Abstract
Introduction
The biochemical mechanisms by which hyperglycemia causes microvascular disease and neuropathy are poorly understood. Experimental studies have established that oxidative stress is present in diabetic rodents with neuropathy, and that antioxidant therapy is protective. Oxidative stress is also present in human diabetes, but its clinical importance is uncertain.
Material and methods
We examined several biochemical measures of oxidative stress in 37 patients with recent-onset (less than 2 years) type 1 diabetes annually in a 3-year longitudinal study. We also performed a comprehensive annual evaluation of somatosensory and autonomic nerve function. A total of 41 control subjects were studied.
Results
Malondialdehyde excretion, a measure of lipid peroxidation, was 1.5l ± .1 μmol/g creatinine in the control subjects, but 2.43 ± . 3 in the diabetic patients in year one, 2.39 ± .2 in year two and 1.92 ± .15 in year three, which was different from controls across all years; p < .005. Serum NOx (nitrate and nitric) was 34.0 ± 4.9 μmol/L in the controls, but 52.4 ± 5 in the diabetics in year one, 50.0 ± 5.1 in year two, and 49.0 ± 5.2 in year three, which was different from controls; p < .01. We measured sudomotor function and observed that the poorly controlled diabetic patients had relatively increased sweating above the waist and relatively decreased sweating below the waist, a typical pattern for sympathetic nerve injury. The ratio of sweating above to sweating below the waist was .385 ± .04 in controls, 0.70 ± .14 in diabetic patients in year one, .51 ± .14 in year two and .496 ± .12 in year three (different from controls; p < .01 across all years). Urinary MDA correlated negatively with total sweat (r = −39, p < .01); NOx also correlated negatively with total sweat (r = −.34, p < .025). Abnormalities in the processing of renin (the renin/prorenin ratio), a test of renal sympathetic neurons, was also documented in early type 1 diabetes.
Conclusions
Oxidative stress and excessive serum NOx are associated with sympathetic dysfunction in early type 1 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most clinical studies of diabetic neuropathy have focused on symptomatic patients who have endured years or decades of hyperglycemia. These individuals have suffered multiple pathological insults including segmental demyelination, axonopathy and degeneration of nerve fibers. Although it is recognized that these problems are driven by hyperglycemia, and may be prevented by suppressing the glucose, the specific biochemical mechanisms which lead to nerve damage and pain are poorly understood. We believe that early nerve dysfunction needs to be more fully understood since it should be more preventable or reversible than the dysfunction suffered by patients with long-standing hyperglycemia. We will present evidence which indicates that oxidative stress can occur very early in type 1 diabetes, and may be associated with adverse effects on autonomic function.
Oxidative stress in diabetes
It is well established that excessive reactive oxygen species are formed in experimental diabetes [5, 26]. Several theories have been advanced to explain how this may promote the development of diabetic complications. The formation of advanced glycation end products, the activation of the aldose reductase pathway, endothelial dysfunction, lipid peroxidation, activation of vascular NADPH oxidases, mitochondrial dysfunction, activation of protein kinase C and programmed cell death have all been linked to oxidative stress in animals [5, 10, 26]. Moreover, all the isoforms of nitric oxide synthase (NOS) when uncoupled, can produce the superoxide anion preferentially over nitric oxide NO [36]. The superoxide anion may then react with nitric oxide and form the toxic intermediate peroxynitrite (Fig. 1) (the only specific product formed from these substrates) which then decomposes to nitrite and OH radicals all of which can nitrate/oxidize biomolecules including DNA. Oxidative damage to DNA, in turn, activates the DNA repair enzyme poly (ADP ribose) polymerase (PARP) [27, 38] (Fig. 1). Stimulation of PARP depletes its substrate (NAD+) which slows glycolysis, depletes ATP and causes cell death [9, 10]. Although the activation of PARP has been demonstrated in diabetic animals, the role of this mechanism has been difficult to test clinically. PARP activation has been described in human lymphocytes [1] and in atheromatous lesions of diabetic patients [25], so it is reasonable to postulate that this mechanism plays a role in clinical microvascular disease and neuropathy.
