Abstract
Purpose
Systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces degeneration of dopaminergic neurons and reproduces the motor features of Parkinson disease (PD); however, the effect of MPTP on extranigral structures has been poorly studied. The aim of this research was to study the cardiac sympathetic innervation of control and MPTP-treated monkeys in order to describe the influence of MPTP toxicity on cardiac tissue.
Methods
Eight monkeys were included in the study and divided into two groups, four monkeys serving as controls and four forming the MPTP group. Sections from the anterior left ventricle were immunohistochemically examined to characterize the sympathetic fibers of cardiac tissue. The intensity of immunoreactivity in the nerve fibers was quantitatively analyzed using ImageJ software.
Results
As occurs in PD, the sympathetic peripheral nervous system is affected in MPTP-treated monkeys. The percentage of tyrosine hydroxylase immunoreactive fibers in the entire fascicle area was markedly lower in the MPTP group (24.23%) than the control group (35.27%) (p < 0.05), with preservation of neurofilament immunoreactive fibers in the epicardium of MPTP-treated monkeys. Alpha-synuclein deposits were observed in sections of the anterior left ventricle of MPTP-treated monkeys but not in control animals, whereas phosphorylated synuclein aggregates were not observed in either controls or MPTP-treated monkeys.
Conclusion
The peripheral autonomic system can also be affected by neurotoxins that specifically inhibit mitochondrial complex I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-motor symptoms are present over the course of Parkinson disease (PD) and they may even precede the motor manifestations of the disease by many years [1,2,3]. Among them, autonomic dysfunction is present in approximately 90% of PD patients [4] and is commonly observed early in the course of the disease [5,6,7,8]. This feature is in line with the description of reduced cardiac uptake of metaiodobenzylguanidine (MIBG), an analog of norepinephrine, and deterioration of cardiovascular reflexes [9] in asymptomatic carriers of leucine-rich repeat kinase 2 (LRRK2) mutations [10]. Indeed, profound cardiac sympathetic denervation has been demonstrated in patients with idiopathic PD [11]. Pathophysiologically, α-synuclein is a pre-synaptic protein involved in the regulation of dopamine synthesis [12, 13] and aggregated forms of this protein are the major component of Lewy bodies (LBs) and Lewy neurites. Although LBs are particularly abundant in the midbrain pigmented nuclei of PD patients [14], several studies have reported α-synuclein aggregates and fewer tyrosine hydroxylase immunoreactive (TH-ir) fibers in various different peripheral tissues including skin, gastrointestinal tract, salivary glands, and cardiac axons [15,16,17]. These facts might account for the autonomic dysfunction of PD patients, since there is a close correlation of density of α-synuclein aggregates with severity of autonomic failure, and also with Braak stage [18]. Interestingly, MIBG uptake is preserved in most patients with multiple system atrophy, progressive supranuclear palsy, or corticobasal degeneration, indicating that cardiac dysfunction may be a specific biomarker of PD [19].
Systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) selectively induces degeneration of dopaminergic neurons in the substantia nigra (SN) and reproduces the main motor features of PD. Nonetheless, to our knowledge neither autonomic symptoms nor protein aggregates of α-synuclein have been studied in monkeys chronically exposed to the neurotoxin, even though changes in the cardiac sympathetic system of MPTP-treated mice have been reported [20, 21]. MPTP may induce histological changes and aggregation of α-synuclein in structures of the autonomic nervous system in animal models. Since MPTP is a neurotoxin that selectively inhibits mitochondrial complex I, our working hypothesis is that cardiac sympathetic denervation is present in macaques with mild motor signs, treated with MPTP. This would support the hypothesis that cardiac dysfunction is a very early alteration in the development of PD, that it precedes the development of the motor disease, and would offer a valid model of PD in which alterations of the autonomic nervous system and non-motor symptoms are also present.
Understanding the pathophysiology of cardiac involvement in the animal model of PD will allow us to better understand the vulnerability of specific subtypes of neurons in the nervous system [22]. We here report for the first time the degeneration of cardiac sympathetic nerve fibers and the presence of α-synuclein aggregates in cardiac tissue of MPTP-treated monkeys. Given the fact that MPTP causes degeneration of sympathetic nerves in the heart, we sought to describe the association between sympathetic denervation and monoamine transporters in the cardiac nerves of control and MPTP-treated monkeys.
