Abstract
The purpose of this study was to determine the micro-tensile bond strength (MTBS) to dentin of seven adhesive systems (total and self-etch adhesives) after 24 h and 5,000 thermocycles. Dentin surfaces of human third molars were exposed and bonded with two total-etch adhesives (Adper Scotchbond 1 XT and XP Bond), two two-step self-etch adhesives (Adper Scotchbond SE and Filtek Silorane Adhesive System) and three one-step self-etch adhesives (G-Bond, Xeno V and Bond Force). All adhesive systems were applied following manufacturers’ instructions. Composite buildups were constructed and the bonded teeth were then stored in water (24 h, 37 °C) or thermocycled (5,000 cycles) before being sectioned and submitted to MTBS test. Two-way ANOVA and subsequent comparison tests were applied at α = 0.05. Characteristic de-bonded specimens were analyzed using scanning electron microscopy (SEM). After 24 h water storage, MTBS values were highest with XP Bond, Adper Scotchbond 1 XT, Filtek Silorane Adhesive System and Adper Scotchbond SE and lowest with the one-step self-etch adhesives Bond Force, Xeno V and G-Bond. After thermocycling, MTBS values were highest with XP Bond, followed by Filtek Silorane Adhesive System, Adper Scotchbond SE and Adper Scotchbond 1 XT and lowest with the one-step self-etch adhesives Bond Force, Xeno V and G-Bond. Thermal aging induced a significant decrease in MTBS values with all adhesives tested. The resistance of resin–dentin bonds to thermal-aging degradation was material dependent. One-step self-etch adhesives obtained the lowest MTBS results after both aging treatments, and their adhesive capacity was significantly reduced after thermocycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Self-etch adhesives are more user friendly, less technique sensitive and increasingly popular [1, 2]. They do not require a separate etching step because they contain a solution of acidic monomers that simultaneously demineralize and infiltrate the dentin [3]. As a result, no discrepancy is expected between demineralization depth and resin infiltration depth [3]. Self-etch adhesives can be two- or one-step according to whether the bonding agent is separate or combined with the self-etch/primer solution. Adhesives that include etching, priming and bonding in a single solution are also called “all-in-one” and are considered the products with the simplest clinical application [1]. However, reports in the literature have demonstrated a negative relationship between step reduction and bond strength, with one-step self-etch adhesives exhibiting very low bond strength values to dentin [4–6] and a marked degradation of bonding effectiveness [7–9].
Various drawbacks compromise the bonding durability obtained with one-step self-etch adhesives. Thus, they contain an increased concentration of hydrophilic monomers that make them less hydrolytically stable [10], behaving as semi-permeable membranes even after activation [11]. Consequently, the adhesive layers created often contain porosities and voids due to osmosis or phase separation [12], with water channels and hydrophilic domains that permit water permeation through the resin–dentin interface [13]. Moreover, the higher concentration of hydrophilic monomers and the presence of residual water and solvents have been related to a lower polymerization conversion, which weakens the adhesive interface [14, 15].
Although hydrolytic degradation of the dentin-bonded interface is more extensive in simplified adhesives [16], a decrease in bond strength over time has also been reported for total-etch and two-step self-etch adhesives [14, 17, 18]. Hence, the durability of the resin–dentin bond appears to be adhesive dependent, regardless of the bonding strategy used [18].
Novel adhesive systems are being continuously developed and launched onto the market without full knowledge of their bonding ability or longevity [4]. Appropriate in vitro bond strength tests include artificial aging techniques to reveal valuable clinical information [19]. Thermocycling in water at temperatures between 5 and 55 °C is considered a suitable method for aging dental materials [20], subjecting the adhesive interface to water infiltration and to expansion and contraction induced by thermal changes. The deterioration of bonding effectiveness is supposed to be caused both by degradation of interface components by hydrolysis as by decreasing the mechanical properties of the polymer matrix, a process known as “plasticization” [21].
