Abstract
We evaluated the degree of selfing and inbreeding depression at the seed and seedling stages of a threatened tropical canopy tree, Neobalanocarpus heimii, using microsatellite markers. Selection resulted in an overall decrease in the level of surviving selfed progeny from seeds to established seedlings, indicating inbreeding depression during seedling establishment. Mean seed mass of selfed progeny was lower than that of outcrossed progeny. Since the smaller seeds suffered a fitness disadvantage at germination in N. heimii, the reduced seed mass of selfed progeny would be one of the determinants of the observed inbreeding depression during seedling establishment. High selfing rates in some mother trees could be attributed to low local densities of reproductive individuals, thus maintenance of a sufficiently high density of mature N. heimii should facilitate regeneration and conservation of the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inbreeding depression, i.e., the reduction in fitness of selfed progeny compared with outcrossed progeny, has been widely recognized in plants (Charlesworth and Charlesworth 1987; Husband and Schemske 1996). Inbreeding depression may affect many different components of fitness in plants, e.g., germination rate, plant size and growth, and seed yield (Charlesworth and Charlesworth 1987; Husband and Schemske 1996; Frankham et al. 2002); all of these adverse effects consequently reduce the fitness of the selfed progeny. The deleterious impacts of inbreeding depression are substantially greater in the field than under experimental conditions, and can severely reduce the fitness and sustainability of natural populations (see Frankham et al. 2002). Thus, levels of selfing and subsequent inbreeding depression should be assessed in natural populations, especially in populations of endangered plant species with high conservation priorities.
Natural levels of selfing (vs outcrossing) and inbreeding depression have been inferred by taking advantage of the recent development of genetic markers. Using highly polymorphic genetic markers such as microsatellites, it is now possible to accurately estimate selfing rates by analyzing the genotypes of mature trees and their progeny. By comparing proportions of selfed progeny in the surviving members of a cohort as they pass through different life stages, we can also investigate the timing and magnitude of the effects of inbreeding depression across these life stages. Moreover, by clarifying the effects of the spatial isolation of reproductive individuals on their selfing rates, potential risks of increasing selfing rates and inbreeding depression associated with selfing after human disturbance (e.g., excessive logging activities) can be predicted.
Vast areas in the forests of Southeast Asia are dominated by dipterocarps, which are predominantly allogamous species pollinated by insects (Bawa 1998). Most dipterocarp species have economic importance due to their timber and, thus, are threatened by logging operations that reduce the numbers of individuals and populations. These human activities may cause not only reductions in population size but also potentially harmful effects such as a reduction in outcrossing rates (e.g., Murawski et al. 1994a; Hall et al. 1996; Lee 2000; Obayashi et al. 2002) and low levels of genetic diversity (Obayashi et al. 2002) in the remnant tree populations. Since the densities of reproductive individuals in tropical canopy trees such as dipterocarps are generally low, even in a primary forest, logging operations are very likely to increase the rate of inbreeding, resulting in a higher risk of inbreeding depression in these populations.
Neobalanocarpus heimii (King) Ashton (Dipterocarpaceae) is a widely distributed canopy tree in mixed dipterocarp forests in Peninsular Malaysia (Ashton 1982). Due to overexploitation (Appanah and Weinland 1993), however, N. heimii now grows only in restricted areas such as forest reserves, and is categorized as “vulnerable” (IUCN 2003). A previous study (Konuma et al. 2000) suggested that N. heimii is predominantly an outcrossing species, with outcrossing rates estimated (based on paternity analysis with four microsatellite primer pairs) at 87.5–97.9% for seedlings and 100% for saplings. However, these estimates may be substantially higher than the outcrossing rates for earlier life stages, since there may have been strong selective pressure against selfed progeny during seed development and seedling establishment. This hypothesis is supported both by models and by empirical observations, suggesting that inbreeding depression has very strong effects on outcrossing species during early stages of their life cycle (Hufford and Hamrick 2003). To clarify the influence of inbreeding depression on survival from seed development to seedling establishment in N. heimii, we investigated the genetic composition (i.e., the proportion of selfed progeny) of a seed and seedling cohort using microsatellite markers. We also examined the influence of inbreeding depression on the fitness components of seeds (i.e., seed mass), the effect of seed mass on germination, and how distance to potential pollen sources influences the degree of selfing in N. heimii.