On the other hand, the oxidative stress that takes place in human diabetes may not be associated with the same biochemical aberrations described in animals. First of all, animals with experimental diabetes are often severely hyperglycemic, catabolic and treated with inadequate doses of insulin. Many studies, for example, have indicated oxidative damage to DNA in experimental diabetes [27], but we have found that 8-OH deoxyguanosine excretion is normal in human diabetes [16]. Thus, multiple metabolic changes, in addition to hyperglycemia and oxidative stress, may be present in animals but not in human diabetes.
Nitrosative stress in diabetes
Although it is widely accepted that oxidative stress takes place in both animal and human diabetes, the presence of nitrosative stress in this setting has been controversial [11] even though many animal studies have indicated that the overproduction of reactive oxygen and reactive nitrogen species are intertwined. The endothelium of diabetic rats, for example, stains positive for nitrotyrosine (evidence of excessive peroxynitrite) which can be suppressed by the antioxidant, alpha lipoic acid [5]. Moreover, mice with inducible NOS deficiency are protected against the deleterious effects of oxidative stress on peripheral nerve function [23, 27]. It has been debated, however, whether oxidative stress drives nitrosative stress or vice versa. Some have argued that hyperglycemia leads to oxidative stress which, in turn, activates NFКB which then augments the gene expression of inducible NOS [26, 29, 38]. Accordingly, oxidative stress damages DNA in animal models, and this activates PARP (as discussed above) which also upregulates inducible NOS [9]. Others have argued that nitrosative stress is the “chicken not the egg”. Inducible NOS-deficient mice, for example, have a deficit in lipid peroxidation which is manifested as decreased free 8-iso-PGF2 alpha [15, 23]. NO over-production, on the other hand, stimulates lipid peroxidation [9]. Despite this abundant animal evidence, the clinical data pertaining to nitrosative stress in diabetes are much less convincing, primarily because the evidence for excess nitrotyrosine is questionable [11]. The peroxynitrite/nitrotyrosine connection in humans is problematic for several reasons. First of all, peroxynitrite is only one of several sources of nitrotyrosine. Myeloperoxidase activity, for example, not peroxynitrite, is the major source of nitrotyrosine in chronic inflammation [8]. Moreover, only a tiny percentage of the peroxynitrite molecules actually nitrate other compounds or macromolecules [11]. Finally, many laboratories (including our own) have experienced problems measuring nitrotyrosine, and the validity of HPLC [19] and immunologically based methods (which have been used in most published studies) has been challenged [11, 30]. Convincing evidence of increased nitrotyrosine in clinical diabetes has been provided by only one laboratory, which used liquid chromatography and electrospray ionization tandem mass spectrometry [33].
Increased NOx has also been documented in the circulation of diabetic patients in several clinical studies, but some have argued that this is a non-specific response to inflammation and not truly indicative of either nitrosative or oxidative stress [11]. Moreover, it has been argued that serum NOx may not truly reflect NO biosynthesis, in part, because dietary nitrate and nitrite can be absorbed into the bloodstream where it may confound the interpretation of NOx. We dispute this argument since pharmacokinetic studies using 15N labeled arginine have documented that NO production is increased in patients with uncomplicated type 1 diabetes [28]. Moreover, most clinical studies, including our own, which have involved measuring NOx, dietary nitrite and nitrate were restricted from the diet. There are other data which indicate that serum NOx provides an indirect measure of oxidative stress: First of all, strong experimental evidence links nitrosative and oxidative stress in animals (as explained above). Secondly, in our own study of early type 1 diabetes (to be detailed subsequently), we observed that NOx was higher in the poorly controlled versus the well controlled patients (Table 1; Fig. 2) The most parsimonious explanation of this finding is that hyperglycemia stimulates oxidative stress, which then activates PARP which, in turn, increases inducible NOS [9]. Thirdly, Pitocco et al. [29] observed an inverse correlation between NOx and uric acid, an endogenous antioxidant, which suggests that patients with increased NOx had greater oxidative stress and therefore decreased uric acid. Fourthly, we have observed a correlation between NOx and free 8-iso-PGFF2 alpha in early type 1 diabetes (Fig. 3). Maejima et al. [22] similarly observed a correlation between plasma NOx and the concentrations of serum lipid peroxides in type 2 diabetes. Finally, we observed that NOx showed a negative correlation with sudomotor function in early diabetes, as will be discussed subsequently, which suggests that toxic metabolites of NO, such as peroxynitrite, have adverse peripheral nerve effects in man similar to those convincingly demonstrated in animals [9]. Maejima et al. [22] similarly observed that type 2 diabetic patients with neuropathy, retinopathy or nephropathy all had increased circulating nitrate. We acknowledge, however, that these considerations provide only indirect evidence for an association between NOx and oxidative stress, and this interpretation of serum NOx has not yet been unequivocally proven to be correct. We also acknowledge that measurement of serum NOx provides only a crude index of NO production in vivo [43].