The actual bonding performance of self-etch adhesives is very variable in comparison with total-etch adhesives, even more if they are submitted to an aging process. Its variation not only depends on the class of self-etch adhesives but also on the type of functional monomers included in their composition [22]; therefore, the objective of this in vitro study was to determine the micro-tensile bond strength (MTBS) of seven commercial total-etch or self-etch adhesive systems after 24 h and 5,000 thermal cycles. The null hypothesis was that dentin MTBS values would not be affected by adhesive type or thermal aging treatment.
Materials and methods
Forty-two extracted human third molars were used in this study. They were hand-scaled, cleaned and stored in a solution of distilled water and thymol at 4 °C for <1 month post-extraction. Each tooth was perpendicularly sectioned to expose a flat mid-coronal dentin surface, which was then polished under running water with 600-grit silicon carbide papers (Buehler, Lake Bluff, IL, USA) to create a standardized and clinically relevant smear layer. The dentin surfaces were verified for the absence of enamel and/or pulp tissue exposure under a light stereomicroscope (Olympus SZX7, Hamburg, Germany).
Then, teeth were divided into seven experimental groups (n = 6), according to the adhesive systems tested: two total-etch adhesives (Adper Scotchbond 1 XT, 3 M ESPE; and XP Bond, Dentsply), two two-step self-etch adhesives (Adper Scotchbond SE, 3 M ESPE; and Filtek Silorane Adhesive System, 3 M ESPE) and three one-step self-etch adhesives (G-Bond, GC; Xeno V, Dentsply and Bond Force, Tokuyama Dental). All adhesive systems were applied in accordance with manufacturers’ instructions (Table 1).
In all cases, composite core buildups were constructed with three incremental layers (2 mm each) of a light-cured universal hybrid resin composite (A3 VITA shade; Filtek Z250, 3 M ESPE, St. Paul, MN, USA), except for Filtek Silorane Adhesive System (chemically incompatible with methacrylate-based resins), which requires its own specific composite (Low Shrink Posterior Restorative). Each increment was photopolymerized for 20 s with a light-emitting diode (LED) Demetron I unit (Kerr, Orange, CA, USA), with a minimum light output of 550 mW/cm².
Bonded teeth were kept intact, with the resin–dentin interface entirely surrounded by resin bonded to the outer enamel rim, and were therefore only indirectly exposed to water. Half of the samples in each group were randomly selected for storage in distilled water for 24 h at 37 °C. The remaining specimens were thermocycled for 5,000 cycles between 5 and 55 °C with a dwell time of 30 s.
Once the two aging treatments were completed, all bonded teeth were sectioned longitudinally with a low-speed diamond saw (Accutom 50, Struers, Copenhagen, Denmark) using copious amounts of water in the “x” and “y” directions, producing stick-shaped specimens with a square bonded area of approximately 1 mm². Up to 15 specimens were collected per tooth.
These specimens were attached with cyanocrylate glue (Loctite Gel, Henkel, Düsseldorf, Germany) to a modified Bencor Multi-T testing apparatus and were individually stressed to failure in tension using a universal testing machine (Instron 3345, Instron Corp., Canton, MA, USA) at a cross-head speed of 1 mm/min. A digital calliper with an accuracy of 0.001 mm (Mitutoyo Corporation, Aurora, IL, USA) was used to measure the sides of the bonding interface and calculate the bonding area in mm². Micro-tensile bond strength data were expressed in megapascals (MPa). All the specimens which experienced a pre-testing failure (whether it occurred during section, manipulation or fixation processes) were discarded for the statistical analysis.