Materials and methods
Study species
Neobalanocarpus heimii (King) Ashton (Dipterocarpaceae) is a canopy tree endemic to Peninsular Malaysia. The population of this species flowers in synchrony for about 2 weeks in most years (Appanah and Weinland 1993), and its flowers are hermaphroditic. Apis and Trigona spp. are assumed to be main pollinators of this species.
The fruits begin to develop after the 11–20 days of flowering period (Appanah 1979) and become mature after 6 months (Appanah and Weinland 1993). As the fruits of Neobalanocarpus heimii are acorn-like and typically hold a single seed, we equated the tree’s fruit with its seed. Thus, in subsequent descriptions of the fruit of N. heimii, we use the term “seed” instead of “fruit” (for the same reason, “seed mass” of N. heimii always refers to “fruit mass” in the following text). The seeds continue to grow in size and weight even after maturation, and mature seeds continue to be dispersed for more than a year (M. Yasuda, Forestry and Forest Research Institute, Tsukuba, Japan, personal communication). The mature seeds without dormancy usually germinate within a week after dispersal (Symington 2004). The observed minimum seed mass required for germination is 1.75 g (M. Yasuda, unpublished data), and the mass of fully developed seeds reaches around 10 g. The final germination percentage of mature seeds gradually increases until about 11 months after flowering (M. Yasuda, personal communication). Therefore, even among mature seeds, there are considerable variations between early- and late-dispersed seeds in terms of both seed mass and germination ability. Early-dispersed seeds appear to be less fit than late-dispersed seeds.
Study site and sample collection
We recently established a permanent 40-ha plot (500 m×800 m) at the natural forest of Pasoh Forest Reserve (2°59′N, 102°18′E, 75–150 m a.s.l.) in Peninsular Malaysia. All dipterocarp trees over 30 cm in diameter at breast height (dbh) in this plot were mapped and identified to species level for our mating system and gene flow studies. This 40-ha plot and the neighboring area were used for the present study (Fig. 1). We found 47 potentially flowering, i.e., mature, N. heimii trees of dbh >30 cm in this study area (Fig. 1). Samples of the inner bark or leaves were collected from all 47 mature trees to determine the genotypes of mother trees and potential pollen parents.
Spatial distribution of Neobalanocarpus heimii [all trees >30 cm diameter at breast height (dbh)] within the 40-ha study plot (inner rectangle) and the neighboring area in the Pasoh Forest Reserve, Malaysia. Our seven focal trees are represented as open circles. The three numbers under the identifier for each tree are (from top): the proportions of selfed progeny in early-dispersed seeds, late-dispersed seeds, and seedlings. The numbers of progeny examined (sample sizes) are shown in parentheses
Sample collection of seeds was started in March 1999, when 19 mature trees were dispersing small but germinable seeds in the 40-ha plot. All these fruiting trees were assumed to have flowered in the same flowering season. Among the fruiting trees, we selected seven focal trees (C3, C4, C7, C8, RP346, WGY307, and YG17; Fig. 1) that we expected to produce enough seeds and seedlings for our study. Since mature seed dispersal of N. heimii continues for a prolonged period, and the genetic composition of seeds possibly changes depending on the dispersal timing of seeds, we collected seeds twice from each focal tree. The first collection for early-dispersed seeds from all of the trees was in March 1999. We subsequently collected late-dispersed, fully developed, seeds from two trees (C7 and WGY307) in October 1999 and from the others (C3, C4, C8, RP346, and YG17) in February 2000, because the timing and intensity of seed dispersal differed among these mother trees. Only green seeds were collected since this coloration indicates newly fallen fresh seeds. In July 2000, the sample collection was completed by collecting seedlings (estimated age: from 5 to 16 months old) from all seven focal trees. The seeds collected in February 2000 were weighed and used for either DNA analysis or germination tests, while other progeny samples were used solely for DNA analysis.
All the seeds and seedlings were collected within a 10-m radius of each focal tree to avoid contamination of our samples by progeny from other conspecific trees.
Germination test
The seeds collected in February 2000 from five mother trees (C3, C4, C8, RP346, and YG17) were used in a germination test. After weighing each seed, the seeds (n=342) were individually planted, with one seed per well, in 63-well flats filled with 100% local river sand. The flats were placed in a nursery at the Forest Research Institute Malaysia (FRIM) and watered to maintain a moisture level sufficient for germination. Each seed was checked for germination, which was defined as the emergence of the radicle from a seed, for about 3 weeks.