Materials and methods
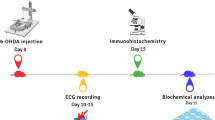
A total of eight male macaques (Macaca fascicularis; 4 years old, weight 3.5–5.5 kg) were included in the study. They were housed in an animal room under standard conditions of air exchange (16 l/min), humidity (50%), and light–dark cycles (8 am–8 pm). They were fed fresh fruit and commercial pellets, with free access to water. Their health was monitored by the attending veterinarian. The animals were euthanized following deep anesthesia, and all efforts were made to minimize suffering. Experimental protocols were in accordance with the European Communities’ Council Directive of 24 November 1986 (86/609/EEC) regarding the care and use of animals for experimental procedures, and under the guidance of the Ethics Committee for Animal Experimentation of the University of Navarra (Permit Number 128/12).
Animals
The animals were randomly assigned to each group. Four animals received weekly intravenous injections of MPTP (Sigma, St Louis, MO; 0.25–0.5 mg/kg) until they developed stable parkinsonism. MPTP was dissolved in sterile saline and given intravenously under light sedation with a mixture of ketamine (5 mg/kg) and midazolam (1 mg/kg). Injections were repeated every week for 10 consecutive weeks up to a cumulative dose of 9–14.4 mg/kg. This MPTP schedule was chosen on the basis of previous studies in our laboratory [23] showing that this regimen elicits 50–60% of dopaminergic cell loss and mild parkinsonian features. Further, the sample size was determined in accordance with general primate research criteria for using as few animals as possible and taking into account the fact that this regimen produces stable motor disability without spontaneous recovery. Cardiac function was not assessed. We studied the heart tissue of four MPTP-treated animals and the other four intact animals served as controls for the histological studies. MPTP-treated monkeys were killed 3 weeks after the last MPTP dose.
Tissue preparation
After deep sedation with a mixture of ketamine (10 mg/kg) and midazolam (1 mg/kg), animals were transcardially perfused with sterile saline. Hearts were immediately removed and fixed in 4% buffered aqueous solution of formaldehyde. For this study, we considered the base of the anterior wall of the left ventricle, since it contains a high density of TH-ir fibers [11, 24, 25]. This region was sampled from the heart and postfixed overnight in a 4% buffered aqueous solution of formaldehyde. No animals showed any features indicative of heart disease in the histological analysis. The tissue blocks were then embedded in paraffin and sectioned at a thickness of 5 µm.
Immunohistochemistry
Serial paraffin sections (5 µm) were stained with primary antibodies. Immunohistochemical assays were performed using a monoclonal antibody against phosphorylated neurofilament (Phosphodetect Anti-Neurofilament, mouse monoclonal; 1:1000; NE1022: Millipore, Billerica, MA, USA) and polyclonal antibody against protein gene product (anti-PGP9.5; 1:500; AB1761: Millipore) as a neuronal marker, to measure heart innervation. PGP9.5 immunohistochemistry was performed to ensure the presence of nerve fascicles. For analysis purposes, only neurofilament (NF) immunohistochemistry was quantified. An antibody against TH (anti-TH, mouse monoclonal; 1:1000; MAB5280: Millipore) was used as a marker of noradrenergic nerve fibers. Two different antibodies against α-synuclein (anti-syn MAB5320; 1:1000: Millipore and anti-syn LB509; 1:1000, Invitrogen, Carlsbad, CA, USA; both mouse monoclonal) and one antibody against phosphorylated α-synuclein (phospho S129, rabbit monoclonal; 1:5000, Abcam, Cambridge, UK) were used as markers of PD pathology. As are a result of inconsistency in the results obtained investigating the presence of synuclein elements, we used two different antibodies against α-synuclein in order to improve the sensitivity to PD pathology [26]. The following antibodies against different neuronal markers were also included to better assess the neurochemical characteristics of the axons in nerve fascicles: anti-norepinephrine transporter (anti-NET, mouse monoclonal; 1:2000, Millipore), mitochondria (anti-mitochondria [MTCO2], mouse monoclonal; 1:200, Abcam, Cambridge, UK), and vesicular monoamine transporter 2 (VMAT2) (anti-VMAT2 rabbit polyclonal; 1:500, Novus Biologicals, Littleton, CO, USA) antibodies. To investigate whether the toxin also affected heart cholinergic innervation, we performed immunohistochemical analysis of sympathetic ganglia using an antibody against choline acetyltransferase (anti-ChAT, goat polyclonal, 1:300, Millipore). Finally, since polo-like kinases appeared to be upregulated in synucleinopathy-diseased brains, we further investigated the expression of this kinase in the autonomic nervous system of MPTP-treated monkeys. Accordingly, sympathetic ganglia were studied using an antibody against PLK2 (H-90, anti-Snk, goal polyclonal, 1:100, Santa Cruz Biotechnology, Dallas, Texas, USA).