When the tensile test ended, fractured sticks were carefully removed from the apparatus and observed by a single operator under a stereomicroscope at a magnification of up to 50× to determine the mode of failure: adhesive (between adhesive and dentin), cohesive (dentin or composite resin) or mixed (simultaneous adhesive and cohesive fractures). Characteristic de-bonded specimens (with MTBS values and failure patterns similar to those most frequently detected in each experimental group) were sputter-coated with gold (SCD 005 Sputter Coater, BalTec, Balzers, Liechtenstein) and observed under scanning electron microscopy (SEM; Hitachi VP-SEM S-3400N, Tokyo, Japan).
The influence of adhesive system, thermal aging and their interactions on MTBS was analyzed by two-way ANOVA. Subsequent comparisons were performed with Tukey HSD and Student’s t tests. All statistical testing was performed at a pre-set alpha of 0.05 using IBM SPSS 19 (IBM Company, Chicago, IL, USA) for Windows software.
Results
The results are displayed in Table 2. Two-way ANOVA test revealed that the MTBS values were significantly influenced by the adhesive system (F = 175.7; p < 0.001), the thermal aging procedure (F = 281.6; p < 0.001) and the interaction of both factors (F = 9.6; p < 0.001).
After 24 h of water storage, MTBS results were ranked into three significantly different subsets (p < 0.05): highest MTBS values were achieved with the total-etch adhesives Adper Scotchbond 1 XT and XP Bond and the two-step self-etch systems Adper Scotchbond SE and Filtek Silorane Adhesive System, with no significant differences among them; intermediate values were observed with the one-step self-etch adhesive Bond Force; and lowest values were obtained with the one-step self-etch adhesives G-Bond and Xeno V, with no significant difference between them.
After thermocycling 5,000×, significantly higher mean MTBS values were achieved with the total-etch adhesive XP Bond than with any other systems tested. Intermediate values were observed with Filtek Silorane Adhesive System, Adper Scotchbond SE and Adper Scotchbond 1 XT, with no significant differences among them. The lowest values were obtained using G-Bond and Xeno V, with the other one-step self-etch adhesive Bond Force showing significantly higher values than G-Bond but similar values to Xeno V.
The influence of thermal aging on MTBS values was analyzed by Student’s t test. After thermocycling, MTBS values were significantly lower than those obtained after 24 h of immersion for all the systems evaluated.
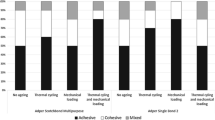
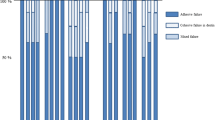
Most of the failures detected were adhesive (Table 2). Pre-testing failures were more frequent after thermocycling with all of the adhesive systems, more markedly with one-step self-etch adhesives G-Bond and Xeno V.
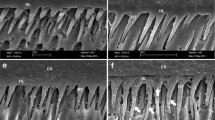
Figures 1, 2, 3, 4 and 5 depict SEM images of fractured surfaces of dentin bonded with Adper Scotchbond SE, Filtek Silorane Adhesive System, G-Bond, Xeno V and Bond Force adhesives after 24 h (a) and 5,000 cycles (b). After 24 h of water storage, failures with the two-step and one-step self-etch adhesives were mainly at the top of the hybrid layer, and the dentin structure was undetectable (Figs. 1a, 2a, 3a, 4a and 5a). We highlight the presence of multiple droplets within the adhesive when G-Bond was applied (Fig. 3a). After thermocycling, most failures were again in the adhesive, with the dentin being completely covered by adhesive (Figs. 2b, 4b and 5b). With Adper Scotchbond SE (Fig. 1b) and G-Bond (Fig. 3b), fractures occurred at the bottom of the hybrid layer and the dentin structure could be detected; tubules appeared occluded and collagen fibrils were observed with the former adhesive, whereas G-Bond tubules were open and few resin tags were visible.