DNA extraction and genotyping
Total DNA was extracted from samples of 47 mature trees and the progeny of seven maternal trees (early-dispersed seeds, late-dispersed seeds, and seedlings), using a modified CTAB method (Tsumura et al. 1996) with extraction buffer containing 2.5% (v/v) β-mercaptoethanol. The extracted DNA was purified using a FastDNA Kit (Bio 101, La Jolla, CA) if the quality was not good enough for polymerase chain reaction (PCR) amplification. We determined the microsatellite genotypes of each sample using four primer pairs (Nhe004, Nhe005, Nhe015, and Nhe018) developed by Iwata et al. (2000) and a further three pairs (Nhe115, Nhe117, and Nhe123; GenBank AY558717–AY558719) developed to improve the accuracy of the paternity assignment. Primer sequences were as follows: Nhe115F, 5′-GCA GGA TGA TAT CTC TGG TAG; Nhe115R, 5′-ACT CAG TAA GGC ATG ATG G; Nhe117F, 5′-GCA TAT CCA TCA CCC AAG C; Nhe117R, 5′-TCA GGC CTT ATA GAC ACA ATG AA; Nhe123F, 5′-TTT CTC CGG CGA CCA GTG; Nhe123R, 5′-AGA GTG AGG CCA GTC CGC TAC.
Polymerase chain reaction amplifications were performed in 10-μl reaction volumes containing 0.3–2 ng genomic DNA, 20 mM Tris–HCl (pH 8.0), 50 mM KCl, 0.2 mM of each dNTP, 1.5 mM MgCl2, 0.2 μM of each primer (one of each pair was fluorescently labeled), and 0.25–0.40 U Taq polymerase [Gibco BRL (Rockville, MD) or Promega Madison, WI)] using a GeneAmp PCR System Model 9700 (Applied Biosystems, Foster City, CA). Two-step PCR was applied, with denaturing at 94°C for 15 s as the first step and annealing as the second step. The annealing temperature and the number of PCR cycles for the primer pairs were as follows: 60–62°C for 30 s and 30–38 cycles for Nhe004; 62°C for 30 s and 33–38 cycles for Nhe115, Nhe117, and Nhe123; and 60°C for 30 s and 34–38 cycles for Nhe005, Nhe015, and Nhe018. After the specified number of cycles, PCR was completed with a 2-min incubation at 72°C. The genotypes were determined using an ABI 310 or 3100 Genetic Analyzer and GeneScan software ver. 3.1.2 or 3.7 (Applied Biosystems).
Data analysis
Genetic diversity
Genetic diversity parameters for the adult population were estimated using genotype data from the 47 mature trees. The observed (Ho) and expected (He) heterozygosity, and the paternity exclusion probability (Q) for each microsatellite locus were estimated using CERVUS 2.0 software (Marshall 2001). The coefficient of inbreeding (FIS) in the adult population was also calculated using the equation: FIS=1−(Ho/He) (Nei 1977). If inbreeding equilibrium is assumed in the adult population, the equation FIS=(1−t)/(1+t) can be solved for t , where t is the outcrossing rate (Nei and Syakudo 1958). We also calculated the outcrossing rate (t) in the adult population using the above equation.
Paternity assignment and direct estimates of selfing rates
Prior to paternity assignment, we excluded four progeny samples (0.45% of the progeny sample) whose genotypes did not match that of the assumed mother and thus were considered to have been dispersed from other conspecific trees. Consequently, we analyzed a total of 891 progenies from the seven mother trees.
The paternity of each seed and seedling was assigned by means of simple exclusion based on the multilocus genotypes of the 47 mature trees. If any progeny lacked any suitable pollen donor candidates, we assumed its pollen donor was located outside the study area. If any progeny had two or more pollen donor candidates, we inferred paternity based on maximum likelihood paternity assignment (Marshall et al. 1998) using the computer program CERVUS 2.0 (Marshall 2001). The allele frequency among all 47 mature trees, rather than the allele frequency among the entire sample, was used for this simulation. The simulation parameters were as follows: 10,000 cycles, 47 candidate parents (the number of all mature trees in the study area), 100% for both the proportion of candidate parents sampled and the proportion of loci typed, 0% for the rate of typing error, and 80.0% for the confidence level. Using this approach to paternity assignment, proportions of self-fertilized (selfed) progeny were determined directly for seeds and seedlings (early- and late-dispersed seed stages and a seedling stage) in each mother tree. Selfing rates were also calculated from the proportion of selfed progeny in the total seed sample obtained from each mother tree.