Immunohistochemistry was performed as follows. Tissue sections containing the left ventricle wall and the surrounding fat tissue were deparaffinized. Slides were heated in an oven at 60 °C for 20 min, submerged in xylene (twice for 5 min each), and then rehydrated with ethanol 100%–ethanol 95%–ethanol 70% and distilled water (5 min each). After that, sections were incubated in PBS with 0.02% hydrogen peroxide (H2O2) for peroxidase inhibition. Then, they were incubated in 0.01 M citrate buffer pH 6.0 for 20 min at 96% for antigen retrieval, in a PT module (Thermo Fisher Scientific). Then, tissue sections were rinsed in 0.1 M PBS and incubated overnight in DAKO antibody diluents containing the corresponding primary antibody (Table 1), rinsed in 0.01 M PBS, and incubated for 30 min in 0.01 M PBS containing the corresponding secondary antibody. Sections were then reacted using 3,3′-diaminobenzidine tetrahydrochloride (Sigma) with H2O2 (DAB Kit, Vector Laboratories) as a chromogen and counterstained with Nissl. Finally, the sections were coverslipped using DPX (BDH) and examined by light microscopy. On the basis of NF immunochemistry, serial tissue sections were collected in each case to ensure that we have selected the same nerve fibers. Control sections were processed without primary antibodies to confirm that their omission led to non-staining. For α-synuclein immunolabeling, serial sections were used as described above. Another set of tissue sections was pre-incubated for 20 min in formic acid (70%) to assess the presence of insoluble forms of α-synuclein in nerve fibers.
Immunofluorescence
To assess the nature and integrity of cardiac nerve fascicles and to estimate the presence of α-synuclein aggregates in these axons, double-labeling assays were performed using various combinations of antibodies against NF, TH, and α-synuclein. Sections were pretreated with preheated 0.01 M citrate buffer (pH 6.0) at 95 °C for 20 min and then washed three times for 5 min in PBS and treated for 15 min with 1% H2O2 in PBS to inactivate the endogenous peroxidase. Sections were then co-incubated overnight with the primary antibody at 4 °C in antibody diluent (DAKO) (NF/TH, TH/syn) (anti-tyrosine hydroxylase, rabbit polyclonal; 1:1000; Millipore). Sections were washed in PBS and subsequently incubated for 120 min in the dark at room temperature with a mixture of secondary antibodies coupled to fluorescent markers, donkey anti-rabbit Alexa Fluor 555 and donkey anti-mouse Alexa Fluor 488 and 568 (1:500, Molecular Probes, Netherlands). Finally, sections were counterstained with a nucleic acid stain for 10 min (TO-PRO-3 iodide, Molecular Probes, Netherlands) and coverslipped with PBS/glycerol (1:1) and examined under a confocal microscope (LSM 510 Meta, Zeiss).
Quantification
Optical density was used for the quantification of NF and TH immunoreactivity in the fibers of cardiac fascicles and also for the quantification of the density of NET, VMAT2, and MTCO2 immunoreactivity in these axons. Images were viewed and digitalized with an Olympus BX-51 microscope equipped with an Olympus DP-70 digital camera at ×20 using CAST grid software package (Olympus, Denmark). This method was used as in previous studies that have investigated the intensity of immunoreactivity using digitalized images [11]. Oval fascicles were eliminated to avoid the quantification of tangentially oriented axons. In all cases, eight ×40 nerve fiber images from each section were randomly digitalized and analyzed using ImageJ software. To ensure that we included the same fascicles in every section, we used serial sections. The contour of the nerve fiber endoneurium was outlined to measure the entire area of the fascicle. Images were converted to 8-bit RGB stacked (blue channel) to process only the dark brown labeling and to remove the blue Nissl staining. A background subtraction procedure was performed equally in each image. The entire immunoreactive area fraction was then measured with the same threshold. Values from eight nerve fascicles from each subject were summed and the result expressed as percentage of the immunoreactive area.