SEM micrographs of the fractured dentin sides of specimens bonded with Adper Scotchbond SE exhibiting adhesive failures (1,500x). a After 24 h of water storage: fracture occurred at the top of the hybrid layer and the dentin surface was completely covered by resin. b After thermocycling 5,000×: fracture occurred at the bottom of the hybrid layer, and most of the tubules were occluded by resin
SEM micrographs of the fractured dentin sides of specimens bonded with Filtek Silorane Adhesive System exhibiting mixed failures after 24 h of water storage (a) and thermocycling 5,000× (b). In both cases, dentin was covered with the adhesive, and failures could be detected between resin composite and adhesive and between adhesive and dentin (1,500x)
SEM micrographs of the fractured dentin sides of specimens bonded with G-Bond exhibiting adhesive failures (1,500x). a After 24 h of water storage: in a magnified image, numerous droplets were observed in the adhesive layer. b Thermocycling 5,000×: most of the tubules were patent, although a few resin tags could be detected
SEM micrographs of the fractured dentin sides of specimens bonded with Bond Force (1,500x). a After 24 h of water storage: a mixed failure was produced, observing an adhesive fracture plus some remnants of composite resin. In a magnified image, a cohesive fracture of the adhesive could be seen. b After thermocycling 5,000×: the dentin surface was completely covered by the adhesive resin
Discussion
According to this study of seven currently available adhesive systems, bond strength is dependent on the system used, while thermal aging has a deleterious effect on the adhesive resistance of all the systems analyzed.
Total-etch and two-step self-etch adhesives exhibited the highest MTBS values after 24 h of water storage, consistent with previous reports [5, 23, 24]. The favorable performance of the total-etch adhesives Adper Scotchbond 1 XT and XP Bond is well documented, and they serve as reference techniques in current research [18, 25–28]. Few data are available on the properties of the two novel two-step self-etch adhesives evaluated, Adper Scotchbond SE and Filtek Silorane Adhesive System [29, 30], although the performance of the second was recently reported to be similar to that of Clearfil SE Bond, considered a gold standard [30]. Both two-step self-etch adhesives exhibited a decline in bond strength after thermocycling in the present study, in agreement with previous research [18].
However, Adper Scotchbond SE and Filtek Silorane Adhesive System differ from typical two-step self-etch adhesives. Thus, Adper Scotchbond SE is similar to a one-step self-etch adhesive where liquid A is a HEMA-water solution without etching capacity [30] that changes color from pink to yellow after application of liquid B, which contains the acidic monomers. The color change confirms that the solutions are adequately mixed and the acidic monomers are activated. Applying water and acidic monomers in separate solutions may help to achieve a stable adhesive interface and lengthen the shelf life of the product [30]. Another favorable factor may be the application of a second coat of liquid B (as instructed by the manufacturer), which provides a hydrophobic solvent-free adhesive layer [9]. Liquid B also contains bonded zirconia nanofiller that helps to develop a uniform and thicker layer, which could improve the relief of contraction stresses and preserve the integrity of the adhesive interface [31–33]. In fact, in the present study, no pre-testing failures were detected after 24 h of water storage with this adhesive, similar to previous reports [30]. For its part, Filtek Silorane Adhesive System requires separate light curing of the primer and bonding, thereby establishing the bonding to dentin mechanism in the first application step, as in one-step self-etch adhesives [34]. There may be several reasons for its good MTBS outcomes. Thus, the vitreous copolymer reinforcement and silane-treated silica filler may promote a dense adhesive interface with high affinity for hard dental tissues. Furthermore, the strong hydrophobia of the adhesive system may endow the bonding layer with exceptional hydrolytic-resistant characteristics and simultaneously optimize its interaction with the composite resin [34]. Finally, the low polymerization shrinkage of silorane composite resin implies less stress and, therefore, a lower likelihood of maladjustment on the bonding interface [35]. The bonding capacity of both two-step self-etch adhesives could be observed in the respective SEM images, in which failures appeared at the top of the hybrid layer within the adhesive resin and the dentin remained covered, with no visualization of its structure (Figs. 1, 2).
