Abstract
Purpose
The average adult stands approximately 50–60 times per day. Cardiovascular responses evoked during the first 3 min of active standing provide a simple means to clinically assess short-term neural and cardiovascular function across the lifespan. Clinically, this response is used to identify the haemodynamic correlates of patient symptoms and attributable causes of (pre-)syncope, and to detect autonomic dysfunction, variants of orthostatic hypotension, postural orthostatic tachycardia syndrome and orthostatic hypertension.
Methods
This paper provides a set of experience/expertise-based recommendations detailing current state-of-the-art measurement and analysis approaches for the active stand test, focusing on beat-to-beat BP technologies. This information is targeted at those interested in performing and interpreting the active stand test to current international standards.
Results
This paper presents a practical step-by-step guide on (1) how to perform active stand measurements using beat-to-beat continuous blood pressure measurement technologies, (2) how to conduct an analysis of the active stand response and (3) how to identify the spectrum of abnormal blood pressure and heart rate responses which are of clinical interest.
Conclusion
Impairments in neurocardiovascular control are an attributable cause of falls and syncope across the lifespan. The simple active stand test provides the clinician with a powerful tool for assessing individuals at risk of such common disorders. However, its simplicity belies the complexity of its interpretation. Care must therefore be taken in administering and interpreting the test in order to maximise its clinical benefit and minimise its misinterpretation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An average adult stands approximately 50–60 times per day [1, 2]. Cardiovascular responses evoked during active standing provide a simple means of assessing short-term neural and cardiovascular function across the lifespan [3, 4]. While head-up tilt testing (HUT) can also be used to assess orthostatic responses in the clinic [4, 5], our focus here is on the first 3 min of the active stand response, since it is a convenient, provocative test that mimics daily activities.
The active stand is used clinically to assess the spectrum of abnormal cardiovascular responses to standing [3, 5, 6] and to evaluate variants of orthostatic hypotension (OH) [7], orthostatic hypertension (OHTN) [8,9,10], causes of unsteadiness and orthostatic intolerance [3, 4], (pre-)syncope [4], autonomic (dys)function [6, 11] and postural orthostatic tachycardia syndrome (POTS) [12]. Active standing may also be used to evaluate the presence of POTS or delayed OH. Under that circumstance, the test duration should be extended to at least 10 minutes.
Emerging research indicates that impaired active stand responses (asymptomatic or symptomatic) are risk factors for injurious falls [13,14,15,16], depression [17, 18], cognitive dysfunction [19,20,21,22,23], incident cardiovascular disease, heart failure [24], stroke and mortality [25,26,27], underscoring the importance of the availability of this measure. Taken together with the growth in the number of methods to measure continuous beat-to-beat non-invasive blood pressure (BP) and heart rate (HR) in clinical and daily living conditions [28, 29], it likey that assessment of the hemodynamic response to active standing will be of increasing importance.
While intermittent auscultatory or oscillometric blood pressure measurements have been used in screening for sustained OH, continuous non-invasive measurement of finger arterial pressure (FinAP) is now the preferred approach for evaluating the active stand response and identification of OH and all its variants [3, 4, 6]. However, no practical guidelines detailing optimal testing protocols and analysis are available for beat-to-beat approaches in this field. In particular, explicit protocols, that assist in measurement and analytical steps required to apply beat-to-beat technologies are lacking. This is reflected in the variability in testing and analysis approaches reported in the literature [7, 22, 27, 30, 31], hindering the clinical utility of the test, its standardisation and broader adoption, and the comparability of past and future research.
Our aim here is to present the reader with a practical guide, based on our combined clinical and research experience in the evaluation of autonomic disorders, which details (1) how to perform active stand measurements, with a focus on using beat-to-beat BP measurement approaches, and (2) how to analyse these beat-to-beat active stand responses. We complement this with background information to help the reader better understand the principles of suggested technical approaches so that they can be applied in an informed manner.
Methods
This paper provides a set of experience/expertise-based recommendations detailing current state-of-the-art measurement and analysis approaches for the active stand test. This information is targeted at those interested in performing and interpreting the active stand test in line with current international standards, including geriatricians, neurologists, cardiologists, clinical nurse specialists, allied health professionals and researchers. Our aim is to foster greater standardisation across clinical practice (and research), thereby improving the comparability and clinical utility of this test. To further assist, we provide detailed supplementary standard operating procedures to support those individuals carrying out the specifics of this test.
Traditional arm-cuff and beat-to-beat BP approaches are complementary, with beat-to-beat approaches providing an extension of the traditional method of classical OH detection and enabling the detection of further subtypes of OH. Both approaches have their advantages and disadvantages (see Table 1). Traditional arm-cuff-based measurements are suited to screening for classical OH in the community and/or non-specialist settings, being simple and relatively low-cost to administer, but are unsuitable for capturing fast transient cardiovascular responses which characterise other important variants of OH and related autonomic disorders. Beat-to-beat approaches are now the preferred option for evaluating such conditions. The primary focus here will therefore be on measurement and analysis of the active stand test using these modern beat-to-beat BP measurement approaches. This approach can also be easily adapted for performing a simplified screening test using traditional arm-cuff methods.
Initially, we provide a high-level overview of the process of measurement and analysis of the active stand response. Where appropriate, we then guide the interested reader to detailed online supplementary information including standard operating procedures for both beat-to-beat and arm-cuff-based approaches.
The paper is structured as follows:
-
1.
Overview of the spectrum of haemodynamic responses to active stand
-
2.
Testing environment, measurement technology, pre-test setup
-
3.
How to perform an active stand measurement
-
4.