The presence of soluble and insoluble protein aggregates of α-synuclein in the epicardial nerve fascicles was assessed semiquantitatively as follows: −, absent (score 0); +, slight (score 1); ++, moderate (score 2); +++, severe (score 3). The PLK and ChAT immunoreactivity in sympathetic ganglia was assessed semiquantitatively as (−) absent, (+) slight, (++) moderate, and (+++) severe. The same researcher (M.C.A.) performed all quantifications in a blinded randomized manner.
Statistical analysis
Statistical analyses were carried out by means of Stata 12.0 software. A t test was used to compare the optical density of NF, TH, NET, VMAT2, and MTCO2 among MPTP-treated monkeys and controls. A p value less than 0.05 was considered significant. Data are presented as mean ± standard deviation and confidence interval.
Results
Sympathetic cardiac innervation is decreased in MPTP-treated monkeys
The results of the quantification of NF and TH immunoreactivity in the axons of control and MPTP-treated monkeys are shown in Table 2. In all tissue sections, NF staining revealed the presence of several nerve fascicles in the epicardium. Briefly, axons showing NF and PGP 9.5 immunoreactivity were present in the fascicles of both MPTP-treated and control animals. NF optical density was similar in both groups (p > 0.05), indicating that axonal loss does not occur in epicardial nerve fascicles of MPTP-treated monkeys. We next studied the percentage of neurofilament immunoreactive (NF-ir) fibers that expressed TH, in other words, the number of TH-ir axons in the heart fascicles. We found that in MPTP-treated monkeys, the ratio of TH-ir to NF-ir axons ranged from 28% to 65% while in control animals the range was 83% to 93%. These findings indicated a substantial loss of TH-ir fibers within the cardiac fascicles of parkinsonian monkeys in spite of having similar innervation measured by the density of NF-ir fibers, this giving rise to lower TH expression by the existing fibers. This is consistent with the fact that the percentage of TH-ir axons in the entire fascicle area was lower in the MPTP group (24.23%) than the control group (35.27%) (p < 0.05). Figure 1 shows the preservation of cardiac nerve fascicles (NF-ir axons) after MPTP exposure and the loss of TH-ir fibers in the axons of MPTP-treated monkeys. Supplementary figure shows a bar chart with mean values of NF-ir and TH-ir in both groups.
Semiquantification of α-synuclein aggregates
Native forms of α-synuclein were observed in the epicardial nerve fascicles of control and MPTP-treated monkeys. Immunohistochemistry using antigen retrieval and protein digestion with formic acid and two different antibodies against α-synuclein (LB509 and MAB5320) revealed the presence of insoluble aggregates of α-synuclein in MPTP-treated monkeys to a moderate degree (score 2), while insoluble forms of α-synuclein were absent in control animals (score 0). It is important to note that we observed similar results using two different antibodies against synuclein (LB509 and MAB5320). On the contrary, phosphorylated synuclein immunoreactivity was not detected in the cardiac nerve fascicles of either MPTP-treated or control animals (Fig. 2).
Immunohistochemistry images of epicardial nerve axons of MPTP-treated monkeys (a–c) and controls (d–f), using antibodies against alpha-synuclein (a, b, d, e). Immunohistochemistry using antigen retrieval and protein digestion with formic acid (b, e) and phospho-alpha-synuclein (c, f). Scale bar 100 μm
Neurochemical characteristics of epicardial nerve fibers
To better characterize the neurochemical characteristics of the epicardial nerve fibers, we studied the presence of immunoreactivity for norepinephrine transporter (NET) and vesicular monoamine transporter (VMAT) using specific markers against VMAT2 and NET. We searched for NF-ir axons and axons expressing TH and analyzed the presence and immunoreactivity of the other markers within the nerve fascicles. VMAT2-ir and NET-ir axons were observed in nerve fascicles of both control and MPTP-treated monkeys. Interestingly, we observed a significantly higher density of VMAT2-ir and NET-ir in nerve fascicles of MPTP-treated monkeys (p < 0.05). Specifically, the density of VMAT2 immunoreactivity in epicardial nerve fibers was 60.19 in MPTP-treated monkeys vs 36.02 in controls. The relative frequency of epicardial nerve fascicles NET-ir was 46.95 and 12.62 in MPTP monkeys and controls, respectively (p < 0.05) (Fig. 3).
