Abstract
Parasenecio auriculata is a woodland perennial herb widely distributed in Northeastern Asia, constituted by a poorly understood polyploidy with a diploid (2n=2x=60) and a tetraploid (2n=4x=120). In this study, for a better understanding of the polyploidal evolution, cytogeography and morphological variation were analyzed in Japanese P. auriculata, including two varieties; var. bulbifera endemic to central Hokkaido and var. kamtschatica widely distributed in northern Honshu and Hokkaido. The occurrence of two polyploidal levels was reconfirmed. While var. bulbifera is predominantly tetraploid, var. kamtschatica is comprised of diploid and tetraploid. Morphological variation among 22 quantitative characteristics is continuous and not distinctive among cytotypes or varieties, but plant size tended to be larger in the order, diploid of var. kamtschatica, var. bulbifera, and tetraploid of var. kamtschatica. The cytotype distribution showed a conspicuous geographical pattern. Besides var. bulbifera endemic to the central Hokkaido, the diploid of var. kamtschatica is mainly found in Southern Hokkaido, and the tetraploid has a disjunct distribution in eastern and northern Hokkaido and northern Honshu. Such a geographical pattern is possibly attributable to the differentiation of climatic preference among cytotypes and varieties, and may have been established in association with the climatic cline along the Japanese archipelago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyploidization has been essential events for the evolution of flowering plants. This event is typically associated with the morphological differentiation of a polyploid (Otte and Whitton 2000) and potentially contributes to the acquisition of new genetic and/or physiological features (Levin 1983). Such evolutionary novelty is expected to result in enhanced competitive ability or ecological tolerance compared to the diploid progenitor(s). Consequently, polyploids tend to show a distinct or peripheral distribution compared to that of the parental diploid along climatic or environmental gradients (Levin 2002; Richards 1997). In this regard, the geographical distribution of polyploid expected to reflect their ecological preferences, and cytogeographical investigations can provide basic information on the evolutionary dynamics and ecological differentiation of polyploid.
Parasenecio auriculata (DC.) J.R. Grant (Senecioneae; Asteraceae) is a perennial herb, widely distributed in Northeastern Asia (Aleutians, Kamchatka, Sakhalin, Kurils, NE China, Korea, and the northern part of Japan). This species is distinguishable from other congeneric species by several morphological characteristics such as narrow panicles, auriculated petioles and reniformed leaves (Koyama 1966). Kitamura (1942) recognized three varieties in this species, two of which are distributed in Japan (Koyama 1995). One variety, P. auriculata var. bulbifera (Koidz.) H. Koyama (referred to here as “var. bulbifera”) is endemic to central Hokkaido and characterized by bulbils at leaf axils. The other variety, P. auriculata var. kamtschatica (Maxim.) H. Koyama (referred to as “var. kamtschatica”), has robust stems, larger auriculates and larger heads compared to the type variety. This variety is widely distributed in Hokkaido and the northern part of Honshu, as well as Sakhalin, Kurils, Kamchatka and Korea, representing a typical geographical range of boreal elements in Japan (Koyama et al. 1971).
In Parasenecio auriculata, the occurrence of two polypoidal races was indicated by previous studies (summarized in Table 1), with 2n=60 (reported from var. auriculata, var. bulbifera and var. kamtschatica) and 2n=120 (found at var. bulbifera and var. kamtschatica). Assuming x=30 in the genus (Robinson et al. 1997), the cytotypes with 2n=60 and 120 are diploid and tetraploid, respectively, while the 2n=118 cytotype was also reported in var. bulbifera. Despite the cytological observations, little is known about the evolutionary outcome of tetraploidization in P. auriculata. Although Koyama (1966) pointed out that the tetraploid of var. kamtschatica has larger phyllaries and florets than the diploid, differentiation of gross morphology between the diploid and tetraploid was not yet to be analyzed in detail. In addition, previous cytological reports (Table 1) have not clarified the geographical trend in the distribution of polyploids in this species, though they have indicated the coexistence of diploids and tetraploids in Japan.