The effect of isolation by distance on the mating pattern of the reproductive trees
To clarify how the degree of isolation by distance from effective pollen donors affected the mating pattern of reproductive trees, we examined the relationship between the selfing rate of each mother tree and the distance from it to its most effective pollen donor using regression analysis. The most effective pollen donor of each mother tree was defined as the mature tree (excluding the respective mother tree) that showed the highest percentage contribution to the progeny of each mother tree as a pollen parent. Since no conspecific tree was closer to mother tree C3 than the nearest border of the study area, the most effective pollen donor for C3 was assumed to be located outside the study area. Thus, we excluded tree C3 from this analysis.
Comparison of the proportion of selfed progeny at different life stages
We compared the proportion of selfed progeny both between seed (collected in March 1999 or February 2000) and seedling stages, and between two seed stages (the early- and late-dispersed seeds), using the Cochran–Mantel–Haenszel test. Since differences in the period of time after seed dispersal might affect the fate of progeny (i.e., seedling establishment success), we did not integrate the two different sampling periods of the late-dispersed seeds (collected in October 1999 or February 2000) when we compared the late-dispersed seeds with the seedlings. Instead, we used the seeds collected in February 2000 as representatives of the late-dispersed seeds for this comparison with the seedling stage.
The effect of cross type on seed mass and the effect of seed mass on germination
To investigate the effect of cross type (selfing vs outcrossing) on seed mass, we performed two-way analysis of variance (ANOVA) using the data for seeds collected in February 2000 from five mother trees (C3, C4, C8, RP346, and YG17). The effect of seed mass on germination was also investigated by fitting a nominal logistic curve for germination to the observed results of the germination test. Since the variance of seed mass showed a highly positive correlation with the mean seed mass among mother trees, we subjected the data to natural logarithmic transformation prior to this statistical treatment. All the standard statistics were calculated using JMP 4.0 software (SAS Institute, Cary, NC).
Results
Microsatellite analysis
We detected 7–14 alleles per locus at the seven microsatellite loci and 74 alleles in total among the 47 mature trees (Table 1). The coefficient of inbreeding (FIS) showed no significant deviations from Hardy–Weinberg expectations at any locus, and the FIS value for the 47 mature trees (adult population) over seven loci was 0.0126. This FIS value was solved for the outcrossing rate (t), which was calculated to be 0.975 in the adult population. The observed heterozygosity for the seven loci averaged 0.790. The total paternity-exclusion probability (Q) over these loci was 0.999, which was high enough to differentiate between selfed and outcrossed progeny.
The effect of isolation by distance on the mating pattern of reproductive trees
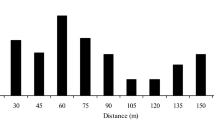
Selfing rates varied greatly, from 1.1 to 46.3% (i.e., outcrossing rates: 53.7–98.9%), among the seven mother trees. Linear regression of the selfing rate on the distance to the most effective pollen donor was significant at the 5% probability level (Fig. 2). The regression coefficient had a positive value, indicating that the selfing rate was higher for the mother tree located farther from its most effective pollen donor.
Comparison of the proportion of selfed progeny at different life stages
The proportions of selfed progeny in the early- and late-dispersed seeds ranged from 0.00 to 50.0 (mean =11.2) and from 0.00 to 44.7 (mean =16.5), respectively, among the seven families (Fig. 1). In contrast, the proportions of selfed progeny at the seedling stage ranged from 0.00 to 10.4 (mean =3.50) and were generally lower than the values for the seed stages. The differences in the proportion of selfed progeny between both of the seed stages (i.e., seeds collected in March 1999 and those collected in February 2000) and the seedling stage were statistically significant (Table 2). The difference in the proportion of selfed progeny between early- and late-dispersed seeds was also significant, indicating that the proportion of selfed progeny was lower in early-dispersed seeds than in late-dispersed seeds.