Transversal sections of the cardiac nerve fascicles processed for vesicular monoamine transporter (VMAT) (a, b) and norepinephrine transporter (NET) (c, d) immunocytochemistry in MPTP-treated monkeys (a, c) and controls (b, d). There is higher VMAT and NET immunoreactivity in the nerves of the MPTP-treated monkeys. Scale bar 100 μm
ChAT immunoreactivity in cardiac ganglionic neurons
To assess cholinergic heart innervation, neurons from cardiac ganglia were studied. Both groups showed a moderate expression of ChAT-ir among ganglion neurons and the average intensity of ChAT immunoreactivity in the cytoplasm of cardiac ganglion neurons was similar in controls and MPTP-treated monkeys (score of 2 in both groups) (Fig. 4).
Cytoplasm mitochondrial immunoreactivity in sympathetic ganglionic neurons
Since MPTP produces neurotoxicity, inducing neuronal cell death by a combination of apoptotic and necrotic mechanisms, due to oxidative stress, we also assessed the presence of mitochondrial abnormalities in cardiac axons using the mitochondrial marker MTCO2, to check for any defects in the respiratory chain.
MTCO2 density was significantly higher in the cardiac TH-ir axons of MPTP-treated monkeys (p < 0.05). Specifically, the mean values of MTCO2-ir were 34.50% in MPTP-treated monkeys and 7.38% in controls, indicating that in cardiac TH-ir axons the higher level of VMAT2 and NET expression is associated with more abundant mitochondrial immunoreactivity (Fig. 5) (p < 0.05). Defects in MTCO2 are a cause of mitochondrial complex deficiency also known as cytochrome c oxidase deficiency, a disorder of the mitochondrial respiratory chain.
PLK expression in sympathetic ganglion neurons
As polo-like-kinase 2 (PLK2) is involved in the phosphorylation of α-synuclein at serine 129, we also studied the expression of PKL2 in neurons of sympathetic ganglia. PLK2-ir neurons were present in sympathetic ganglia of the epicardium of MPTP-treated monkeys, whereas neurons of control animals did not express this kinase. Semiquantitative analysis of this marker showed a moderate degree of PLK2 expression in MPTP-treated monkeys (score of 2), with a clearly different pattern in control monkeys (score of 0, indicating a lack of expression) (Fig. 6).
Discussion
Cardiac sympathetic denervation
In this study, we have demonstrated that subacute MPTP intoxication causes degeneration of cardiac TH-ir fibers in monkeys, as reflected by the presence of significantly fewer TH-ir axons. More importantly, we have shown that MPTP exposure is able to induce, in addition to nigral dopaminergic cell loss, the degeneration of cardiac TH-ir fibers. These results are consistent with previous studies in PD patients, showing degeneration of cardiac nerve fibers and decreased numbers of TH-ir axons. Decreases in NF-ir fibers have also been reported in PD, suggesting that the TH-ir fibers degenerate early in a progressive process in which the axons will eventually degenerate. In fact, in MPTP-treated monkeys, we observed profound sympathetic denervation in cardiac fascicles (fewer TH-ir fibers) with preservation of nerve fibers (measured by means of NF-ir fiber density). In this regard, other authors have described the effect of MPTP treatment on myocardial tissue in mice, namely, cardiac sympathetic dysfunction with preservation of myocardial nerve fibers [21]. An explanation for these discrepancies may lie in the differences in the effect of MPTP exposure depending on the scheme of administration, in contrast with the slow degenerative process underlying PD. It could be that a slower chronic MPTP administration schedule would also produce the degeneration of NF-ir axons resembling the pathological findings in PD. On the other hand, the demonstration that a selective dopamine neurotoxin is able to produce, in addition to dopaminergic cell loss in the SN, marked degeneration of the autonomic nervous system, supports the hypothesis that the degenerative process in PD might be triggered by a neurotoxin that can reach the brain through peripheral structures like the gut or olfactory bulb. Although dopaminergic cell loss in the SN was not quantified in our monkeys, previous studies in our lab have demonstrated that this regimen of MPTP administration produces nearly 60% nigral cell loss and mild parkinsonian features. Consequently, our results indicate that this MPTP model with mild parkinsonism and evident degeneration of sympathetic cardiac fibers is able to mimic an early stage of the disease in which cardiac denervation is already present with mild nigral cell loss and parkinsonian features. In fact, our MPTP-treated monkeys developed a mild degree of parkinsonism, measured by means of the motor disability scale (score 9–10/24), suggesting an early stage of the disease.