Effects of glycemic control on oxidative stress, NOx and serum uric acid in patients with well controlled diabetes (open bars) and poorly controlled diabetes (closed bars) versus controls. Different from control subjects, *P < 0.05, **P < 0.01. Patients in good control were different from those in poor control, † P < 0.05. Patients in good control across all years were different from those in poor control, ‡ P < 0.05
Although our discussion of NO in diabetes has thus far assumed that the bioavailability of nitrogen-free radicals (including peroxynitrite and perhaps nitrogen trioxide) is increased and leads to detrimental consequences [5, 9], others have argued, to the contrary, that the bioavailability of NO is decreased in clinical diabetes. It is widely recognized, for example, that NO has beneficial effects on vascular tone, gastrointestinal motility and erectile function. There is evidence that diabetes compromises these desirable effects presumably because the overabundance of the superoxide anion inactivates NO by converting it to peroxynitrite, which, in turn, is broken down to nitrate, nitrite and oxygen [9, 11]. This is consistent with the animal studies [5] and clinical data just reviewed which have shown that the NOx is increased in patients with diabetes [15, 29]. How can NO be both excessive and deficient? Evidence indicating that hyperglycemia causes nitric oxide deficiency derives from studies of the endothelium, where NO synthesis is regulated by endothelial NOS [10]. Several investigators have shown that NO is decreased in diabetic endothelial cells, because hyperglycemia leads to decreased biopterin, an essential cofactor for endothelial NOS, which, in turn, uncouples the enzyme and causes it to produce the superoxide anion rather than NO [36]. NO synthesized elsewhere, for example in macrophages, epithelial cells, hepatocytes and vascular smooth muscle derives from inducible NOS [38]. The gene expression of inducible NOS is mediated by NFКB, which, in turn, is activated by hyperglycemia and oxidative stress, as described above [27, 29, 38]. Inducible NOS is a quantitatively more important source of NO in the whole patient than endothelial NOS, and this may explain the discrepancy between the physiological studies of endothelial cells (where NO has decreased bioavailability) and the biochemical studies of the whole patient (where NOx is excessive). Animal models have been developed in which there is evidence of both peroxynitrite excess (nitrotyrosine staining of the endothelium) and NO deficiency (failure of acetylcholine-induced NO release) [5]. The same conundrum can be seen in clinical data. Studies of endothelial function and skin blood flow [39] have indicated that the bioavailability of NO is decreased in diabetes, whereas others have reported that nitrotyrosine (a marker of toxic NO metabolites) in plasma proteins is increased [33]. Increased serum NOx is accompanied by increased free 8-iso-PGF2alpha (Figs. 2, 3) and urinary malondialdehyde, another index of lipid peroxidation, is also increased in poorly controlled patients with early diabetes [11] (Table 1; Fig. 2). This is of interest because the formation of lipid peroxides in nerve membranes has adverse effects on fluidity, electrical conductivity and function.
Endogenous antioxidants in diabetes
Glutathione is generally regarded as the major endogenous water soluble intracellular antioxidant, and there is evidence that its depletion in diabetes places vulnerable tissues at risk for developing oxidative injury [38]. Multiple enzymes counteract the overproduction of reactive oxygen species. Superoxide dismutase, which converts the superoxide anion to hydrogen peroxide, and catalase, which converts the latter to water, are the most important, but difficult to assess in vivo. Urate is the most important extracellular antioxidant [2] but has been mostly ignored in the diabetes literature. We became interested in urate because of reports that it acts as a scavenger of peroxynitrite and may have a neuroprotective role in the human brains of patients with Alzheimer’s disease and parkinsonism [14]. Furthermore, birds, which have high urate are protected from hyperglycemia associated oxidative stress [20]. Similarly, intravenous administration of 1 g of urate to patients with type 1 diabetes enhances the bioavailability of NO released in the forearm, and improves endothelial dysfunction [41]. We believe this means that urate neutralizes superoxide anion formation in the endothelium; as a result, more locally produced NO acts as a vasodilator, because less is converted to toxic NO metabolites.