In considering the geographical range of Parasenecio auriculata in Japan, climate is an important factor in the determination of plant distribution. This region is located in a climatic cline from cool-temperate to sub-arctic zones, as one end of the gradual shift initiated from the sub-tropical zone along the Japanese archipelago. Correspondingly, the forest vegetation in this region is represented by the transition from broad-leaved deciduous forests to sub-alpine coniferous forests along the longitudinal or altitudinal axis (Kira et al. 1967). A value for Kira’s (1948) warmth index of 45(°C month) is considered to roughly accord with the conditions at the border between two forest zones (Nogami and Ohba 1991), while the development of “pan-mixed forest” is also recognized in the southern part of the Kuromatunai depression as an intermediate zone (Tatewaki 1955). Such a vegetational shift is attributable to the differentiation of the geographical range among plant species according to their climatic tolerance (Masuda 1972; Sakai and Kurahashi 1975; Uemura et al. 1986). In this regard, Uemura et al. (1986) indicated that P. auriculata var. kamtschatica was distributed under conditions of “warm and small rain” in Hokkaido. However, their study site did not represent the whole geographical range of P. auriculata in Japan, and is not enough to discriminate the climatic relationship to the polyploidal distribution in this species.
In this study, for a better understanding of the polyploidal evolution in Parasenecio auriculata, the geographical distribution of the diploid and tetraploid was examined in Japan. The influence of climate on the distribution of the polyploids is also discussed based on the results obtained. In addition, a morphological analysis was performed to confirm the correlation between ploidal level and morphological differentiation in this species.
Materials and methods
Plant materials
Plant materials from Parasenecio auriculata for chromosome observation were collected at 44 localities in Japan, with 11 localities of var. bulbifera and 33 localities of var. kamtschatica (Table 2). Four of the 44 localities were analogous to the collection sites of previous reports on chromosome number (see Table 1), though the precise location of the site is unclear. In Sounkyo, Koyama (1966) reported the chromosome number of var. kamtschatica (2n=120; Table 1), but the present study dealt with samples of var. bulbifera. For each locality, 1–15 individuals (average, 6.11) were collected, which were cultivated at the botanical garden or at the laboratory of the Graduate School of Science, Kyoto University. Voucher specimens deposited in the herbarium of Kyoto University (KYO) were obtained from materials collected in the field and additionally from cultivated plants. Unfortunately, specimens were not available for all individuals examined, because some cultivated plants were senesced before flowering.
Chromosome observations
Somatic chromosomes were examined with the standard aceto-orcein squash method using root tips. Root tips were pretreated with a 0.005 M-hydroxyquinoline solution for 24 h at 4°C and fixed with a 45% acetic acid solution for 15–30 min. Then, they were macerated in a 1:2 mixture (v/v) of 45% acetic acid and 1 N HCl for 30 s at 60°C, and stained and squashed with a 1% of acetic orcein solution. The exact number of chromosomes was determined by observation under an Olympus BX-51 microscope.
Climatic conditions
Climatic data such as monthly mean temperature, annual mean temperature, monthly precipitation, annual precipitation and monthly snow depth were obtained from the 44 localities from the Japan Metrologial Agency (2002). These variables were estimated for each 1-km2 mesh covering the whole of Japan, based on 30 years (1971–2000) of census data collected by automated meteorological data acquisition system (AMeDAS) using multiple regression analysis. Utilizing this data, climatic conditions at the localities were summarized by six indexes; Kira’s (1948) warmth index [WI (°C·month)], mean temperature in the coldest month [MTCM (°C)], annual mean temperature [AMT (°C)], precipitation in summer [May to September; PS (mm)], precipitation in winter [October to May; PW (mm)], and maximum snow depth [MSD (cm)]. To analyze the relevance to polyploidal distribution, localities were grouped according to the main cytotype for each variety or to distinctive geographical ranges within the varieties (see “Results”), and differences among the groups were tested with the Kruskal–Wallis test using Statview Version 5.0 (SAS Institute, USA) followed by non-parametric multiple comparisons according to the procedure described by Zar (1999).