Synuclein aggregates as a hallmark of PD
Insoluble forms of synuclein were observed on TH-ir fibers of the anterior left ventricle of MPTP-treated monkeys. However, phosphorylated synuclein aggregates were not observed in MPTP-treated monkeys. The lack of LBs in toxin-induced PD models has been considered a weakness of these models, since they do not reproduce the main neuropathological hallmark of PD [27]. Nonetheless, several studies have shown intraneuronal inclusions of synuclein aggregates in MPTP-treated mice [28], baboons [29], and squirrel monkeys [30]. These synuclein aggregates might be considered early stages of LB formation and reflect a critical step in the neuronal degenerative process. LB formation is a dynamic process and probably the final step of several processes that requires the activation, among others, of genetic factors and of other pathways such as those of oxidative stress and synuclein phosphorylation. All of these processes are age dependent and could be more evident in aged monkeys. On the basis of our results, we suggest that protein aggregates containing synuclein in degenerating fibers may reflect an early stage of LB formation.
Monoamine neurotransmitters
Vesicular uptake of neuronal catecholamine is decreased in LB diseases and vesicular monoamine transport has been suggested as a target for treatment and neuroprotection in PD since it seems to be impaired in this disease [31]. MPTP crosses the blood–brain barrier and is metabolized into MPP+ by action of the enzyme MAO-B. Then, MPP+ is taken up by the dopamine transporter (DAT) located in dopaminergic terminals and stored in vesicles by the vesicular monoamine transporter (VMAT2). Normally, the function of VMAT2 is to maintain dopamine in synaptic vesicles, avoiding abnormally high cytoplasmic levels. High levels of cytoplasmic dopamine will promote the generation of excessive reactive oxidative species (ROS), mitochondrial dysfunction, and neuronal death.
In this study, we have found a higher density of VMAT2 and NET in nerve fibers of the cardiac fascicles of MPTP-treated monkeys. As our monkeys exhibited very mild parkinsonism, it might be suggested that in early stages of the disease, undamaged fibers increase the expression of VMAT2 and NET to prevent further damage, by promoting a compensatory presynaptic dopamine mechanism. In this regard, the increase in the reuptake of dopamine from the synaptic cleft provoked by the increase of VMAT2 and NET might be an acute response in this early stage of the disease produced by the toxin administration.
Accordingly, an increase in the release and reuptake of dopamine in DJ-1 knockout rats has also been described early in the course of nigral degeneration. In this regard, in early stages of the disease there is increased expression of VMAT2 and NET in non-degenerated nerve terminals, probably reflecting a compensatory mechanism to the axonal damage.
Cholinergic innervation
No differences were observed between MPTP-treated monkeys and controls in terms of cardiac cholinergic innervation. Neurons of cardiac ganglia were immunoreactive for choline acetyltransferase, in both parkinsonian and control animals, indicating that in MPTP-treated monkeys there is a preservation of cholinergic innervation, at least when the animals are mildly affected.
It has been demonstrated that cardiac impairment in PD patients is related to sympathetic noradrenergic dysfunction whereas the cholinergic system remains unimpaired [32, 33], indicating that in PD there is a specific pattern of cardiac dysfunction, catecholaminergic innervation being the only affected system. Accordingly, PET studies undertaken in PD using 5-[11C]methoxy-donepezil to measure the acetylcholinesterase activity of the peripheral nervous system have shown a reduction in intestinal and pancreatic cholinergic activity, whereas cholinergic activity in the heart remains unaffected and similar to that of healthy individuals [34]. Our results in MPTP-treated monkeys exhibiting an early phase of the disease are in line with these observations and support the hypothesis that sympathetic noradrenergic denervation is causing autonomic non-motor symptoms in PD patients.