Thermocycling samples 5,000× in water at temperatures between 5 and 55 °C corresponds to approximately 6 months of in vivo functioning [36]. The aging effect induced by thermocycling relies on the ability of hot water to accelerate the hydrolysis of non-protected collagen and leaching of poorly polymerized resin monomers and to generate repetitive contraction/expansion stresses at the bonding interface [19, 37]. The interface undergoes a process known as plasticization, with a decrease in its mechanical properties [21]. Accordingly, thermocycling produced a reduction in bond strength for all adhesives evaluated in the present study and caused an increase in pre-testing failures for all adhesive systems tested.
Previous studies reported that the resin–dentin interface remained stable with total-etch adhesives due to the protective effect of the surrounding enamel rim, hindering water diffusion [17, 18]. Nevertheless, we found significant differences between the total-etch adhesives after the aging treatment. Micro-tensile bond strength values of the samples bonded with Adper Scotchbond 1 XT showed a marked reduction in MTBS values after thermocycling, evidencing hydrolytic degradation. The previous version of this adhesive, Scotchbond 1, was found to produce hybrid layers without complete infiltration of the mineral-depleted collagen layer [38], attributed to the presence of polyalkenoic acid co-polymer in its composition [38, 39]. This acid reacts with calcium and forms a gel that limits infiltration of the collagen matrix by resin monomers [40, 41]. In vivo degradation of the interface generated with this adhesive was reported after 1 year [40].
Although the bond strength values of XP Bond also decreased after thermocycling, they did not show such an accentuated deterioration. XP Bond mainly differs from Adper Scotchbond 1 XT in the type of solvent, as its novel chemistry incorporates butyl alcohol, which has similar vapor pressure to ethanol (used with water in Adper Scotchbond 1 XT), but does not carry the risk of esterification of functional monomers [6]. This component may be responsible for the higher stability and lower hydrolytic degradation of the adhesive interface produced by XP Bond in comparison with Adper Scotchbond 1 XT. Another reason could be the chemical interaction of XP Bond with dentin components. It is hypothesized that this interaction is due to the formation of calcium phosphate complexes derived from mineral apatite in the dentin and phosphate esters in the adhesive functional group [25]. One-step self-etch adhesives have been widely reported to yield the lowest bond strength values, regardless of the aging treatment used, consistent with the deficient performance of these adhesive systems [2, 6, 8, 9, 18, 39]. Their high hydrophilicity and extensive water sorption have been reported to affect the mechanical stability of the adhesive interface [4, 10, 18].
However, we found marked differences among the three one-step self-etch adhesives after 24 h of water storage. Thus, in comparison to Bond Force, MTBS values were significantly lower for Xeno V and G-Bond and their percentage of pre-testing failures was higher, consistent with a recent report of inferior outcomes with G-Bond [4, 6]. In contrast, other authors reported similar MTBS values for Xeno V, Bond Force and XP Bond after 24 h of water storage [5], although they did not use the same resin with all systems, unlike in the present study (with the exception of one product), and their results would have been influenced by the different stiffness and contraction of the restorative composites at the adhesive interface [42].
According to the manufacturer, Bond Force produces a multiple point interaction with dentin calcium due to the presence of several functional groups per molecule, which may improve the bond strength. Moreover, Bond Force contains HEMA, whereas Xeno V and G-Bond do not, and this water-soluble monomer facilitates infiltration of the collagen matrix, thereby enhancing bond strength to dentin, and improves the miscibility of the adhesive components, avoiding a phase separation between the solvent and monomers [33, 43, 44].
A further difference among these one-step self-etch adhesives is the degree of acidity, with Bond Force and Xeno V being considered mild and G-Bond intermediately strong. We highlight that pre-testing failures of up to 25 % were observed with Xeno V and G-Bond. All three one-step self-etch adhesives showed adhesive fractures after thermocycling 5,000×, but fractures with Bond Force and Xeno V were at the top of the hybrid layer and the dentin remained covered by the adhesive (Figs. 4b, 5b), whereas fractures with G-Bond were at the bottom of the layer and the dentin structure was visible, with only a few tubules occluded by the adhesive (Fig. 3b).