How to analyse and interpret the active stand response
Spectrum of haemodynamic responses to active standing
Figure 1 depicts a normal BP and HR response to active stand and its key features. For a comprehensive review of the underlying physiological mechanisms involved in the active stand response, please refer to the companion article by van Wijnen et al. [3]. Here we provide a synopsis only.
Blood pressure (top) and heart rate response (bottom) to active standing identifying the key features of interest used to derive clinical definitions in subsequent steps. Refer to Supplementary Table 1 for full details of how these features are defined and calculated. a = supine SBP/DBP/HR average 60 to 30 s prior to standing (see Sect. 2.2 for rationale); b = immediate BP peak; c = BP nadir; d = BP overshoot; en = steady-state values at n seconds after standing; f = maximum HR; g = minimum HR; ΔSBP = difference between the SBP/DBP baseline (a) and the nadir (c); HRmax − HRmin = difference between maximum and minimum HR; HRR = speed of heart rate recovery, i.e. HR10 – HR20; tr = recovery time, i.e. the time taken for the SBP/DBP to recover back to within a pre-determined fraction of the baseline. Here it is depicted as the time taken to return to baseline value
On active standing, a characteristic biphasic initial (first 30 s) HR and BP response is observed. The initial BP decline, reaching a minimum within 10 s of standing, is a reflection of a mismatch between an increase in cardiac output resulting from leg muscle pumping and abdominal compression, and instantaneous vasodilatation in the active leg muscle [3, 6]. Short-term baroreflex mechanisms result in quick increases in HR (within 1–3 s), cardiac contractility (within 3–8 s), and peripheral arterial and venous vasoconstriction (within 10–30 s), leading to arterial BP recovery. In the majority of healthy individuals, these compensatory mechanisms result in BP re-stabilisation to supine values within 20–30 s [3]. The biphasic BP and HR response on active standing is far less or absent on passive tilting. At stabilisation approximately 500 ml of blood has been translocated to areas below the heart and diaphragm [32].
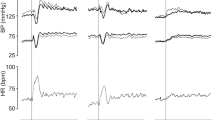
Figure 2 illustrates the spectrum of haemodynamic responses to active stand with the following abnormal responses most commonly identified.
Spectrum of blood pressure responses to active standing [systolic blood pressure (red), diastolic blood pressure (blue)] and heart rate (green). Six common clinical patterns are shown: a normal recovery; b initial orthostatic hypotension—note the larger initial BP drop; c delayed recovery—note the delayed recovery back to baseline and also motion artefact affecting the initial standing HR and BP data in this response; e orthostatic hypertension—note the sustained BP overshoot after standing; f postural orthostatic tachycardia syndrome—note the sustained HR increase in the absence of orthostatic hypotension. The blue vertical line at 0 s indicates the initial stand time. All data have been filtered by a ±1-s moving average filter to remove signal noise
-
Initial orthostatic hypotension (IOH): IOH is defined as a transient decrease in systolic BP (SBP) of > 40 mmHg and/or > 20 mmHg in diastolic BP (DBP) within 15 s of standing in the absence of sustained OH (Fig. 2b) [3, 5, 32].
-
Delayed recovery: Delayed recovery has been defined to date as the inability of SBP to recover to ≤ 20 mmHg of supine baseline values at 30–40 s after standing but not meeting the criteria of classical OH (see below). The delay can be considerable, but recovery occurs by definition within 3 min of standing (Fig. 2c) [3, 7].
-
Classical orthostatic hypotension (OH): Classical OH has been defined as a sustained decrease of ≥ 20 mmHg in SBP (or ≥ 30 mmHg in those with baseline hypertension) or ≥ 10 mmHg (≥ 15 mmHg in those with baseline hypertension) in DBP (or SBP < 90 mmHg) occurring between 60 and 180 s of standing (Fig. 2d) [3, 5, 7].
-
Orthostatic hypertension (OHTN) This is the converse of classical OH and is defined as a sustained increase of ≥ 20 mmHg in SBP or ≥ 10 mmHg (or > 140/90 mmHg if patient is normotensive supine). Sustained is defined as occurring at all time points occurring 60–180 s after the standing (Fig. 2e) [8,9,10].
-
Postural orthostatic tachycardia syndrome (POTS): Sustained tachycardia after standing > 30 bpm (> 40 bpm in those aged < 18) over baseline or > 120 bpm without concurrent OH. Sustained tachycardia after standing > 30 bpm (> 40 bpm in those aged < 18) over baseline or > 120 bpm without concurrent OH. A 10 min standing/upright tilt recording is necessary to confirm and/or rule out POTS (and delayed OH) definitively (Fig. 2f) [12].
Unsurprisingly given the natural underlying variability in the autonomic nervous system, active stand HR and BP responses demonstrate low to moderate test–retest reliability (ICC ≈ 0.5–0.8), and relatively wide values of minimum detectable change (25 mmHg SBP; 12–16 bpm HR) [33]. The nadir values (and derived changes from baseline) tend to be the least reliable (ICC ≈ 0.5), with more reliable values occurring later in the steady-state period of the stand (ICC ≈ 0.8) [33, 34]. Applying the results of active stand responses over longitudinal periods, for example in tracking treatment changes and/or disease progression, therefore warrants careful consideration.
Testing environment and measurement technology
Testing environment
In order to minimise stimuli affecting autonomic nervous system function, the test should be performed in a quiet, dimly lit room at a comfortable temperature maintained at 21–23 °C [6]. Higher temperatures can lead to vasodilation, increasing the probability of OH, with colder temperatures having the opposite effect.