Morphological variation
Morphological variation in Parasenecio auriculata was analyzed with voucher specimens for which chromosome numbers were known. In the analysis, specimens of cultivated plants were not included because their variation in size seems to be dependent on the conditions of cultivation such as availability of sunlight or pot size. As a result, one to four specimens were available for each locality (a total of 83 specimens), although for some localities no specimens were available (Table 2). For each specimen, 16 quantitative, primary characters were measured, and six derived characters (i.e., ratio between characters) were calculated (Fig. 1; Table 3). Because of the reniform or ovate-reniform leaves of this species, two leaf lengths (Nos. 5 and 6 in Table 3) were defined, and the foliar characters (Nos. 4–9 in Table 3) were measured on the largest leaf of the specimen. The floral characters (i.e., those associated with heads or florets; Nos. 12–22 in Table 3) were represented by the means for measurements of three to five (mostly five) heads, phyllaries or florets randomly taken from the inflorescence.
Definition of characters for morphological analysis. a largest leaves. b heads, phyllaries and florets. Numbers are equivalent for those in Table 3
For individual characters, the difference in means among cytotypes or varieties was tested by one-way analysis of variance (ANOVA). Prior to the statistical test, the variables were logarhystic- or arcsine-transformed to meet the assumption of a normal distribution (Sokal and Rohlf 1995). Computations of one-way ANOVA and Schffé’s multiple comparisons were performed with Statview Version 5.0 (SAS Institute). A principal components analysis (PCA) was also performed with a specimen as an operational unit. From 22 characters, either of the two characters used to calculate the derived characters were excluded from the data set, resulting in a set of 16 characters (Table 3). Before the analysis, each of the values were standardized by ranging. The PCA was carried out by Excel Statistics Version 1.1 (Social Survey Research Information Co. Ltd.).
Results
Cytotype distribution
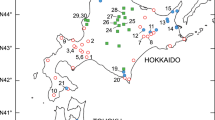
In total, six cytotypes were observed among 269 individuals of Parasenecio auriculata examined in this study (Figs. 2, 3; Table 2). For var. bulbifera, all individuals had a chromosome number of 2n=120 (Fig. 2a) except for one individual in Maruseppu with 2n=121 (Fig. 2b), and the cytotypes with 2n=118 reported by Nishikawa (1980) and 2n=60 by Koyama (1995) were not found in this study. For var. kamtschatica, the diploid cytotype with 2n=60 (Fig. 2c) is most frequently observed (60.2% of 227 samples), while small numbers of diploidal aneuploids with 2n=61 (Fig. 2d) and 62 (Fig. 2e) were also found. In addition, one individual in Onneto had a chromosome number of 2n=90 (Fig. 2f), a triploid cytotype. Finally, tetraploids with 2n=120 (Fig. 2 g) comprised 34.8% of the samples, representing the second major cytotype in this variety.
At most of the 44 localities, samples were mostly single polyploidal. Although sample sizes in several localities were limited to one or a few (Table 2), the site could be categorized as a diploid or tetraploid locality. The coexistence of different polyploidal levels was observed at two localities. In Onneto, one sample was a triploid with 2n=90, while the other 14 samples were diploid (2n=60) or diploidal aneuploid (2n=61 and 62). In addition, four samples from Mt. Hakodate included three diploids and one tetraploid. At the localities included in the previous reports, no difference was detected between those reports and the samples examined in this study (see Table 1).
The geographical distribution of cytotypes based on the data available from previous studies (Table 1) and this study (Table 2) were shown in Fig. 4b. As mentioned above, var. bulbifera is predominantly tetraploid, and endemic to central Hokkaido. In var. kamtschatica, diploids were distributed relatively continuously in Southern Hokkaido, while Koyama (1961) reported 2n=60 in Towada, northern Honshu (Table 1). In addtion, diploidal aneuploids with 2n=61 and 62 were found only in eastern Hokkaido. On the other hand, tetraploids of this variety were distributed disjunctly in northern and eastern Hokkaido and also the northern part of Honshu, except for the one individual at Mt. Hakodate (Southern Hokkaido). However, there was a less evident pattern in the altitudinal distribution of cytotypes (Fig. 4c). While the localities of var. bulbifera (central Hokkaido) and tetraploid of var. kamtschatica in northern Honshu tended to be distributed at higher altitudes than those of diploid and tetraploid of var. kamtschatica in Hokkaido, altitudinal ranges of the cytotypes or the varieties were overlapping one another, with the wider range for the tetraploid of var. kamtschatica from 5 m at Bekkai to 1,500 m at Mt. Hakkoda.