Several factors may have contributed to the reduced durability of the interface generated by Xeno V and G-Bond. In the case of Xeno V, no published data are available on the durability of the interface with dentin substrate, although a low degree of conversion with high concentrations of hydrophilic monomers was reported for previous versions of this product [14, 45]. It should be noted that incompletely cured acidic monomers produce a continuous etching of the dentin during water storage [46]. G-Bond contains 4-MET as functional monomer, which seems to have a low affinity for chemical bonding to hydroxyapatite [47], and it is HEMA-free, therefore the water separates from the other ingredients [12, 43]. For this reason, it is recommended to use an air-drying technique [15, 48, 49] to remove water-filled droplets retained in the adhesive layer and solvent residues [15], which would otherwise contribute to the formation of voids [43]. However, it was reported that even a strong air-drying technique was unable to eliminate all droplets at the dentin surface [48]. In the present study, SEM images of fracture specimens revealed multiple droplets within the interface produced with G-Bond (Fig. 3a). Moreover, due to the absence of HEMA, a high concentration of acetone is included in this adhesive. Although this solvent is extremely volatile when it binds to water, its evaporation ratio is low and complete evaporation of water/solvent cannot be achieved, even with strong air drying [49, 50]. The presence of residual solvent impairs adequate polymerization [50] and has been related to the increased permeability found with one-step self-etch adhesives [14].
The results of this in vitro study revealed that the lowest MTBS results were obtained by one-step self-etch adhesives after both aging treatments; thus, a reduction in steps decreases the MTBS values and increases the susceptibility of the adhesive interface to water degradation. Micro-tensile bond strength values were significantly reduced after thermocycling for all the adhesives tested. Further studies of these novel materials are needed to consolidate these results.
References
Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, Van Landuyt K, Lambrechts P, Vanherle G. Buonocore Memorial Lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215–35.
Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864–81.
Watanabe I, Nakabayashi N, Pashley DH. Bonding to ground dentin by a phenyl-P self-etching primer. J Dent Res. 1994;73:1212–20.
Perdigão J, Gomes G, Gondo R, Fundingsland JW. In vitro bonding performance of all-in-one adhesives. Part I. microtensile bond strengths. J Adhes Dent. 2006;8:367–73.
Yazici AR, Celik C, Ozgünaltay G, Dayangaç B. Bond strength of different adhesive systems to dental hard tissues. Oper Dent. 2007;32:166–72.
Sarr M, Kane AW, Vreven J, Mine A, Van Landuyt KL, Peumans M, Lambrechts P, Van Meerbeek B, De Munck J. Microtensile bond strength and interfacial characterization of 11 contemporary adhesives bonded to bur-cut dentin. Oper Dent. 2010;35:94–104.
Hashimoto M, Tay FR, Ito S, Sano H, Kaga M, Pashley DH. Permeability of adhesive resin films. J Biomed Mater Res B Appl Biomater. 2005;74:699–705.
De Munck J, Van Landuyt K, Coutinho E, Poitevin A, Peumans M, Lambrechts P, Van Meerbeek B. Micro-tensile bond strength of adhesives bonded to class-I cavity-bottom dentin after thermo-cycling. Dent Mater. 2005;21:999–1007.
Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101.
Yiu CK, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MR, Tay FR. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–96.
Tay FR, Pashley DH, Suh BI, Carvalho RM, Itthagarun A. Single-step adhesives are permeable membranes. J Dent. 2002;30:371–82.
Van Landuyt KL, Snauwaert J, De Munck J, Coutinho E, Poitevin A, Yoshida Y, Suzuki K, Lambrechts P, Van Meerbeek B. Origin of interfacial droplets with one-step adhesives. J Dent Res. 2007;86:739–44.