Measurement technology: the FinAP method
The FinAP method enables continuous non-invasive BP measurements and is based on pulsatile unloading of the finger arterial walls using an inflatable finger cuff with built-in plethysmograph [35]. The plethysmograph measures changes in arterial blood volume, and the inflatable cuff pressure is controlled so that a constant arterial blood volume is maintained (‘volume-clamped’) [29]. Non-invasive continuous FinAP recordings are similar in appearance to intra-arterial BP recordings and have been validated for tracking orthostatic changes in BP, but these measurements are not identical [35]. This is because arterial waveforms in the finger differ from more central arteries.
Modern devices report the brachial pressure reconstructed from the finger pressure, and correct for the above effects. The accuracy of this approach is maintained by a combination of a dynamic set-point controller (Finometer, Nexfin [36, 37], ClearSight, Nano systems all use ‘Physiocal’ [38]; Task Force monitor uses ‘VERIFI’ [39]), a height correction unit which counteracts hydrostatic pressure differences between finger and heart, and reconstruction filters to transform finger pressure to brachial pressure waveforms. Despite these systems, systolic BP values in individual patients may still deviate significantly (underestimate) from the ‘true’ intra-arterial (radial or brachial) values. Recent studies suggest that reconstructed FinAP pressure levels lie between invasively measured pressures and auscultatory pressures, with FinAP measurements remaining accurate at low pressures [40]. We therefore use the FinAP approach to measure changes in BP specifically.
Proper use of the FinAP approach requires attention to a number of key issues. At regular time intervals, the set-point controller can lead to brief signal interruptions during critical time periods, such as nadir. These can be avoided by temporarily switching the set-point controller off. We usually leave this off for 1 min only, but no more than 3 min, as the measurements may become unreliable after this period [40]. Peripheral BP is also affected by the vertical distance from the measurement site to the heart, due to the mid-axillary line (Fig. 3). The measurement arm is often supported by a loose sling, which has the added benefit of reducing the likelihood of movement artefact contamination of the BP signal.
In patients with extensive vascular disease such as atherosclerosis or systemic vasculitis, or conditions associated with severe peripheral vasoconstriction (e.g. patients who feel uncomfortable, feel peripheral cold, are nervous, are in pain or under light anaesthesia), it is not always possible to obtain a reliable registration of the continuous BP [35, 41]. Warming the hand with warm water, a warm cloth, or a glove or mitten filled with warm water can improve measurements in these situations [36] (see Supplementary Table 1).
Haemodynamic parameters such as stroke volume (SV), cardiac output (CO), and systemic vascular resistance (SVR) can also be derived from the measured BP waveforms (sampled at 200 Hz, filtered between 0.01 and 100 Hz) using mathematical estimates of these parameters [42, 43]. This approach captures trend variations in CO and SVR, with absolute values less accurate [37, 44], requiring calibration with a suitable reference standard CO measurement technique (e.g. thermodilution, rebreathing, echocardiography, MRI). All CO methods have large errors (approximately 20% when expressed as standard deviation/mean); however, the errors in trend variations for Windkessel-based methods are typically 10–30% [42, 43, 45].
How to perform an active stand measurement
A graphical description of the protocol timeline typically employed is shown in Fig. 4. In addition, an active stand protocol video can be found at: https://youtu.be/NwWFie1_ddU?t=397 (6:36-7:06). Supplementary Table 1 provides detailed standard operating procedures to inform testing. See Supplementary Table 2 detailing standard operating procedures for arm-cuff-based approaches only [46].
Active stand indications
The active stand should be considered in patients with recurrent symptoms associated with orthostasis, syncope and pre-syncope [4], falls or unexplained falls [47], in the investigation of autonomic function in peripheral autonomic neuropathies, e.g. diabeteic neuropathy [48], central and peripheral neurodegenerative disorders, as well as suspected POTS [12]. It can be performed independently to evaluate symptom reproduction, or as part of an autonomic function test battery [6]. Additionally, it can be used to evaluate the effectiveness of therapeutic interventions (e.g. medication modifications, lifestyle interventions) when the repeatability is carefully considered (see previous section on repeatability) [4, 49] and for biofeedback-based patient education, i.e. demonstrating the haemodynamic changes associated with standing and physical counter manoeuvres [50].
Active stand contraindications
Active stand testing is safe, with no known contraindications. Assistance with standing should be available for those who are unsteady or at a risk of falls and should be supervised by qualified personnel who have been trained in active stand procedures and in managing any safety issues/clinical events that arise (e.g. CPR-trained, trained in managing syncopal events). While very rare in our experience, some patients may be unable to stand (e.g. due to severe mobility issues). In these rare situations, a short HUT test (3–5 min) is used to ascertain HR and BP responses to orthostasis [4, 5]. The HUT response can be used to diagnoses classical OH since the steady-state BP and HR responses are similar to the active stand. It can also be used for identification of POTs, vasovagal syncope and delayed OH. However, since the initial HR and BP transient is absent and/or much attenuated [51] during HUT it is not suitable for identifying initial orthostatic hypotension (IOH), delayed BP recovery and indices of autonomic dysfunction that rely on the initial transient HR response to standing (e.g. speed of heart rate recovery (HRR), HRmax − HRmin, HRmax/HRmin Ewing’s 30:15 ratio) [3]. Ultimately these two methods for assessing orthostatic intolerance (or tolerance) can be considered complimentary.