Climatic conditions
Six climatic indexes for 44 localities are summarized in Table 4. Kruskal–Wallis tests revealed a significant difference among localities for var. bulbifera (tetraploid), and the diploid and tetraploid of var. kamtschatica, in all indexes except for PS. Note that Mt. Hakodate (No. 40 in Table 2), where the diploid and tetraploid were symmetrically distributed, was not included in the tests. Three parameters related to thermal conditions (WI, MTCM and AMT) indicated that var. bulbifera is distributed under colder climates than var. kamtschatica, within which the tetraploid was distributed under colder climates than the diploid. On the other hand, PW and MSD indicated the tetraploid of var. kamtschatica was distributed in the most snowy environment, followed by var. bulbifera, and the diploid of var. kamtschatica.
Likewise, Kruskal–Wallis tests were also performed to test the difference of climatic conditions among three geographically distinct regions for tetraploid of var. kamtschatica; northern Honshu, northern and eastern Hokkaido (see Fig. 4). As a result, no significant difference was found at the three thermal parameters; WI (H=3.17; P>0.05), MTCM (H=3.74; P>0.05) and AMT (H=1.92; P>0.05). All of the mean values of these parameters for three distinct regions ranged between those for var. bulbifera and dipolid of var. kamtschatica, expect for the lower WI of eastern Hokkaido [WI=40.7 (±5.1); mean (±SE)]. In contrast, three parameters on precipitation showed statistical significance among the three regions; PS (H=9.90; P=0.007), PW (H=8.86; P=0.012) and MSD (H=6.76; P=0.034). Northern Honshu was characterized by the highest precipitation in summer and winter [PS=937.7 (±192.6), PW=559.4 (±64.2), MSD=219.5 (±74.2); mean (±SE)]. Multiple comparisons showed significantly lower summer rainfall in northern Hokkaido [PS=508.3 (±53.3), PW=370.2 (±54.4), MSD=161.3 (±50.3); mean (±SE)] and lower snowfall in eastern Hokkaido [PS=651.6 (±111.6), PW=282.7 (±98.0), MSD=88.8 (±38.8); mean (±SE)]. This relationship on precipitation was obviously inconsistent with the results of comparisons between the cytotypes and the varieties (see Table 4).
Morphological variation
Table 5 summarizes the morphological variation among 22 characters analyzed in 83 specimens of Parasenecio auriculata, classified into three groups, var. bulbifera (tetraploid) and the diploid and tetraploid of var. kamtschatica. To simplify the analysis, three specimens of var. kamtschatica with 2n=61 were included in the diploid group. For all 16 primary characters, the variation was continuous among these groups, but significant differences were also detected by one-way ANOVA except for numbers of leaves, heads and phyllaries per head (Nos. 2, 3, and 12 in Table 5). For most of the characters, the tetraploid of var. kamtschatica exhibited larger values than the diploid, while var. bulbifera tended to display an intermediate range of values. Two exceptions were the number of heads, which was largest in the diploid of var. kamtschatica, and the floral tube length, which was longest in var. bulbifera. Likewise, all derived characters varied continuously and were indistinguishable among the three groups of specimens, despite that the ANOVA revealed a significant difference in three of six characters (Nos. 11, 15 and 21).