Chersoni S, Suppa P, Grandini S, Goracci C, Monticelli F, Yiu C, Huang C, Prati C, Breschi L, Ferrari M, Pashley DH, Tay FR. In vivo and in vitro permeability of one-step self-etch adhesives. J Dent Res. 2004;83:459–64.
Cadenaro M, Antoniolli F, Sauro S, Tay FR, Di Leonarda R, Prati C, Biasotto M, Contardo L, Breschi L. Degree of conversion and permeability of dental adhesives. Eur J Oral Sci. 2005;113:525–30.
Ikeda T, De Munck J, Shirai K, Hikita K, Inoue S, Sano H, Lambrechts P, Van Meerbeek B. Effect of air-drying and solvent evaporation on the strength of HEMA-rich versus HEMA-free one-step adhesives. Dent Mater. 2008;24:1316–23.
Carvalho RM, Chersoni S, Frankenberger R, Pashley DH, Prati C, Tay FR. A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occurs in self-etch adhesives. Biomaterials. 2005;26:1035–42.
De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P, Vanherle G. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82:136–40.
Osorio R, Pisani-Proenca J, Erhardt MC, Osorio E, Aguilera FS, Tay FR, Toledano M. Resistance of ten contemporary adhesives to resin–dentine bond degradation. J Dent. 2008;36:163–9.
Abdalla AI, Feilzer AJ. Four-year water degradation of a total-etch and two self-etching adhesives bonded to dentin. J Dent. 2008;36:611–7.
International Organization for Standardization. ISO/TS 11405:2003 Dental materials—testing of adhesion to tooth structure. WHO, Geneva, Switzerland
Ferracane JL, Berge HX, Leung BW. In vitro aging of dental composites in water—effect of degree of conversion, filler volume, and filler/matrix coupling. J Biomed Mater Res. 1998;42:465–72.
Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28.
Oliveira SS, Pugach MK, Hilton JF, Watanabe LG, Marshall SJ, Marshall GW Jr. The influence of the dentin smear layer on adhesion: a self-etching primer vs. a total-etch system. Dent Mater. 2003;19:758–67.
Hürmüzlü F, Ozdemir AK, Hubbezoglu I, Coskun A, Siso SH. Bond strength of adhesives to dentin involving total and self-etch adhesives. Quintessence Int. 2007;38:206–12.
Lattaa MA. Shear bond strength and physicochemical interactions of XP Bond. J Adhes Dent. 2007;9(suppl 2):245–8.
Perdigão J, Lopes MM, Gomes G. In vitro bonding performance of self-etch adhesives: II-ultramorphological evaluation. Oper Dent. 2008;33:534–49.
Saboia VP, Silva FC, Nato F, Mazzoni A, Cadenaro M, Mazzotti G, Giannini M, Breschi L. Analysis of differential artificial ageing of the adhesive interface produced by a two-step etch-and-rinse adhesive. Eur J Oral Sci. 2009;117:618–24.
Margvelashvili M, Goracci C, Beloica M, Papacchini F, Ferrari M. In vitro evaluation of bonding effectiveness to dentin of all-in-one adhesives. J Dent. 2010;38:106–12.
Duarte S Jr, Phark JH, Varjão FM, Sadan A. Nanoleakage, ultramorphological characteristics, and microtensile bond strengths of a new low-shrinkage composite to dentin after artificial aging. Dent Mater. 2009;25:589–600.
Mine A, De Munck J, Cardoso MV, Van Landuyt KL, Poitevin A, Kuboki T, Yoshida Y, Suzuki K, Lambrechts P, Van Meerbeek B. Bonding effectiveness of two contemporary self-etch adhesives to enamel and dentin. J Dent. 2009;37:872–83.