Oxidative stress and autonomic nerve function in early diabetes
Previous studies of the effects of oxidative stress on the natural history of diabetes have focused on patients with long-standing disease and many of the patients have had hypertension, dyslipidemia, insulin resistance or endothelial dysfunction, each of which has been linked to oxidative stress even in nondiabetic patients. Thus, in chronic diabetes these confounding factors make it difficult to assess the significance of oxidative stress and document its relationship with hyperglycemia. In order to minimize the impact of these covariables, we have focused on 37 young patients (mean age at diagnosis 20.3 years) with recently diagnosed type 1 diabetes (mean disease duration at entry into the study of 10.4 months) who underwent a 3-year longitudinal study (Fig. 1). Because of their young age and short duration of diabetes they were in excellent health, except for hyperglycemia, and minimally affected by the above-mentioned cardiovascular risk factors that have been linked to oxidative stress in patients with long-standing disease. Our patient population, therefore, provided a unique opportunity to focus on the relationship between hyperglycemia, oxidative stress and autonomic nerve function [17]. All subjects gave informed consent allowing us to proceed with this study. The patients and controls were admitted to West Virginia University Hospital annually for 24–36 h to control the diet, glucose and activity of the patients. Urinary-free malondialdehyde excretion was derivatized with thiobarbituric acid, and the resulting adduct was purified by HPLC [21]. Serum nitrate was converted to nitrite by nitrate reductase, which was reacted with the Griess reagent and detected colorimetrically [34]. Free 8-iso-PGF2 alpha was measured by the ELISA method using a kit from Cayman Laboratories (Ann Arbor, Michigan) [6]. Urinary 8-OH deoxyguanosine was measured as previously described [16]. We performed a comprehensive assessment of peripheral nerve function [15] but focused on the autonomic nervous system. We assessed parasympathetic innervation of the heart by measuring high frequency (0.15–0.4 Hz) power spectral analysis of heart rate variability [15, 17]. We also measured beat to beat variation with deep breathing, an alternative index of parasympathetic cardiovascular control [18]. We assessed sympathetic function by measuring the heart rate response to the Valsalva maneuver (tachycardia/bradycardia ratio), renin biosynthesis (the renin/prorenin ratio), and sudomotor function (the Quantitative Sudomotor Axon Reflex, QSART) [17]. We measured renin and prorenin and calculated the renin/prorenin ratio which reflects the integrity of the sympathetic innervation of the kidneys [18]; the QSART assesses the integrity of the sympathetic nerve terminals which innervate sweat glands [17]. We assessed cardiac sympathetic function by measuring intermediate frequency (0.04–0.15 Hz) power spectral analysis of heart rate variability. These latter tests of sympathetic function were incorporated into the study to address the concept that parasympathetic dysfunction develops prior to sympathetic dysfunction in patients with diabetes [3]. We questioned this perspective because it is based on the comparison of sensitive tests of parasympathetic function, such as the heart rate variability with deep breathing, with insensitive tests of sympathetic function, such as the hemodynamic response to orthostatic stress or isometric hand grip.