In the principal component analysis, the first two components (PC1 and PC2) accounted for 32.1 and 13.8% of the total variance. The character loading on these components were given in Table 5. The PC1 is positively associated with the characters related to plant size such as shoot height, leaf width, auriculate diameter, phyllary length and style length (Nos. 1, 4, 9, 13 and 22 in Table 5). The PC2 has a positive loading for ratio between phyllary width and length and the ratio between floral tube width and floret length (Nos. 15 and 21), and a negative loading for number of heads, phyllary length and floret length (Nos. 3, 13 and 17). A plot of the scores of PC1 and PC2 is shown in Fig. 5. In the plot, the ranges of specimens of var. bulbifera, and the diploid and tetraploid of var. kamtschatica largely overlap one another. However, specimens tended to be located along the PC1 axis in the order, diploid of var. kamtschatica, var. bulbifera and tetraploid of var. kamtschatica, supporting the plant size differentiation indicated by the univariate analysis. In addition, var. bulbifera tended to concentrate on the negative side of the PC2 axis. Three specimens of var. kamtschatica with 2n=61 (represented by stars) were positioned within the range of the diploid (2n=60). On the other hand, the ranges of specimens of three disjunct regions for tetraploid of var. kamtschatica (assigned different symbols in Fig. 5) overlaps one another, reflecting the continuous morphological variation among the three regions. However, specimens from northern Hokkaido (filled circles) tend to show higher PC1 scores, indicating a tendency for the larger plant size at the region.
Discussion
The occurrence of two polyploidal levels in Parasenecio auriculata, a diploid with 2n=60 and a tetraploid with 2n=120 (Table 1), was confirmed in this study (Table 2). The cytotypes with 2n=60 and 120 accounted for 51.6 and 44.6% of the samples overall, obviously representing two major cytotypes in this species. As for varietal basis, var. bulbifera is predominantly comprised of tetraploids, while var. kamtschatica includes both diploids and tetraploids. This indicates the disconcordance of polyploidal constitution between the two varieties. On the other hand, small numbers of aneuploids with 2n=61, 62 or 121 were also observed. However, it is difficult to speculate on the evolutionary significance of these aneuploids because their inheritance or reproductive ability is largely unknown. In addition, one triploid individual with 2n=90 was also found at Onneto (Table 2). This cytotype could potentially be derived from the intercytotype mating between the diploid (2n=60) and tetraploid (2n=120), despite that the tetraploid was not found in the samples either this locality or adjacent sites (such as Mt. Oakan, Teshikaga and Sokodai). Since the cytotype is also likely to be derived from the occasional formation of an unreduced gametophyte, the origin of the triploid remains unclear.
The morphological analysis revealed significant differentiation of quantitative characters among the cytotypes of Parasenecio auriculata (Table 5). While the ranges of the characters overlapped one another, the tetraploid of var. kamtschatica has the largest plant size, followed by var. bulbifera (tetraploid), and the diploid of var. kamtschatica. This relationship was confirmed by the PCA (Fig. 5). Whereas moderate differentiation of plant size variation was also revealed among three geographically distinct regions for tetraploid of var. kamtschatica (cf. Fig. 5), this exemplified size enlargement at higher polyploid levels in P. auriculata, which is consistent with the tendency found for other diploid-tetraploid pairs in other taxa (e.g., Mosquin 1967; Gauthier et al. 1998; Naiki and Nagamasu 2004). However, it also clearly illustrated that cytotypes could not be distinguished based only on quantitative characters, although var. bulbifera is clearly distinguishable from var. kamtshatica by bulbils at leaf axils. Grant (1981) pointed out that morphological resemblance between a polyploid and its putative progenitor is one of the most important characteristics of autopolyploidization, while cytological or genetic evidences are also important to clarify the origin of polyploids. In this regard, the continuous morphological variation among cytotypes in var. kamtschatica could be interpreted as a support for a autotetraploidal origin of the 2n=120 cytotype from the 2n=60 cytotype. Correspondingly, var. bulbifera, which showed a continuous variation on quantitative characters with var. kamtschatica, may be an autotetraploidal variety in P. auriculata, despite its phylogenetic relationship with var. kamtschatica or other varieties in this species still remains uncertain.