Patient preparation
Patients are typically required to fast (minimum 2 h after the last meal) before testing to avoid the confounding effects of postprandial hypotension [52]. Testing in the morning is preferable, since test sensitivity is increased, and also volume depletion-related OH more likely [5] and fasting more easily achieved. Factor that affect test performance include time of day [6, 53,54,55], caffeine, nicotine intake (withhold for 3–4 h), alcohol, taurine-containing beverages,. e.g. Red Bull (withhold for 8–12 h) [56]. and vigorous exercise (avoid for 12 h) [57, 58], These should be controlled where repeatability (see previous section on repeatability) of testing is of importance, e.g. evaluating the effects of treatment or in research setting.
Medications
Medications affecting the cardiovascular and autonomic nervous system, particularly antihypertensive drugs and those likely to affect intravascular volume, should be noted. For purposes of initial test interpretation, it is best to continue the medications that the patient was taking when symptoms occurred, although modification (withdrawal) of culprit medications is a frequent intervention for observed OH, syncope and falls and to assess disease state, and may be required for specific research protocols [4].
Instructions and setup
Prior to testing, the procedure is explained to the patient. Particular attention is drawn to instructing participants to avoid unnecessary movement and talking throughout testing, since changes in intrathoracic pressure or external pressures on the finger cuff or arm movement can influence BP waveform measurement accuracy. During this setup period, patient history of orthostatic symptoms can be recorded.
The patient should sit upright on a standard clinical examination table or tilt-table style bed while the FinAP system (and traditional arm cuff) is attached as per manufacturer guidelines. Sitting during setup helps to ensure that the duration of lying can be maintained to within 5–10 min. Alternatively, some units perform a practice stand for the purposes of test familiarisation and controlling the duration of lying. Both of these approaches may not always be possible in a busy clinical environment, and instrumentation may be more convenient to perform in the supine position. In this case duration of lying should be controlled to 5–10 min (see next section). It is essential that finger cuff (and arm cuff) size selection is appropriate for each patient to maximise measurement accuracy. The height correction system should be nulled and securely fastened at heart level (the fifth intercostal space (mid-sternum) in the mid-axillary line) and on the finger cuff, with the measurement arm supported by a loose sling (see Fig. 3). The calibrating arm cuff should be placed above the elbow at the level of the heart (on the contralateral or ipsilateral side depending on the system employed). A separate ECG system may then be applied to record leads I, II and III and derive the RR interval or HR variability.
Supine baseline measurements
After setup, the patient is asked to lie supine for 5-10 min, with the FinAP system turned on at the beginning of this supine period. This allows for adequate cardiovascular stabilisation [59]. A 5-min rest period is considered sufficient to achieve stable baseline BP values, since there is little difference in the initial nadir after supine rest between 5 and 10 min, while a very short (< 5 min) period could lead to an unstable baseline, and very long periods of supine rest (> 10 min) may lead to larger initial BP drops [60].
At this stage, all effort should be made to ensure that the recorded BP is stable and that the set-point controller interval reaches > 30 beats and system calibration is performed using arm-cuff BP measurements. This reduces the likelihood of artefacts during standing. Note that this resting period can be used to identify supine hypertension and derive 5-min resting measurements of BP and HR variability (BPV and HRV), which are markers of global autonomic (dys)function [61]. From this resting data, it is also possible to derive spontaneous resting baroreflex sensitivity measures [62], which are used to indicate the integrity of short-term baroreflex HR control. Ninety seconds prior to standing, a standard oscillometric or auscultatory BP measurement on the contralateral arm should be taken to assess absolute baseline BP values.
Standing measurements
After rest, patients are asked to stand up as quickly as possible during inspiration (where possible) to minimise straining and arm movement, which can be achieved by asking patients to neither hold their breath or strain. Just prior to standing (−30 s), the set-point controller should be switched off to avoid signal interruption/loss during large initial BP transients. Should signal loss occur it is can be necessary to repeat the stand.
Some centres pre-empt the stand using a countdown procedure of 5–4–3–2–1, while others use a verbal command. The stand time (i.e. the time at which the patient begins to move by lifting their torso) must then be accurately marked. This can be achieved using a manual event mark or an automated approach such as bed-based pressure sensors. The stand can be timed with a stopwatch if using a traditional arm-cuff system for screening. Standing typically takes < 3 s for younger, healthier subjects, while frail adults may take longer, with assistance often necessary. Individuals are asked to remain standing unaided for 3 min. After 1 min standing (and BP has sufficiently stabilised), the set-point controller should be turned back on. If this results in a significant BP correction (> 10 mmHg change), the BP values during this set-point controller ‘off’ period should be interpreted with caution. A repeat measurement may be required to authenticate the quality of the recording in this case. At this time point (1 min), an arm-cuff measurement should be taken if a screening test is being performed. After 3 min, a final brachial arm-cuff BP measurement can be taken. Patients are then asked to sit or lie down to allow BP to return to baseline (usually this is for 1 min). In instances where delayed OH and/or POTs is suspected, the active stand can be prolonged to 5–10 min to increase its sensitivity to these conditions.
Timing of the symptoms should be marked in real time using suitable event markers. Patients are asked to describe their symptoms using a grading system (mild, moderate, severe) at 1 and 3 min after standing (see Supplementary Appendix 1 on Symptom Assessment). All talking by the patient should be avoided during the testing where possible especially during the transient changes with symptom cue cards used to minimise talking (see Supplementary Appendix 2 for sample cue cards). Testing is terminated by the operator if pre-syncope/syncope ensues or if an individual cannot stand for the full duration of the test.
