-ketoprofen at pH 2. Biotechnol Lett 21:143−146
Liu YY, Xu JH, Hu Y (2000) Enhancing effect of Tween-80 on lipase performance in enantioselective hydrolysis of ketoprofen ester. J Mol Catal B Enzym 10:523−529
Margolin AL (1993) Enzyme in the synthesis of chiral drugs. Enzyme Microb Technol 15:266−279
Plou FJ, Sodro P, Calvo MV, Burguillo FJ, Ballesteros A (1997) Kinetic and enantioselective behaviour of isoenzymes A and B from Candida rugosa lipase in the hydrolysis of lipids and esters. Biocatal Biotransform 15:75−89
Rhys-Williams W, McCarthy F, Baker J, Hung YF, Thomason MJ, Lloyd AW, Hanlon GW (1998) A mechanistic investigation into the microbial chiral inversion of 2-arylpropionic acids using deuterated derivatives of 2-phenylpropionic acid. Enzyme Microb Technol 22:281−287
Shen D, Xu JH, Gong PF, Wu HY, Liu YY (2001) Isolation of an esterase-producing Trichosporon brassicae and its catalytic performance in kinetic resolution of ketoprofen. Can J Microbiol 47:1101−1106
Shen D, Xu JH, Wu HY, Liu YY (2002) Significantly improved esterase activity for ketoprofen resolution by 2-propanol treatment of Trichosporon brassicae cells. J Mol Catal B Enzym 18:219−224
Wu HY, Xu JH, Liu YY (2001) A practical enzymatic method for preparation of (S)-ketoprofen with a crude Candida rugosa lipase. Synth Commun 31:3491−3496
Xiong ZG (1995) Principle of fermentation technology. China Medicinal Publishing, Beijing
Acknowledgements
Financial support from the National Natural Science Foundation of China (grant 20176011) and the Shanghai Commission of Science and Technology (grant 98QB14042) is gratefully acknowledged. We also thank Professor Qin Ye at our State Key Laboratory of Bioreacotor Engineering for helpful discussions and suggestions in the improvement of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, HY., Xu, JH., Shen, D. et al. Improved production of (S)-ketoprofen ester hydrolase by a mutant of Trichosporon brassicae CGMCC 0574. J IND MICROBIOL BIOTECHNOL 30, 357–361 (2003). https://doi.org/10.1007/s10295-003-0053-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-003-0053-1