Mitochondrial abnormalities
Toxic agents such as MPTP specifically inhibit mitochondrial complex I activity and consequently increase the production of ROS, and both events clearly contribute to MPTP-induced cell death [35]. As is known, dysfunctional mitochondria are related to oxidative damage [36] and mitochondrial dysfunction has a role in the pathophysiology of PD [37]. We sought to explore whether mitochondrial impairment was associated with cardiac denervation in this animal model, where oxidative stress is present because of a toxic effect. Our results in the cardiac tissue of MPTP-treated monkeys support this hypothesis. Differences in immunoreactivity were found between the groups studied. Surprisingly, we found a higher mitochondrial signal in cardiac nerve fascicles of parkinsonian monkeys, indicating that in MPTP-treated monkeys, as occurs in PD, a mitophagy failure might promote the accumulation of damaged mitochondria that cannot be efficiently degraded, or possibly, the increase in mitochondrial signal could appear as a result of a compensatory increase in mitochondrial activity due to the presence of oxidative stress in a toxic environment. In control animals, there is a low level of expression of MTCO2, but it is detectable. In fact, we suggest that the higher immunoreactivity of mitochondrial elements in MPTP-treated monkeys may be the result of a compensatory mechanism triggered by toxicity. That is, there is a higher mitochondrial signal as a consequence of an excessive turnover of mitochondria and decreased elimination [38].
Role of kinases
We found that PLK2 was present only in ganglionic neurons of cardiac tissue of MPTP-treated monkeys. PLK2 has been described as being upregulated in the brain of synucleinopathies [39], where it seems to play a role in synuclein phosphorylation, particularly at serine 129, the main component of LBs [40, 41]. Once they have been phosphorylated, kinases act as regulators of the cell cycle. In particular, PLK2 interacts with, phosphorylates, and enhances synuclein autophagy [42]. This function has been associated with a neuroprotective effect in synucleinopathies and hence PLK2 may also be playing a role as a potent modulator of synuclein turnover in cardiac tissue. The fact that we failed to detect PLK2 activity in control animals may indicate that PLK2 levels are related to a cascade of events triggered by the presence of the toxic effect of MPTP. We suggest that MPTP inhibits mitochondrial complex I and resulting in its dysfunction. In this scenario, PLK2 is upregulated in order to prevent cell death, by acting as an antioxidant [43] and regulating the cell cycle; however, in an attempt to achieve cell survival the phosphorylation of kinases would have a pathogenic role, promoting the aggregation of this protein.
In this animal model, we found a higher level of kinase activity, but we did not find LBs in cardiac fascicles. Moreover, protein aggregates did not contain phosphorylated synuclein. The lack of LBs may be due to the fact that there is a need for not only a toxic effect but also other factors, such as age and other post-translational changes in α-synuclein.
Conclusion
As occurs in PD, the peripheral nervous system is also affected in MPTP-treated monkeys. In these animals, there were notably few TH-ir fibers with preservation of NF-ir in the epicardium. VMAT and NET expression were significantly higher in the TH-ir fibers of MPTP-treated monkeys than controls (p < 0.05) and native forms of synuclein were observed in both groups, pointing to the possibility of an early phase of the disease in which the peripheral autonomic system may also be affected by neurotoxins that specifically inhibit mitochondrial complex I.