We found that the poorly controlled patients had increased malondialdehyde excretion, increased serum NOx and increased serum-free 8-iso-PGF2alpha (Fig. 2) but normal urinary 8-OH deoxyguanosine (contrary to expectations) (Table 1). The diabetic patients had normal parasympathetic function early in their disease. The beat to beat variation with deep breathing, in contrary to expectations, was actually increased in the patients with early diabetes [18]. The high frequency power spectra of heart rate variability were no different in the diabetic patients versus the controls. By contrast, we observed that renin/prorenin was decreased even at the first evaluation of the diabetic patients and it deteriorated further in the poorly controlled patients during the course of the 3-year study (Fig. 4). The analysis of sudomotor function in early diabetes is complex. It is well known that chronic diabetes causes sympathetic denervation, atrophy of sweat glands and eventually anhidrosis in the feet. Paradoxically, however, patients with diabetic neuropathy typically sweat excessively above the waist, especially in the head and neck and in this regard they resemble patients who experience hyperhidrosis following sympathectomy [32]. We therefore postulated that there would be a relative deficiency in sweat production below the waist, but a relative excess of sweat production above the waist. To test this, we measured sweat in four locations, including the L forearm, the proximal L leg, the distal L leg and the proximal L foot, as detailed previously [18]. We observed that sudomotor function was normal in the well controlled patients, but in the poorly controlled the ratio of sweating above the waist divided by sweating below the waist was increased at each of three annual evaluations (Fig. 4). We believe this redistribution of sudomotor responses is an early sign of sympathetic neuropathy. Grandenetti et al. [13] observed a similar pattern of responses in patients with impaired glucose tolerance. We also observed changes in more traditional tests of sympathetic function in early diabetes. The post Valsalva bradycardia was slightly decreased in the poorly controlled patients at each evaluation (Fig. 1), and 3-methoxy-4-hydroxymandelic acid excretion, a measure of norepinephrine production by the sympathetic nervous system, was decreased by about 25% in the poorly controlled patients at the time of the third evaluation [18]. We interpret these results to indicate that the sympathetic nerves are especially vulnerable to the damaging effects of chronic hyperglycemia. Moreover, we observed that several markers of oxidative stress correlated negatively with sympathetic function. Total sweat production correlated negatively with malondialdehyde excretion (P < 0.01), and NOx (P < 0.025) (Fig. 5). A negative correlation between free 8-iso-PGF-2alpha and sweating approached statistical significance (P = 0.06). Malondialdehyde excretion also showed a negative association (P < 0.01) with intermediate frequency power spectral analysis of heart rate, an index of cardiovascular sympathetic function [17].
Effect of glycemic control on sympathetic function in early diabetes. Mean results ± SE are presented for patients whose HgbA1 values were below (open bars) or above (closed bars) the median for the group, respectively, versus the control subjects (hatched bars). Different from control subjects, *P < 0.05, **P < 0.01. Different from patients with low HgbA1, † P < 0.05, †† P < 0.025, Diabetic patients with high versus low HgbA1 across all years were different, ‡ P < 0.01
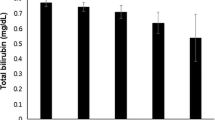
We also measured urate in our longitudinal study of diabetes, and demonstrated that it was decreased by 25% in early type 1 diabetes (Table 1). Moreover, we and Pitocco [29] documented a negative correlation between urate and glycosylated hemoglobin which indicates that the suppression of the former is a compensatory response to hyperglycemia related oxidative stress. Peroxynitrite is the major oxidant which reacts with urate, but the superoxide anion and hydrogen peroxide can also do so, but they are not as potent oxidizers as is peroxynitrite [31]. Moreover, Pitocco [29] also observed a negative correlation between NOx and uric acid. This is an important observation since it suggests that other reactive nitrogen-free radicals, perhaps dinitrotrioxide, can also react with and degrade uric acid. The suppression of urate in early diabetes, however, is multifactorial. Increased inducible NOS activity and increased NO formation, for example, stimulate glomerular hyperfiltration which causes enhanced uricosuria [15]. Thus, the direct effects of increased NO metabolites were more important initially in our study, because there was no hyperfiltration or hyperuricosuria in our patients at their baseline evaluation [15]. Indirect renal effects of NO probably play a contributory role subsequently; at the final evaluation, both hyperfiltration and increased uricosuria were documented. NO which mediates hyperfiltration is a critical component of this indirect mechanism, since it mediates hyperfiltration in early diabetes. Chiarelli [4] reported and we confirmed [15] that NOx correlated with creatinine clearance in early diabetes. The loss of urate, an endogenous antioxidant, is disadvantageous, and could, in theory, worsen multiple complications of diabetes (Fig. 1). We acknowledge that the uricosuria may be important only transiently, since hyperfiltration at the renal glomerulus takes place only in early diabetes. Pitocco et al. [29] studied patients with a longer history of type 1 diabetes (up to 10 years), and observed a 38% reduction of serum urate in this cohort with normal uric acid excretion. In our study, patients with suppressed urate showed a relative increase in sweating above the waist divided by sweating below the waist, a marker of sympathetic injury. Patients with suppressed urate also showed a decreased renin/prorenin ratio, a marker of renal sympathetic nerve activity (Fig. 6). These results, taken together, demonstrate that oxidative stress, increased NOx and lipid peroxidation (Fig. 3) disrupt sympathetic function in early type 1 diabetes. These changes, though clinically silent, are nevertheless of potential importance since sympathetic nerve damage in diabetes can progress to cause multiple adverse consequences including arteriovenous shunting and ischemia of the distal capillary networks, [35] and increased blood flow to skin and bone which may translate into neuropathic arthropathy or neuropathic edema [42]. Sympathetic denervation may also cause vascular calcification, medial degeneration, vascular rigidity and eventually peripheral arterial disease [12]. Eventually sympathetic injury can lead to gustatory sweating or orthostatic hypotension, which can be disabling and carries a poor prognosis [3, 42]. Finally, iatrogenic hypoglycemia leads to activation of the sympathetic nervous system [7], and we have postulated that sympathetic nerve damage compromises the awareness of hypoglycemia [17].