The effect of cross type on seed mass, and effect of seed mass on germination
Since there appeared to be no selfed progeny from mother tree YG17 in the late-dispersed seeds (Fig. 1), we excluded its progeny from the analyses examining the effect of cross type on seed mass and the effect of seed mass on germination. In all, we used data from 164 seeds from the DNA analysis to investigate the effect of cross type on seed mass, and data from 277 seeds from the germination experiment to investigate the effect of seed mass on germination.
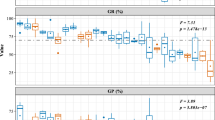
The mean seed masses of the selfed progeny were smaller than those of the outcrossed progeny for each of the four mother trees (Fig. 3), although the differences were not statistically significant except for the progeny of tree C3 (P=0.008, unpaired Student’s t test). The cross type explained a significant amount of the variation in seed mass, but mother tree explained a larger proportion of the variation (Table 3).
Comparison of the mean seed mass for the selfed and outcrossed progeny of four mother trees of Neobalanocarpus heimii. Error bars represent standard deviations of the seed mass for each mother tree. The differences were not statistically significant except for the progeny of the tree C3 (C3: P=0.008, C4: P=0.643, C8: P=0.087, RP346: P=0.447; unpaired Student’s t test). *P <0.05
Germination probability was positively correlated with seed mass (Fig. 4). The results of a nominal logistic fit for germination and the Wald test showed that the effects of both log-transformed seed mass and mother tree on germination were statistically significant (Table 4).
Discussion
Outcrossing rate
The estimates of outcrossing rates varied widely, from 53.7 to 98.9% (mean =87.7%), among mother trees, but were within the reported range for various dipterocarp populations or individuals, such as Dryobalanops aromatica Gaertn. f. (78.7–85.6%; Kitamura et al. 1994; 55.1–92.3%; Lee 2000), Stemonoporus oblongifolius Thwaites (84.4%; Murawski and Bawa 1994), Shorea megistophylla Ashton (71.3–86.6%; Murawski et al. 1994a), S. trapezifolia (Thwaites) Ashton (54.4–61.7%; Murawski et al. 1994b); S. leprosula Miq. (55.0–100%; Lee et al. 2000; 77.0–100%; Nagamitsu et al. 2001) and S. curtisii Dyer ex King (32.4–100%; Obayashi et al. 2002). Results of these previous studies have shown that dipterocarps are predominantly outcrossing species, but have considerable potential for selfing. Our results with Neobalanocarpus heimii accorded well with those of previous studies.
The result of regression analysis (Fig. 2) indicates that selfing rates tended to increase in parallel with the degree of isolation by distance from effective pollen donors. The relatively high selfing rates observed in RP346, C3 and C4 are likely to have been caused by low local densities of effective pollen donors, which reduces the inter-tree movement of pollinators and promotes selfing. Since low densities of flowering individuals have strongly increased selfing rates in some tropical canopy tree species (e.g., Murawski et al. 1990; Murawski and Hamrick 1991; Obayashi et al. 2002), the density of reproductive trees can be a critical factor affecting the mating pattern of tropical canopy tree species, which usually depend on animal vectors for their pollination.
Inbreeding depression in the early life stages
In the present study, the proportion of selfed progeny among the early-dispersed seeds was significantly smaller than that in the late-dispersed seeds (Fig. 1; Table 2), suggesting that the genetic composition of seeds may change depending on the dispersal timing of seeds in Neobalanocarpus heimii. However, the lower selfing rates in the early-dispersed seeds did not support the hypothesis that early dispersal of developing mature seeds was due to inbreeding depression during seed development. To ensure absence of inbreeding depression during seed development in N. heimii, aborted/immature seeds should also be examined in a further study.
Inbreeding depression in postdispersal seeds was also examined. The highly significant differences in the proportion of selfed progeny found between the seed and seedling stages (Table 2) provide strong evidence that inbreeding depression affected the viability of postdispersal seeds during seedling establishment. Hufford and Hamrick (2003) also found that the most intense selection occurred between the seed and seedling stages in a wild population of the Neotropical tree, Platypodium elegans Vogel (Fabaceae). Inbreeding depression can explain a large amount of regeneration failure in early life stages among tropical tree species that are predominantly outcrossing but have considerable potential for selfing.