Signal quality assessment
A signal quality checklist (see Supplementary Table 1 Sect. 8) can be employed to assist in optimising quality or in identifying reasons for suboptimal-quality records [46]. Additionally, regular feedback and discussions focused on signal quality improves knowledge, experience and quality. Training can also target troubleshooting ‘difficult’ recording situations, e.g. making measurements in those with poor peripheral circulation due to cold fingers, Raynaud’s syndrome, heavy smokers, those with exceptionally thin/short fingers, arthritic fingers, Parkinson’s disease (which can cause rigidity and/or excessive tremor), or peripheral artery disease [36, 63]. These patients are of greatest clinical interest but are also difficult to obtain good-quality recordings in, and thus clinicians must be prepared to deal with these technical challenges. See Supplementary Table 1 for troubleshooting tips in these challenging cases.
Operator training
Training of operators should be performed under the supervision of an experienced operator until proficiency in testing and troubleshooting common problems is achieved. In our experience, training for 30–50 stands provides sufficient experience for an operator to work independently.
Of paramount importance is the recognition of common quality and BP waveforms issues [64]. Continual quality assessment is a particularly important part of the methodology which is often left unaddressed in the literature. During training, particular attention should be focused on:
-
1.
Identification of stable, high-quality BP records which are free of artefacts when recording baseline and transient BP measurements.
-
2.
Ensuring the height correction unit is properly attached and zeroed before the test commences.
-
3.
Recognising and minimising motion and external pressures being applied to the finger pressure cuff during standing, as this often leads to over-reading and significant artefacts during the active stand.
-
4.
Accurate marking of events and recording of symptoms.
How to analyse and interpret the active stand response
Specific details and standardisation are often lacking in the literature surrounding active stand data processing using beat-to-beat responses. This section provides an overview of this process (see Fig. 5 and [64] for more detail). Ultimately, the goal of this process is to extract features from the beat-to-beat and filtered responses which are representative of the BP and HR patterns of primary clinical interest.
Analysis requires the following steps: (1) data preprocessing and filtering, (2) feature extraction and (3) determining clinical definitions. Each of these steps can be easily performed in manufacturer-supplied clinical software such as NOVAScope or Nexfin@PC, or a standard data analytics package such as MATLAB®. While the manufacturer software is designed for clinical use, further expertise in biomedical engineering and/or advanced data analysis is recommended if analysing very large databases. These steps are not required if using traditional arm-cuff measurements, since filtering and analysis is performed automatically.
Data preprocessing and filtering
Prior to determining the clinical features of importance, a number of preprocessing steps must be performed, including stand time determination, data quality assessment, artefact rejection and filtering.
Accurate extraction of the stand time is important, since some clinical features of interest rely on the time after stand [3, 13, 64]. Stand time is defined as the moment when the patient lifts their upper torso off the bed and is usually identified using a manual or automated event mark. Additional sensor data can also be used to detect the start of the stand (e.g. height correction unit, bed occupancy pressure sensors, initiation of HR and BP transients during standing) [64].
The next step is to identify poor-quality waveform data (similarly to ECG analysis). Recognition of these abnormalities can be performed by scanning baseline and pre-stand, the transition (first 30 s) and standing steady-state (30–180-s) periods. Specific noise sources of note include motion artefacts, calibration artefacts (‘Physiocal’ or brachial-cuff calibration), the height correction unit has fallen off or is inappropriately connected, misidentification of beats (e.g. large reflected waves may be misreported as a beat), pulse pressure dampening (low pulse pressure < 20 mmHg), signal oscillations (due to inappropriate cuff fit), and indications of difficulty in finding a set-point like frequent and/or long staircases in Physiocal. The presence of arrhythmias may appear similar to a non-physiological source of artefacts. It is recommended also to monitor the height correction unit signal to identify large unwanted motion artefacts/fast arm movements.
While clinical software does not explicitly allow the removal of artefact beats, application of basic filtering approaches is often sufficient to minimise their effects [7, 26, 64]. Shorter time windows (±1-s averages) are recommended if beat-to-beat values are of most interest (e.g. nadir/peak HRs) [64] in the presence of noise. A ±5-s averaging method as described by van der Velde et al. [65] is appropriate when interested in slower BP recovery patterns, which are dominated by slower (≈10 s) baroreflex-driven sympathetic modulation of arterial [3, 27, 66] BP and venous tone (≈30 s [66]). In more severe cases of artefact, beat removal and interpolation may be required prior to filtering. This advanced analysis requires signal processing expertise.
The presence of frequent ectopic beats, atrial fibrillation or other rhythm disturbances makes interpretation of the active stand challenging, since it may mask the overall response pattern (see Fig. 6). These arrhythmias increase the beat-to-beat variability of the BP and HR signals but reflect the true physiological state of the patient [67]. In our experience, applying a 5–7-beat moving average filter can assist in visually identifying the underlying active stand responses of interest, i.e. delayed recovery, classical OH, and enables a full analysis to be completed. However, it should be noted that measuring specific beat-to-beat changes in HR or BP (e.g. maximum HR change or nadir) may be underestimated when filters are applied, and so any analysis should note the level of filtering incorporated to aid interpretation.
Systolic (red) and diastolic (blue) blood pressure (top) and heart rate response (bottom) to active standing in an individual with atrial fibrillation (AF), where the dashed lines indicate the unfiltered beat-to-beat values, while the solid lines depict the filtered responses (±2.5-s moving average filter). Arrhythmias such as atrial fibrillation are physiological noise sources, i.e. the high variability is physiological. However, this masks the overall BP and HR response, making it difficult to identify the blood pressure response pattern, the nadir values and time to recovery. Applying a ±2.5-s moving average window here clarifies that the blood pressure pattern during active stand looks to be a borderline case, i.e. a normal initial blood pressure drop, with normal to mild delay, taking approximately 30 s to recover to baseline. Despite filtering, it remains difficult to characterise the heart rate response in those with AF
Clinical feature extraction
Since the original definitions of OH were based on the oscillometric approach, beat-to-beat definitions of impaired BP and HR responses to standing are less standardised in the literature [5]. Here, to guide this process, we propose to invoke tools from systems and control theory and analysis which are suitable for capturing the majority of BP and HR responses that are of primary clinical interest [68]. This analytical framework is flexible and can be extended to describe further patterns which may be of future clinical interest, such as increased BP variability during standing [9, 69].