Uric acid and autonomic function. Mean results ± SE are illustrated for patients categorized according to whether their uric acid was above or below the gender specific median uric acid at that evaluation. Different from control subjects, *P < 0.05, **P < 0.01. Diabetic patients with suppressed uric acid were different from those with normal uric acid across all years and were different from controls † P < 0.05. Diabetic patients with suppressed versus normal uric acid were different across all years †† P < 0.025, ††† P < 0.0025. Different from patients with normal uric acid, ‡ P < 0.025, § P < 0.01
Parasympathetic activity was assessed by measuring the beat to beat variation with deep breathing and high frequency power spectral analysis of heart rate variability, as described above. Although neither were decreased in early diabetes, we observed, interestingly, that patients with suppressed urate had worse performance on both tests than did patients with normal urate (Fig. 6). Thus, it appears likely that oxidative stress plays a role in the initiation of both parasympathetic and sympathetic damage. Parasympathetic dysfunction causes impotence in diabetic males, and oxidative stress must take place in the penis since impotent diabetic males have higher penile malondialdehyde than do their nondiabetic counterparts [37]. Cardiovascular parasympathetic dysfunction in chronic diabetes is of great clinical concern. Vinik et al. [40] have performed a meta-analysis of several studies and demonstrated that decreased heart rate variability in diabetes is associated with silent cardiac ischemia and increased mortality.
The biochemical markers of oxidative stress correlated weakly with somatosensory function in early diabetes. Although malondialdehyde excretion correlated with the peroneal motor nerve amplitudes [17], this relationship was not observed in the ulnar or median nerves.
Gender and oxidative stress
All measures of oxidative stress were higher in the diabetic females than in diabetic males (Fig. 7). Marra [24] also observed this gender effect in type 1 diabetes. The biochemical mechanism of the gender impact on oxidative stress in diabetes is unknown. We postulate that this phenomenon reflects the well known fact that females have less urate than men. In our study, for example, the mean urate in the diabetic females was 208 ± 8 μmol/L while urate in the diabetic males was 237 ± 14 (P < 0.05).
Effects of gender on oxidative stress. Female diabetic patients were different from male diabetic patients across all years, *P < 0.05. Female diabetic patients were different from nondiabetic females (**P < 0.01) and diabetic males, P < 0.01. Diabetic males were different from diabetic females, † P < 0.025. Nondiabetic males were different from nondiabetic females, †† P < 0.02. Female diabetic patients were different from female controls, ‡ P < 0.01
In conclusion, we have observed that the sympathetic nervous system is especially vulnerable to the adverse consequences of chronic hyperglycemia. We observed aberrant sudomotor responses, a relative deficit of sudomotor responses below the waist, and a relative increase in sudomotor responses above the waist, at the time of the each evaluation of the diabetic patients. Deficits in the processing of renin were demonstrable in the diabetic patients throughout the longitudinal study. Moreover, serum NOx, urinary malondialdehyde, and serum-free 8-iso-PGF2alpha correlated negatively with sudomotor function. Finally, the suppression of urate, an endogenous extracellular antioxidant, showed a correlation with both sympathetic and parasympathetic dysfunction in early type 1 diabetes.