The components of inbreeding depression during seedling establishment
Seed size is an important trait because it affects many features of seeds and seedlings (e.g., germination rate, seedling size and competitive ability) and often determines the viability of progeny. Many studies on intra-specific variation in seed size have shown that large seeds have advantages over smaller seeds during the early stages of seedling establishment (e.g., Stanton 1984; Tripathi and Khan 1990; Bonfil 1998; Seiwa 2000; Khan 2004). In fact, larger seeds showed higher germination probability in Neobalanocarpus heimii (Fig. 4; Table 4). Like many other tree species (Khan 2004 and references therein), larger seeds may also confer greater seedling survival and growth that may be linked with the large reserves of nutritive substances. Thus, our observation that selfed seeds were smaller than outcrossed ones (Table 3; Fig. 3) suggests that reduced seed mass was one of the factors contributing to the observed inbreeding depression from the seed to the seedling stage in N. heimii.
Implications for conservation of the remnant populations of Neobalanocarpus heimii
Our study has demonstrated that inbreeding depression affects survival rates from the postdispersal seed to the seedling stage of N. heimii. Konuma et al. (2000) reported that inbreeding depression might occur between the seedling and sapling stage of N. heimii because they detected selfed progeny in seedlings, but not in saplings. Furthermore, the very high outcrossing rate of the adult population (97.5%) detected in the present study provides further evidence that inbreeding depression continues to occur after seedling establishment, almost completely eliminating the selfed progeny from the adult population.
Given all these results, very few selfed progeny may survive to reach reproductive maturity as a result of inbreeding depression throughout the life stages of N. heimii. Moreover, our results also indicate that any reduction in the density of reproductive trees will tend to increase self-fertilization, increasing the risk of inbreeding depression in future generations. Thus, if the density of mature trees decreases due to logging or other human activities, remnant populations of N. heimii may be prone to increasing regeneration failure through inbreeding depression, thereby increasing the extinction probability of its populations. To maintain sound and sustainable populations of N. heimii, forest management strategies must aim to maintain high scope for outcrossing among reproductive trees by preventing their isolation, in terms of potential pollination, from conspecific reproductive trees.
References
Appanah S (1979) The ecology of insect pollination of some Malaysian rain forest trees. PhD Thesis, University of Malaya
Appanah S, Weinland G (1993) Planting quality timber trees in peninsular Malaysia—a review. Forest Research Institute of Malaysia, Kuala Lumpur
Ashton PS (1982) Dipterocarpaceae. Flora Malesiana Ser I Spermatophyta 9:237–552
Bawa KS (1998) Conservation of gengetic resources in the Dipterocarpaceae. In: Appanah S, Turnbull JM (eds) A review of dipterocarps: taxonomy, ecology and silviculture. Center for International Forestry Research, Bogor, pp 45–55
Bonfil C (1998) The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am J Bot 85:79–87
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst 18:237–268
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Hall P, Walker S, Bawa KS (1996) Effect of forest fragmentation on genetic diversity and mating system in a tropical tree, Pithecellobium elegans. Conserv Biol 10:757–768
Hufford KM, Hamrick JL (2003) Viability selection at three early life stages of the tropical tree, Platypodium elegans (Fabaceae , Papilionoideae). Evolution 57:518–526
Husband BC, Schemske DW (1996) Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50:54–70
IUCN (2003) IUCN Red List of Threatened Species. http://www.redlist.org. Downloaded on 3 March 2004
Iwata H, Konuma A, Tsumura Y (2000) Development of microsatellite markers in the tropical tree Neobalanocarpus heimii (Dipterocarpaceae). Mol Ecol 9:1684–1685
Khan ML (2004) Effects of seed mass on seedling success in Artocarpus heterophyllus L., a tropical tree species of north-east India. Acta Oecologica 25:103–110
Kitamura K, Rahman MYBA, Ochiai Y, Yoshimaru H (1994) Estimation of the outcrossing rate on Dryobalanops aromatica Gaertn. f. in primary and secondary forests in Brunei, Borneo, Southeast Asia. Plant Species Biol 9:37–41