Visual assessment of patterns
Figure 1 describes the key BP and HR patterns of interest. The first goal of interpretation should be to recognise three key periods of the response, i.e. baseline, initial transient and early standing steady-state periods, and how they deviate from a normal response. This will help prevent misinterpretation of the numerical values extracted at the next stage of analysis. The presence/absence of significant oscillations/variability and any periods of artefacts can also be noted at this stage. If the baseline is normotensive, the initial BP drop is small, the transient period is complete within 20 s, and steady-state values are similar to normotensive baseline, then a normal response is likely. This first ‘eyeballing’ evaluation should then enable an initial classification of the response as a normal or abnormal response (or other).
BP and HR feature extraction
Figure 1 depicts the key features used to characterise the beat-to-beat BP and HR responses. Please refer to Supplementary Table 3 for the specific definitions of each of these points. Firstly, the baseline is derived as the mean of values occurring 60 to 30 s prior to standing. This value minimises the influence of the anticipatory rise and movement artefacts on subsequent drops [3, 64] and is used to identify the presence of supine hypertension. Secondly, the beat-to-beat values (used in defining IOH, HRmin, HRmax) which fall within the first 30 s of standing can be easily measured using the measurement tool of the manufacturer-supplied software. If noisy data are present, consider using a ±1-s moving average filter to measure the beat-to-beat parameters. Once these initial points are measured, turn on the ±5-s moving average filter and record the steady-state values at 30-s intervals. These can then be used for identifying delayed recovery and/or sustained OH and the supine baseline values.
These values can also be used to derive other measures that assess the integrity of the autonomic nervous system including HR recoverability measures (e.g. speed of heart rate recovery (HRR), HRmax − HRmin, HRmax/HRmin Ewing’s 30:15 ratio) and baroreflex sensitivity measures (e.g. delta HR/delta SBP). The later may also be useful in differentiating between neurogenic and non-neurogenic causes of OH [70].
Determining the clinical classification
Once the key features have been calculated from the response, these values can be used to numerically confirm the clinical definitions of interest, i.e. normal BP and HR recovery, IOH, delayed BP recovery, classical OH and POTS. Table 2 contains definitions that apply criteria to beat-to-beat data for commonly identified responses of clinical interest (Fig. 2). The presence of an impaired HR response to standing in the presence of OH can also enable identification of neurogenic OH. Supine baseline BP values can also be used to identify if the patient has coexisting supine hypertension. These definitions can also be combined with symptom assessment to identify whether the active stand response is symptomatic or asymptomatic (see Supplementary Appendices 1 and 2), with recent evidence suggesting that symptomatic OH at 30 s after standing has prognostic value in predicting the development of depression in older adults [18]. Note that if using a traditional arm-cuff approach, only the definitions of OH, OHTN and POTs can be applied (Table 2).
Conclusion
Impairments of neurocardiovascular control are an attributable cause of instability, falls and syncope across the lifespan. The simple active stand test coupled with non-invasive beat-to-beat continuous blood pressure measurement provides the clinician with a powerful tool for assessing individuals at risk of such common disorders. However, its simplicity belies the complexity of the underlying neurocardiovascular responses observed during standing. Care must therefore be taken in administering and interpreting the test to maximise its clinical benefit and minimise its misinterpretation. This paper presents a clear and concise protocol and analysis procedure to assist with operationalising procedures for the active stand test and symptom capture. This should foster better harmonisation and standardisation of clinical practice and research studies, and thus improve the comparability and clinical utility of the test.