References
Adaikalakoteswari A, Rema M, Mohan V, Balasubramanyam M (2007) Oxidative DNA damage and augmentation of poly (ADP-ribose) polymerase/nuclear factor-kappa b signaling in patients with type 2 diabetes and microangiopathy. Int J Biochem and Cell Biol 39:1673–1684
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA 78:6858–6862
Bennett T, Hosking DJ, Hampton JR (1975) Cardiovascular control in diabetes mellitus. Br J Med 2:585–587
Chiarelli F, Cipollone F, Romano F, Tumini S, Costantini F, DiRicco L, Pomilio M, Pierdomenico SD, Marini M, Cuccurullo F, Mezzeti A (2002) Increased circulating nitric oxide in young patients with type I diabetes and persistent microalbuminuria. Diabetes 49:1258–1263
Coppey LJ, Gellet JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA (2001) Effect of anti oxidant treatment on streptozotocin-induced diabetic rats on endoneurial blood flow, nerve conduction velocity, and vascular reactivity of epineural arterioles of the sciatic nerve. Diabetes 50:1927–1937
Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C (1999) In vivo formation of 8-iso-prostaglandin F2 and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 99:224–229
DeRosa MA, Cryer PE (2004) Hypoglycemia and the sympathoadrenal system: neurogenic symptoms are largely the result of sympathetic neural rather than adrenomedulary activation. Am J Phys Endocr Metab 287:E32–E41
Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, Vazquez-Corzo S, Perez-Molina G, Gallegos-Sandova R, Moreno R, Garg NJ (2009) Increased myeloperoxidase activity and proteín nitration are indicators of inflammation in patients with Chagas’ disease. Clin Vaccine Immunol 16:660–666
Drel VR, Lupachyk S, Shevalye H, Vareniuk I, Xu W, Zhang J, Delamere NA, Shahidullah M, Slusher B, Obrosova IG (2010) New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PARP inhibitor, nitrotyrosine, and tumor necrosis factor-alpha. Endocrinology 151:1–9
Feldman E (2003) Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest 111:431–433
Giustarini D, Dalle-Donne I, Tsikas D, Rossi R (2009) Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci 46:241–281
Goss DE, Trafford JC, Roberts VC, Flynn MD, Edmonds ME, Watkins PJ (1989) Raised ankle/brachial pressure index in insulin-treated diabetic patients. Diabetes Med 6:576–578
Grandenetti A, Chow DC, Sletten DM, Oyama JK, Theriault AG, Schatz IJ, Low PA (2007) Impaired glucose tolerance is associated with postganglionic sudomotor impairment. Clin Auton Res 17:231–233
Hensley K, Maidt ML, Yu Z, San H, Merkeshery WR, Floyd RA (1998) Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer’s brain indicates region specific accumulation. J Neurosci 18:8126–8132
Hoeldtke RD (2006) Oxidative stress in type I diabetes: a clinical perspective. In: Opara E (ed) Nutrition and diabetes: pathophysiology and treatment. Taylor and Francis, Boca Raton, pp 319–344
Hoeldtke RD, Bryner KD, Corum LL, Hobbs GR, VanDyke K (2009) Lipid peroxidation in early type I diabetes mellitus is unassociated with oxidative damage to DNA. Metab Clin Exp 58:731–734
Hoeldtke RD, Bryner KD, Hoeldtke ME, Christie I, Ganzer G, Hobbs G, Riggs J (2006) Sympathetic sudomotor disturbance in early type I diabetes mellitus is linked to lipid peroxidation. Metabolism Clin Exp 55:1524–1531
Hoeldtke RD, Bryner KD, Horvath GG, Phares RW, Broy LF, Hobbs GR (2001) Redistribution of sudomotor responses is an early sign of sympathetic dysfunction in Type I diabetes. Diabetes 50:436–443
Hoeldtke RD, Bryner KD, McNeill DR, Hobbs GR, Riggs JE, Warehime SS, Christie I, Ganser G, VanDyke K (2002) Nitrosative stress, uric acid and peripheral nerve function in Early type I diabetes. Diabetes 51:2817–2825
Klandorf H, Rathore DS, Iqbal M, Shi X, VanDyke K (2001) Accelerated tissue aging and increased oxidative stress in broiler chickens fed allopurinol. Comp Biochem Physiol Toxicol Pharmacol 129:93–104
Kosugi H, Kojima T, Kikugawa K (1993) Characteristics of the thiobarbituric acid reactivity of human urine as a possible consequence of lipid peroxidation. Lipids 28:337–343