In Parasenecio auriculata in Japan, the cytotype distribution showed a clear geographical pattern (Fig. 4b). While the diploid of var. kamtschatica was continuously distributed in southern Hokkaido, the tetraploid distributed disjunctly in northern and eastern Hokkaido, and also at the northern Honshu. In addition, var. bulbifera, predominantly tetraploid, was endemic to central Hokkaido. Comparisons of the climatic indexes among the localities revealed differences in habitat among the cytotypes and the varieties (Table 4). Statistical significance was detected for the parameter associated with warmth and snowfall. Moreover, among three disjunct regions for the tetraploid of var. kamtschatica, significant differences were detected for rainfall and snowfall conditions. Such climatic relationships reflects the differentiation of climatic preferences within and among the cytotypes or the varieties, and is likely explain geographical distribution of cytotypes in Japanese P. auriculata. However, precipitation conditions seem to be less implicative for explanation of the cytotype distribution, because the relationship among the cytotypes and the varieties was not concordant with those among the disjunct ranges for the tetraploid of var. kamtschatica. In contrast, the relationship for thermal condition was sufficiently consistent; var. bulbifera inhibited at the coldest condition, followed by the tetraploid of kamtschatica and the diploid. This exemplifies a confident tendency of the tetraploid to be distributed under colder climate than the diploid within this species. In this regard, warmthness, rather than precipitation, may be a critical factor to account for the cytogeographical structure of Japanese P. auriculata.
Interestingly, the mean for Kira’s (1948) WI among localities for the tetraploid of var. kamtschatica is 45.4, while for var. bulbifera and the diploid of var. kamtschatica it is 41.1 and 49.7, respectively. This means that the geographical distribution of cytotypes in Japanese P. auriculata is formed around WI=45, which is considered the boundary between broad-leaved deciduous and sub-alpine coniferous forests (Nogami and Ohba 1991). Indeed, comparison with a vegetation map (Miyawaki 1977) revealed that the geographical range of both the tetraploid of var. kamtschatica and var. bulbifera largely overlaps that of sub-alpine coniferous forests in Hokkaido, whereas the range of the diploid of var. kamtschatica overlaps that of broad-leaved deciduous forests (Fig. 6). However, the relationship is less evident in Honshu, and the delimitation by vegetation does not necessarily accord with local forest types at the localities (Nakagawa personal observation). Nonetheless, the cytogeographical structure of Parasenecio auriculata in Hokkaido roughly corresponds to the shift in vegetation associated with the climatic cline along the Japanese archipelago, and the climatic gradient might play an important role in determining the geographical distribution of cytotypes in this species.
A schematic illustration of the distribution of Parasenecio auriculata var. bulbifera (tetraploid) and the diploid and tetraploid of P. auriculata var. kamtschatica in Japan. Shaded areas represent ranges of subalpine coniferous forests (partly including the alpine zone) based on Miyawaki (1977)
Consequently, cytogeographical structure and its climatic relevance in Japanese Parasenecio auriculata imply that the tetraploidization is likely associated with an acquisition of the tolerance to colder climate. On the other hand, the chromosome number of 2n=60 or associated numbers were also reported at Sakhalin, southern Primosky and Koyak in Russia (Table 1). This means that the geographical distribution of the diploid extends further into the northern region than Hokkaido. In this regard, it is questionable whether such a widespread distribution is accompanied by greater climatic adaptability of the diploid than is to be expected from its distribution in Japan. In addition, the tetraploid has been reported only in Japan, and the distribution within the species range is largely unknown. Thus, more cytological information from a wider geographical range is still required, although results obtained in this study provides a foundation to elucidate the ecological significance of polyploidization in P. auriculata.