References
Smith L, Hamer M, Ucci M, Marmot A, Gardner B, Sawyer A et al (2015) Weekday and weekend patterns of objectively measured sitting, standing, and stepping in a sample of office-based workers: the active buildings study. BMC Public Health 17(15):9
Dall PM, Kerr A (2010) Frequency of the sit to stand task: an observational study of free-living adults. Appl Ergon 41(1):58–61
van Wijnen VK, Finucane C, Harms MPM, Nolan H, Freeman RL, Westerhof BE et al (2017) Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med 282(6):468–483
Brignole M, Moya A, Lange D, Deharo J-C, Elliott PM et al (2018) 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 39(21):1883–1948
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I et al (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21(2):69–72
Wieling W, Karemaker JM (2013) Measurement of heart rate and blood pressure to evaluate disturbances in neurocardiovascular control. In: Mathias CJ, Bannister SR (eds) Autonomic failure, 5th edn. Oxford University Press, Oxford
Finucane C, O’Connell MD, Fan CW, Savva GM, Soraghan CJ, Nolan H et al (2014) Age related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation 130(20):1780–1789
Fessel J, Robertson D (2006) Orthostatic hypertension: when pressor reflexes overcompensate. Nat Rev Nephrol 2(8):424–431
Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T et al (2002) U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol 40(1):133–141
Fedorowski A, Ostling G, Persson M, Struck J, Engström G, Nilsson PM et al (2012) Orthostatic blood pressure response, carotid intima-media thickness, and plasma fibrinogen in older nondiabetic adults. J Hypertens 30(3):522–529
Smit AAJ, Halliwill JR, Low PA, Wieling W (2017) Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol 519(1):1–10
Low PA, Sandroni P, Joyner M, Shen W-K (2009) Postural Tachycardia Syndrome (POTS). J Cardiovasc Electrophysiol 20(3):352–358
Finucane C, O’Connell MDL, Donoghue O, Richardson K, Savva GM, Kenny RA (2017) Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc 65(3):474–482
Shaw BH, Claydon VE (2014) The relationship between orthostatic hypotension and falling in older adults. Clin Auton Res 24(1):3–13
Juraschek SP, Daya N, Appel LJ, Miller ER, Windham BG, Pompeii L et al (2017) Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens 30(2):188–195
Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER, Windham BG et al (2017) Comparison of early versus late orthostatic hypotension assessment times in middle-age adults. JAMA Intern Med 177(9):1316–1323
Briggs R, Kenny RA, Kennelly SP (2017) Does baseline hypotension predict incident depression in a cohort of community-dwelling older people? Data from the Irish Longitudinal Study on Ageing (TILDA). Age Ageing 46(4):648–653
Briggs R, Carey D, Kennelly SP, Kenny RA (2018) Longitudinal association between orthostatic hypotension at 30 seconds post-standing and late-life depression. Hypertension 71:946–954
Frewen J, Savva GM, Boyle G, Finucane C, Kenny RA (2014) Cognitive performance in orthostatic hypotension: findings from a nationally representative sample. J Am Geriatr Soc 62(1):117–122
O’Hare C, Kenny R-A, Aizenstein H, Boudreau R, Newman A, Launer L et al (2017) Cognitive status, gray matter atrophy, and lower orthostatic blood pressure in older adults. J Alzheimers Dis 57(4):1239–1250
Frewen J, Finucane C, Savva GM, Boyle G, Kenny RA (2014) Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J Gerontol Ser A 69(7):878–885
Hayakawa T, McGarrigle CA, Coen RF, Soraghan CJ, Foran T, Lawlor BA et al (2015) Orthostatic blood pressure behavior in people with mild cognitive impairment predicts conversion to dementia. J Am Geriatr Soc 63(9):1868–1873
Holm H, Nägga K, Nilsson ED, Melander O, Minthon L, Bachus E et al (2017) Longitudinal and postural changes of blood pressure predict dementia: the Malmö Preventive Project. Eur J Epidemiol 32(4):327–336
Fedorowski A, Engström G, Hedblad B, Melander O (2010) Orthostatic hypotension predicts incidence of heart failure: the Malmö preventive project. Am J Hypertens 23(11):1209–1215
Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O (2010) Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmö Preventive Project). Eur Heart J 31(1):85–91
McCrory C, Berkman L, Nolan H, O’Leary N, Foley M, Kenny RA (2016) Speed of heart rate recovery in response to orthostatic challenge: a strong risk marker of mortality. Circ Res 119(5):666–675
Lagro J, Schoon Y, Heerts I, Meel-van den Abeelen ASS, Schalk B, Wieling W, et al. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J Gerontol A Biol Sci Med Sci. 2014;69(4):471–8
Chung E, Chen G, Alexander B, Cannesson M (2013) Non-invasive continuous blood pressure monitoring: a review of current applications. Front Med 7(1):91–101
Truijen J, van Lieshout JJ, Wesselink WA, Westerhof BE (2012) Noninvasive continuous hemodynamic monitoring. J Clin Monit Comput 26(4):267–278
Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW (2011) Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc 59(4):655–665
Finucane C (2008) Identifying blood pressure response subtypes following orthostasis using pattern recognition techniques. http://www.tara.tcd.ie/handle/2262/66730. Accessed 3 Jul 2018
Wieling W, Krediet CTP, van Dijk N, Linzer M, Tschakovsky ME (2007) Initial orthostatic hypotension: review of a forgotten condition. Clin Sci Lond Engl 1979. 112(3):157–65
Finucane C, Savva GM, Kenny RA (2017) Reliability of orthostatic beat-to-beat blood pressure tests: implications for population and clinical studies. Clin Auton Res 27(1):31–39
Lipsitz LA, Storch HA, Minaker KL, Rowe JW (1985) Intra-individual variability in postural blood pressure in the elderly. Clin Sci 69(3):337–341
Imholz BP, Wieling W, van Montfrans GA, Wesseling KH (1998) Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 38(3):605–616
Edwards Lifesciences BMEYE (2008) Nexfin HD Operator’s manual. Edwards Lifesciences BMEYE, Amsterdam
Bartels SA, Stok WJ, Bezemer R, Boksem RJ, van Goudoever J, Cherpanath TGV et al (2011) Noninvasive cardiac output monitoring during exercise testing: Nexfin pulse contour analysis compared to an inert gas rebreathing method and respired gas analysis. J Clin Monit Comput 25(5):315–321