Maejima K, Nakano S, Himeno M, Tsuda S, Makiishi H, Ito T, Nakagawa A, Kigoshi T, Ishibashi T, Nishio M, Uchida K (2001) Increased basal levels of plasma nitric oxide in Type 2 diabetic subjects. Relationship to microvascular complications. J Diabetes Complicat 15:135–143
Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD (2000) Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem 275:13427–13430
Marra G, Cotroneo P, Pitocco D, Manto A, DiLeo MA, Ruotolo V, Caputo S, Giardna B, Ghirlandu G, Santini SA (2002) Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type I diabetes: a case for gender difference. Diabetes Care 25:370–375
Martinet W, Knaapen MWM, DeMeyer G, Herman AG, Kockx MM (2002) Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 106:927–932
Nishikawa T, Edelstein D, Brownlee MA (2000) Missing link: a single unifying mechanism for diabetic complications. Kidney Int 58(Suppl 77):S26–S30
Obrosova IG (2008) Diabetes and the peripheral nerve (2009). Biochem Biophys Acta 1792:931–940
O’Bryne S, Forte P, Roberts LJ II, Morrow JD, Johnston A, Anggard E, Leslie RDG, Benjamin N (2000) Nitric oxide synthesis and isoprostane production in subjects with type 1 diabetes and normal urinary albumin excretion. Diabetes 49:857–862
Pitocco D, DiStasio E, Romitelli F, Zaccardi F, Tavazzi B, Manto A, Caputo S, Musella T, Zuppi C, Santini SA, Ghirlanda G (2008) Hypouricemia linked to overproduction of nitric oxide is an early marker of oxidative stress in female subjects with type I diabetes. Diabetes Metab Res Rev 24:318–323
Safinowski M, Wilhelm B, Reimer T, Weise A, Thome N, Hanel H, Forst T, Pfutzner A (2009) Determination of nitrotyrosine concentrations in plasma samples of diabetes mellitus patients by four different immunoassays leads to contradictive results and disqualifies the majority of the tests. Clin Chem Lab Med 47:483–488
Santos CXC, Anjos EI, Augusto O (1999) Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid peroxidation. Arch Biochem Biophys 372:285–294
Shelley WB, Florence R (1960) Compensatory hyperhidrosis after sympathectomy. New Engl J Med 263:1056–1058
Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce KeaneyJF, Penn MS, Sprecher DL, Vita JA, Hazen SL (2003) Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 289:1675–1680
Suto T, Losonczy G, Qiu C, Hill C, Samsell L, Ruby J, Charon N, Venuto R, Baylis C (1995) Acute changes in urinary excretion of nitrite + nitrate do not necessarily predict renal vascular NO production. Kidney Int 48:1272–1277
Tesfaye S, Malik R, Harris N, Jakubowski JJ, Mody C, Rennie IG, Ward JD (1996) Arteriovenous shunting and proliferating new vessels in acute painful neuropathy of rapid glycemic control (insulin neuritis). Diabetologia 39:329–335
Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J (2007) Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 56:666–674
Tuncayengin A, Biri H, Onaran M, Seni I, Tucayengin O, Polat F, Erbas D, Bozkirli I (2003) Cavernosal tissue nitrite, nitrate, malondialdehyde and glutathione levels in diabetic and on diabetic erectile function. Int J Androl 26:250–254
Vincent AM, Russell JW, Low P, Feldman EL (2004) Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25:612–628
Vinik AI, Erbas T, Park TS, Stansberry KB, Scanelli JA, Pittenger GL (2001) Dermal neurovascular dysfunction in type 2 diabetes. Diabetes Care 24:1468–1475
Vinik AI, Maser RE, Mitchell BD, Freeman R (2003) Diabetic autonomic neuropathy. Diabetes Care 26:1553–1579
Waring WS, McKnight JA, Webb DJ, Maxwell SR (2006) Uric acid restores endothelial function in patients with type I diabetes and regular smokers. Diabetes 55:3127–3132
Watkins PJ (1992) Clinical observations and experiments in diabetic neuropathy. Diabetologia 35:2–11
Zeballos GA, Bernstein RD, Thompson CI, Forfia PR, Seyedi N, Shen W, Kaminsi PM, Wolin MS, Hintze TH (1995) Pharmacodynamics of plasma nitrate/nitrite as an indication of nitric oxide formation in conscious dogs. Circulation 91:2982–2988
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoeldtke, R.D., Bryner, K.D. & VanDyke, K. Oxidative stress and autonomic nerve function in early type 1 diabetes. Clin Auton Res 21, 19–28 (2011). https://doi.org/10.1007/s10286-010-0084-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-010-0084-4