References
Stephenson R, Siderowf A, Stern MB (2009) Premotor Parkinson’s disease: clinical features and detection strategies. Mov Disord 24 Suppl 2(2):S665–S670
Wolters E (2009) Non-motor extranigral signs and symptoms in Parkinson’s disease. Parkinsonism Relat Disord 15 Suppl 3(3):S6–S12
Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R et al (2009) Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 15 Suppl 3(3):S1–S5
Kaufmann H, Goldstein DS (2013) Autonomic dysfunction in Parkinson disease. Handb Clin Neurol 117:259–278
Kaufmann H, Nahm K, Purohit D, Wolfe D (2004) Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology 63(6):1093–1095
Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M et al (2007) Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders? A cohort study. Neurology 68(23):2012–2018
Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K (2007) Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113(4):421–429
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601
Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO 3rd (2000) Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med 133(5):338–347
Tijero B, Gomez-Esteban JC, Lezcano E, Fernandez-Gonzalez C, Somme J, Llorens V et al (2013) Cardiac sympathetic denervation in symptomatic and asymptomatic carriers of the E46K mutation in the alpha synuclein gene. Parkinsonism Relat Disord 19(1):95–100
Amino T, Orimo S, Itoh Y, Takahashi A, Uchihara T, Mizusawa H (2005) Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol 15(1):29–34
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22(8):3090–3099
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25(1):239–252
Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61(5):413–426
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL et al (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119(6):689–702
Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P et al (2014) Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 82(15):1362–1369
Wang N, Gibbons CH, Lafo J, Freeman R (2013) α-Synuclein in cutaneous autonomic nerves. Neurology 81(18):1604–1610
Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K et al (2008) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131(Pt 3):642–650
Orimo S, Suzuki M, Inaba A, Mizusawa H (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord 18(5):494–500
Ren J, Porter JE, Wold LE, Aberle NS, Muralikrishnan D, Haselton JR (2004) Depressed contractile function and adrenergic responsiveness of cardiac myocytes in an experimental model of Parkinson disease, the MPTP-treated mouse. Neurobiol Aging 25(1):131–138
Amino T, Uchihara T, Tsunekawa H, Takahata K, Shimazu S, Mizusawa H et al (2008) Myocardial nerve fibers are preserved in MPTP-treated mice, despite cardiac sympathetic dysfunction. Neurosci Res 60(3):314–318
Metzger JM, Emborg ME (2019) Autonomic dysfunction in Parkinson disease and animal models. Clin Auton Res. https://doi.org/10.1007/s10286-018-00584-7
Pérez-Otaño I, Oset C, Luquin MR, Herrero MT, Obeso JA, Del Río J (1994) MPTP-induced parkinsonism in primates: pattern of striatal dopamine loss following acute and chronic administration. Neurosci Lett 175(1–2):121–125
Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K et al (2002) Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry 73(6):776–777
Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T et al (2005) Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol 109(6):583–588
Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW et al (2008) Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 116(3):277–288
Blesa J, Przedborski S (2014) Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat 8(155):155
Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S (2000) Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem 74(2):721–729
Kowall NW, Hantraye P, Brouillet E, Beal MF, McKee AC, Ferrante RJ (2000) MPTP induces alpha-synuclein aggregation in the substantia nigra of baboons. Neuroreport 11(1):211–213
Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA (1986) Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol 20(4):449–455
Goldstein DS, Holmes C, Kopin IJ, Sharabi Y (2011) Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest 121(8):3320–3330
Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS (2008) Generalized and neurotransmitter-selective noradrenergic denervation in Parkinson’s disease with orthostatic hypotension. Mov Disord 23(12):1725–1732
Sharabi Y, Li ST, Dendi R, Holmes C, Goldstein DS (2003) Neurotransmitter specificity of sympathetic denervation in Parkinson’s disease. Neurology 60(6):1036–1039
Gjerloff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, Jacobsen S et al (2015) Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain 138(Pt 3):653–663
Przedborski S, Tieu K, Perier C, Vila M (2004) MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr 36(4):375–379
Kahle PJ, Waak J, Gasser T (2009) DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med 47(10):1354–1361
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1(8649):1269
Batlevi Y, La Spada AR (2011) Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol Dis 43(1):46–51
Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M et al (2010) Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem 285(4):2807–2822
Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schobel S, Frigon NL et al (2009) Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem 284(5):2598–2602
Bergeron M, Motter R, Tanaka P, Fauss D, Babcock M, Chiou SS et al (2014) In vivo modulation of polo-like kinases supports a key role for PLK2 in Ser129 alpha-synuclein phosphorylation in mouse brain. Neuroscience 256:72–82
Oueslati A, Schneider BL, Aebischer P, Lashuel HA (2013) Polo-like kinase 2 regulates selective autophagic alpha-synuclein clearance and suppresses its toxicity in vivo. Proc Natl Acad Sci USA 110(41):E3945–E3954
Li J, Ma W, Wang PY, Hurley PJ, Bunz F, Hwang PM (2014) Polo-like kinase 2 activates an antioxidant pathway to promote the survival of cells with mitochondrial dysfunction. Free Radic Biol Med. 73:270–277
Funding
This study was partially co-funded by the Instituto de Salud Carlos III through project PI12/01730 from the Spanish Health Research Fund (FIS) and the Association of Friends (ADA) of the University of Navarra (MCA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carmona-Abellan, M., Martínez-Valbuena, I., DiCaudo, C. et al. Cardiac sympathetic innervation in the MPTP non-human primate model of Parkinson disease. Clin Auton Res 29, 415–425 (2019). https://doi.org/10.1007/s10286-019-00620-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-019-00620-0