Wesseling KH, De Wit B, Van der Hoeven GMA, Van Goudoever J, Settels JJ (1995) Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis 36:67–82
Fortin J, Marte W, Grüllenberger R, Hacker A, Habenbacher W, Heller A et al (2006) Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med 36(9):941–957
Martina JR, Westerhof BE, Van Goudoever J, De Jonge N, Van Lieshout JJ, Lahpor JR et al (2010) Noninvasive blood pressure measurement by the Nexfin monitor during reduced arterial pulsatility: a feasibility study. ASAIO J 56(3):221
Rongen GA, Bos WJW, Lenders JWM, Montfrans V, A G, Lier V et al (1995) Comparison of intrabrachial and finger blood pressure in healthy elderly volunteers. Am J Hypertens 8(3):237–48
Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ (1993) Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol Bethesda Md 1985 74(5):2566–73
Bogert LWJ, Wesseling KH, Schraa O, Van Lieshout EJ, de Mol BA, van Goudoever J et al (2010) Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia 65(11):1119–25
Ameloot K, Palmers P-J, Malbrain MLNG (2015) The accuracy of noninvasive cardiac output and pressure measurements with finger cuff: a concise review. Curr Opin Crit Care 21(3):232–239
Truijen J, Westerhof BE, Kim Y-S, Stok WJ, de Mol BA, Preckel B et al (2018) The effect of hemodynamic and peripheral vascular variability on cardiac output monitoring: thermodilution and non-invasive pulse contour cardiac output during cardiothoracic surgery. Anaesthesia 73(12):1489–1499
The Irish Longitudinal Study on Ageing (2013) TILDA Wave 3 health assessment standard operating procedure. Unpublished
Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society (2011) Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 59(1):148–57
Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop-Busui R et al (2015) Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev 27(7):654–664
Wieling W, Colman N, Krediet CTP, Freeman R (2004) Nonpharmacological treatment of reflex syncope. Clin Auton Res 14(Suppl 1):62–70
Groothuis JT, van Dijk N, Ter Woerds W, Wieling W, Hopman MTE (2007) Leg crossing with muscle tensing, a physical counter-manoeuvre to prevent syncope, enhances leg blood flow. Clin Sci Lond Engl 1979 112(3):193–201
Sprangers RL, Veerman DP, Karemaker JM, Wieling W (1991) Initial circulatory responses to changes in posture: influence of the angle and speed of tilt. Clin Physiol Oxf Engl 11(3):211–220
Jansen RWMM (1995) Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med 122(4):286
Fan CW, Savva GM, Finucane C, Cronin H, O’Regan C, Kenny RA et al (2012) Factors affecting continuous beat-to-beat orthostatic blood pressure response in community-dwelling older adults. Blood Press Monit 17(4):160
Hayano J, Sakakibara Y, Yamada M, Kamiya T, Fujinami T, Yokoyama K et al (1990) Diurnal variations in vagal and sympathetic cardiac control. Am J Physiol Heart Circ Physiol 258(3):H642–H646
Pal MD, Benarroch EE (eds.) (2008) Clinical autonomic disorders, 3rd edition. LWW, Philadelphia
van de Borne P, Mark AL, Montano N, Mion D, Somers VK (1997) Effects of alcohol on sympathetic activity, hemodynamics, and chemoreflex sensitivity. Hypertension 29(6):1278–1283
Goldsmith RL, Bloomfield DM, Rosenwinkel ET (2000) Exercise and autonomic function. Coron Artery Dis 11(2):129
Halliwill JR, Taylor JA, Eckberg DL (1996) Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol 495(Pt 1):279–288
Frith J, Rn PR, Newton JL (2015) Length of time required to achieve a stable baseline blood pressure in the diagnosis of orthostatic hypotension. J Am Geriatr Soc 61(8):1414–1415
Mader SL, Palmer RM, Rubenstein LZ (1989) Effect of timing and number of baseline blood pressure determinations on postural blood pressure response. J Am Geriatr Soc 37(5):444–446
Ewing DJ, Neilson JM, Shapiro CM, Stewart JA, Reid W (1991) Twenty four hour heart rate variability: effects of posture, sleep, and time of day in healthy controls and comparison with bedside tests of autonomic function in diabetic patients. Heart 65(5):239–244
Westerhof BE, Gisolf J, Stok WJ, Wesseling KH, Karemaker JM (2004) Time-domain cross-correlation baroreflex sensitivity: performance on the EUROBAVAR data set. J Hypertens 22(7):1371
FMS, Finapres Medical Systems BV. Finometer User Guide. Arnheim, The Netherlands; 2002
Soraghan CJ, Fan CW, Hayakawa T, Cronin H, Foran T, Boyle G et al (2014) TILDA Signal Processing Framework (SPF) for the analysis of BP responses to standing in epidemiological and clinical studies. In: IEEE-EMBS international conference on biomedical and health informatics (BHI), pp 793–6
van der Velde N, van den Meiracker AH, Stricker BHC, van der Cammen TJM (2007) Measuring orthostatic hypotension with the Finometer device: is a blood pressure drop of one heartbeat clinically relevant? Blood Press Monit 12(3):167–171
Heldt T, Shim EB, Kamm RD, Mark RG (2002) Computational modeling of cardiovascular response to orthostatic stress. J Appl Physiol Bethesda Md 1985 92(3):1239–1254
Berkelmans GFN, Kuipers S, Westerhof BE, Spoelstra-de Man AME, Smulders YM (2018) Comparing volume-clamp method and intra-arterial blood pressure measurements in patients with atrial fibrillation admitted to the intensive or medium care unit. J Clin Monit Comput 32(3):439–446
Chui CK, Chen G (2012) Signal processing and systems theory: selected topics. Softcover reprint of the original 1st ed. 1992 edn. Springer, Berlin
Julien C (2006) The enigma of Mayer waves: facts and models. Cardiovasc Res 70(1):12–21
Norcliffe-Kaufmann L, Kaufmann H, Palma J-A, Shibao CA, Biaggioni I, Peltier AC et al (2018) Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol 83(3):522–531
Funding
BEW was supported by NWO-VICI (918.16.610).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BEW previously worked for Edwards Lifesciences, Amsterdam, The Netherlands.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Finucane, C., van Wijnen, V.K., Fan, C.W. et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res 29, 427–441 (2019). https://doi.org/10.1007/s10286-019-00606-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-019-00606-y