Van Meerbeek B, Willems G, Celis JP, Roos JR, Braem M, Lambrechts P, Vanherle G. Assessment by nano-indentation of the hardness and elasticity of the resin–dentin bonding area. J Dent Res. 1993;72:1434–42.
Choi KK, Condon JR, Ferracane JL. The effects of adhesive thickness on polymerization contraction stress of composite. J Dent Res. 2000;79:812–7.
Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85.
Mine A, De Munck J, Van Ende A, Cardoso MV, Kuboki T, Yoshida Y, Van Meerbeek B. TEM characterization of a silorane composite bonded to enamel/dentin. Dent Mater. 2010;26:524–32.
Bouillaguet S. Biological risks of resin-based materials to the dentin–pulp complex. Crit Rev Oral Biol Med. 2004;15:47–60.
Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999;27:89–99.
De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–32.
Osorio R, Ceballos L, Tay F, Cabrerizo-Vilchez MA, Toledano M. Effect of sodium hypochlorite on dentin bonding with a polyalkenoic acid-containing adhesive system. J Biomed Mater Res. 2002;60:316–24.
Shirai K, De Munck J, Yoshida Y, Inoue S, Lambrechts P, Suzuki K, Shintani H, Van Meerbeek B. Effect of cavity configuration and aging on the bonding effectiveness of six adhesives to dentin. Dent Mater. 2005;21:110–24.
Koshiro K, Inoue S, Sano H, De Munck J, Van Meerbeek B. In vivo degradation of resin–dentin bonds produced by a self-etch and an etch-and-rinse adhesive. Eur J Oral Sci. 2005;113:341–8.
Eliades G, Vougiouklakis G, Palaghias G. Heterogeneous distribution of single-bottle adhesive monomers in the resin–dentin interdiffusion zone. Dent Mater. 2001;17:277–83.
De Munck J, Arita A, Shirai K, Van Landuyt KL, Coutinho E, Poitevin A. Microrotary fatigue resistance of a HEMA-free all-in-one adhesive bonded to dentin. J Adhes Dent. 2007;9:373–9.
Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, Inoue S, Peumans M, Suzuki K, Lambrechts P, Van Meerbeek B. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84:183–8.
Van Landuyt KL, Snauwaert J, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. The role of HEMA in one-step self-etch adhesives. Dent Mater. 2008;24:1412–9.
Nunes TG, Ceballos L, Osorio R, Toledano M. Spatially resolved photopolymerization kinetics and oxygen inhibition in dental adhesives. Biomaterials. 2005;26:1809–17.
Wang Y, Spencer P. Continuing etching of an all-in-one adhesive in wet dentin tubules. J Dent Res. 2005;84:350–4.
Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, Inoue S, Tagawa Y, Suzuki K, De Munck J, Van Meerbeek B. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83:454–8.
De Munck J, Ermis RB, Koshiro K, Inoue S, Ikeda T, Sano H, Van Landuyt KL, Van Meerbeek B. NaOCl degradation of a HEMA-free all-in-one adhesive bonded to enamel and dentin following two air-blowing techniques. J Dent. 2007;35:74–83.
Monticelli F, Osorio R, Pisani-Proença J, Toledano M. Resistance to degradation of resin–dentin bonds using a one-step HEMA-free adhesive. J Dent. 2007;35:181–6.
Torkabadi S, Nakajima M, Ikeda M, Foxton RM, Tagami J. Bonding durability of HEMA-free and HEMA-containing one-step adhesives to dentin surrounded by bonded enamel. J Dent. 2008;36:80–6.
Acknowledgments
The authors wish to thank Yudi Gómez for the technical support in the laboratory of the University of Granada (Spain).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baracco, B., Fuentes, M.V., Garrido, M.A. et al. Effect of thermal aging on the tensile bond strength at reduced areas of seven current adhesives. Odontology 101, 177–185 (2013). https://doi.org/10.1007/s10266-012-0073-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-012-0073-2