References
Arano H (1964) Cytological studies in Subfamily Carduoideae (Compositae) of Japan XVII. The Karyotype analysis in Cacalia and Syneilesis. Bot Mag Tokyo 77:86–97 (In Japanese)
Gauthier P, Lumaret R, Bédécarrats A (1998) Genetic variation and gene flow in Alpine diploid and tetraploid populations of Lotus (L. alpinus (D.C.) Schleicher/L. corniculatus L.). I. Insights from morphological and allozyme markers. Heredity 80:683–693
Grant V (1981) Plant speciation, 2nd edn. Columbia University Press, NY
Japan Metrologial Agency (2002) Mesh climatic data of Japan. Japan Metrologial Business Support Center, Tokyo (in Japanese; CD-ROM)
Kira T (1948) On the altitudinal arrangement of climatic zones in Japan. A contribution to the rational land utilization in cool highlands. Agric Sci N Temp Reg 2:143–173
Kira T, Shidei T, Numata M, Yoda K (1967) Vegetation of Japan. Science (Tokyo/Kagaku) 46:235–247 (in Japanese)
Kitamura S (1942) Compositae Japonicae IIIb:209–212
Koyama H (1961) Chromosome numbers in some Japanese species of Cacalia and the allied genera. Acta Phytotax Geobot 19:18–19 (in Japanese)
Koyama H (1966) Cytotaxonomic studies of Compositae. 2. On Cacalia auriculata var. kamtschatica. Acta Phytotax Geobot 22:11–14
Koyama H (1995) Parasenecio. In: Iwatsuki K, Yamazaki T, Boufford DE, Ohba H (eds) Flora of Japan III. Kodansha, Tokyo, pp 47–53
Koyama H, Fukuoka N, Kurosaki N (1971) Taxonomical and geographic studies on the Japan-Sea side elements. I. On the northern species (Sympetalae). Mem Natl Sci Mus (Tokyo) 4:87–94 (in Japanese)
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122:1–25
Levin DA (2002) The role of chromosome change in plant evolution. Oxford University Press, NY
Masuda H (1972) Tree distribution and temperature factor. Natural distribution of Hokkaido native coniferous trees and warmth index. Jpn J For Environ 8:7–16 (in Japanese)
Miyawaki A (1977) Vegetation of Japan—compared with other region of world. Gakken, Tokyo
Mosquin T (1967) Evidence for autopolyploidy in Epilobium angustifoloium (Onagraceae). Evolution 21:713–719
Naiki A, Nagamasu H (2004) Correlation between distyly and ploidy level in Damnacnathus (Rubiaceae). Am J Bot 91:664–671
Nishikawa T (1980) Chromosome counts of flowering plants of Hokkaido (4). Rep Taisetsuzan Inst Sci 15:23–28 (in Japanese)
Nishikawa T (1982) Chromosome counts of flowering plants of Hokkaido (6). Rep Taisetsuzan Inst Sci 17:9–16 (in Japanese)
Nishikawa T (1984) Chromosome counts of flowering plants of Hokkaido (7). J Hokkaido Univ Educ (Ser IIB) 35:31–42 (in Japanese)
Nogami M, Ohba H (1991) Warmth index and vegetation of Japan. Science (Tokyo/Kagaku) 61:136–149 (in Japanese)
Otte SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34:401–437
Richards AJ (1997) Plant breeding systems, 2nd edn. Chapman and Hall, London
Robinson H, Carr GD, King RM, Powell AM (1997) Chromosome numbers in Compositae. XVII: Senecioneae III. Ann MO Bot Gard 84:893–906
Sakai A, Kurahashi A (1975) Freezing resistance of conifers in Japan with special reference to their distributions. Jpn J Ecol 25:192–200 (in Japanese)
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, NY
Sokolovskaya AP (1960) Georaficheskoje rasprostranenie poliploidnykh vidov rastenii (issledovanije flory o.Sakhalin). Vestn Leningrodsk Univ No. 4 Ser Biol 21:42–58 (in Russian)
Sokolovskaya AP (1966) Georaficheskoje rasprostranenie poliploidnykh vidov rastenii (issledovanije flory Primorskogo Kraya). Vestn Leningrodsk Univ No. 3 Ser Biol 1:92–106 (in Russian)
Sokolovskaya AP (1968) A karyological investigation of the flora of the Koriakian land. Bot Zhul 53:99–105 (in Russian)
Tatewaki M (1955) Pan-mixed forest zone in East Asia. N For 7:240–243
Uemura S, Takeda Y, Nakanishi S (1986) Behaviors of the main temperate plant in Hokkaido along climatic gradients. Jpn J Ecol 36:141–152
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, NJ
Acknowledgements
The author thanks the staff of the botanical garden of the Graduate school of Science, Kyoto University for their support in the cultivation of plant materials; and Dr. J. Noguchi (Kyoto University) and Dr. Y. Kimoto (Research Institute for Humanity and Nature) for technical advice on chromosome observations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakagawa, M. Ploidy, geographical distribution and morphological differentiation of Parasenecio auriculata (Senecioneae; Asteraceae) in Japan. J Plant Res 119, 51–61 (2006). https://doi.org/10.1007/s10265-005-0239-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-005-0239-x