Konuma A, Tsumura Y, Lee CT, Lee SL, Okuda T (2000) Estimation of gene flow in the tropical-rainforest tree Neobalanocarpus heimii (Dipterocarpaceae), inferred from paternity analysis. Mol Ecol 9:1843–1852
Lee SL (2000) Mating system parameters of Dryobalanopus aromatica Gaertn. f. (Dipterocarpaceae) in three different forest types and a seed orchard. Heredity 85:338–345
Lee SL, Wickneswar R, Mahani MC, Zakari AH (2000) Mating system parameters in a tropical tree species, Shorea leprosula Miq. (Dipterocarpaceae) from Malaysian lowland dipterocarp forest. Biotrop 32:693–702
Marshall TC (2001) CERVUS ver. 2.0 Available at http://helios.bto.ed.ac.uk/evolgen
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Murawski DA, Bawa KS (1994) Genetic structure and mating system of Stemonoporus oblongifolius (Dipterocarpaceae) in Sri Lanka. Am J Bot 81:155–160
Murawski DA, Hamrick JL (1991) The effect of the density of flowering individuals on the mating systems of nine tropical tree species. Heredity 67:167–174
Murawski DA, Hamrick JL, Hubbell SP, Foster RB (1990) Mating systems of two Bombacaceous trees of a neotropical moist forest. Oecologia 82:501–506
Murawski DA, Gunatilleke IAUN, Bawa KS (1994a) The effect of selective logging on inbreeding in Shorea megistophylla (Dipterocarpaceae) from Sri Lanka. Conserv Biol 8:997–1002
Murawski DA, Dayanandan B, Bawa KS (1994b) Outcrossing rates of two endemic Shorea species from Sri Lankan tropical rain forests. Biotrop 26:23–29
Nagamitsu T, Ichikawa S, Ozawa M, Shimamura R, Kachi N, Tsumura Y, Norwati M. (2001) Microsatellite analysis of the breeding system and seed dispersal in Shorea leprosula (Dipterocarpaceae). Int J Plant Sci 162:155–159
Nei M (1977) F-statics and analysis of gene diversity in subdivided populations. Ann Hum Genet 41:225–233
Nei M, Syakudo K (1958) The estimation of outcrossing in natural populations. Jpn J Genet 33:46–51
Obayashi K, Tsumura Y, Ihara-Ujino T, Niiyama K, Tanouchi H, Suyama Y, Washitani I, Lee CT, Lee SL, Norwati M. (2002) Genetic diversity and outcrossing rate between undisturbed and selectively logged forests of Shorea curtisii (Dipterocarpaceae) using microsattellite DNA analysis. Int J Plant Sci 163:151–158
Seiwa K (2000) Effects of seed size and emergence time on tree seedling establishment: importance of developmental constraints. Oecologia 123:208–215
Stanton ML (1984) Seed variation in wild radish: effect of seed size on components of seedling and adult fitness. Ecology 65:1105–1112
Symington CF (2004) Forester’s manual of dipterocarps (2nd edn., revised by Ashton PS, Appanah S). Malayan Forest Records no. 16. pp. 519. Forest Research Institute Malaysia and Malaysian Nature Society, Kuala Lumpur
Tripathi RS, Khan ML (1990) Effects of seed weight and microsite characteristics on germination and seedling fitness in two species of Quercus in a subtropical wet hill forest. Oikos 57:289–296
Tsumura Y, Kawahara T, Wickneswari R, Yoshimura K (1996) Molecular phylogeny of Dipterocarpaceae in Southeast Asia using RFLP of PCR-amplified chloroplast genes. Theor Appl Genet 93:22–29
Acknowledgements
We thank Dr. M. Yasuda for helpful discussions and permission to use his unpublished data; members of the Genetics Unit of FRIM for their assistance with laboratory work and nursery experiments; and Ms. Y. Kawamata, Ms. K. Obayashi, and Ms. Y. Takeuchi for their assistance during sample processing and collection. We also thank Mr. Quah and Ms. K Obayashi for their assistance in plot construction and field observations. Many thanks are also due to the members of the Genome Analysis Laboratory of the Forestry and Forest Products Research Institute and Tohoku University, and two anonymous reviewers for their invaluable comments and helpful discussions. This study was part of a joint research project undertaken by FRIM, Universiti Putera Malaysia, and Japan’s National Institute for Environmental Studies (Global Environment Research Program supported by the Ministry of Environment in Japan, grant no. E-4). The study was also partly supported by a Grant-in-Aid for Scientific Research (No. 15405026) provided by the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naito, Y., Konuma, A., Iwata, H. et al. Selfing and inbreeding depression in seeds and seedlings of Neobalanocarpus heimii (Dipterocarpaceae). J Plant Res 118, 423–430 (2005). https://doi.org/10.1007/s10265-005-0245-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-005-0245-z