Abstract
The etiology of rheumatoid arthritis (RA) is thought to involve dysfunction of the programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) pathway; PD-1 negatively regulates autoimmunity by interacting with its ligand, PD-L1. We therefore investigated PD-1/PD-L1 expression in synovial tissue of patients with RA. We immunohistochemically stained synovial specimens from 51 patients with RA and assessed the association between PD-1/PD-L1 expression and rheumatoid factor (RF), the total count of infiltrating T cells, C-reactive protein (CRP), and Krenn’s synovitis score. PD-1 expression on infiltrating lymphocytes was detected in 34/51 RA cases (66.7%), while PD-1 expression was very mildly correlated only with the number of total infiltrating T cells (R2 = 0.1011, P = 0.0230). On the other hand, PD-L1 expression on synovial lining cells was observed in 37/51 RA cases (72.5%). Furthermore, a higher PD-L1 expression was significantly associated with RF positive state (P = 0.0454), and the correlations between PD-L1 expression and the number of infiltrating T cells (R2 = 0.5571, P < 0.0001), CRP (R2 = 0.4060, P < 0.0001), and Krenn’s synovitis score (R2 = 0.7785, P < 0.0001) were confirmed. PD-1 was expressed on infiltrating lymphocytes, while PD-L1 was expressed on synovial lining cells; the expression of PD-L1 on synovial lining cells was significantly correlated with the active state of the disease. These data suggest that PD-1/PD-L1 pathway may have an important role in the pathogenesis of RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammation of the joints due to autoimmune disorder. RA is prevalent in ~ 1% of the population, and many patients endure long-term destruction of bone and cartilage, resulting in severe deformity and joint instability [1, 2]. Treatments have advanced dramatically with the advent of methotrexate (MTX) and biological agents. However, RA pathophysiology is complicated, and therapeutic effects remain unsatisfactory [3]. Rheumatoid arthritis synovial tissue is characterized by villous proliferation of synoviocytes and infiltration of inflammatory cells, chiefly comprising lymphocytes [4], mainly CD4+ or CD8+ T cells, which both have cytotoxic function [5, 6]. Nonetheless, studies have suggested that CD4+ T cells play an especially important role [3].
Programmed cell death 1 (PD-1), a member of the CD28 family, is a 55 kDa transmembrane protein [7]. PD-1 is expressed on activated T cells, B cells, and monocytes [8]; PD-1 negatively regulates the T cell receptor (TCR) signal by recruiting src homology two-domain-containing tyrosine phosphatase 2 (SHP-2) to the phosphorylated tyrosine residue in the cytoplasmic region [9]. Interestingly, PD-1 −/− mice develop autoimmune diseases. For instance, C57BL/6 mice develop glomerulonephritis and arthritis [10], while BALB/c mice develop autoimmune dilated cardiomyopathy [11]. Programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2), both ligands for PD-1, belong to the B7 superfamily [12, 13]. PD-L1, a type 1 transmembrane protein, inhibits proliferation and activation of T cells by interacting with PD-1 [14]. Recently, it has been demonstrated that various malignant tumors express PD-L1 and escape from the host immunity by suppressing T cell activity [15]. Furthermore, in Sjögren’s syndrome (SS), PD-L1 expression on salivary gland ductal and acinar epithelial cells was observed and correlated with the degree of lymphocyte infiltration [16]. These results suggest that the PD-1/PD-L1 pathway is critical to the regulation of immunity and may be closely related to the pathogenesis of malignant tumors and autoimmune diseases.
In RA, PD-1 expression on T cells infiltrating the synovium, synovial fluid, and peripheral blood [17,18,19] and PD-L1 expression on synovial tissue and synovial fluid [3, 18] have been reported. However, particularly with respect to PD-1 expression on T cells, there are reports of both elevation [17,18,19] and suppression [20]; no definitive understanding has been obtained. Additionally, the relationship between clinicopathological characteristics and PD-1/PD-L1 expression in RA synovium has not been revealed to date. We therefore evaluated PD-1/PD-L1 expression in the synovial tissues of patients with RA using immunohistochemical (IHC) staining, and we statistically analyzed associations between clinicopathological features and PD-1/PD-L1 expression.
Materials and methods
Patients and samples
We investigated 51 RA patients who underwent total joint replacement surgery at Kurume University and Kurume University Medical Center from 1995 to 2009, by retrieving formalin-fixed, paraffin-embedded (FFPE) synovial tissues. Two rheumatologists (K.M. and S.Y.) re-evaluated the patients, and all of them met the 2010 American College of Rheumatology/European League Against Rheumatism criteria [21]. Synovial tissues of 22 patients with radiologically diagnosed osteoarthritis (OA) were used as controls. Samples were reviewed by two pathologists (K.O. and H.M.) who were blinded to any clinical information and classified according to Krenn’s synovitis score [22, 23]. Clinical data were obtained from patients’ medical records. This study was approved by the Research Ethics Committee of Kurume University, and all patients provided written informed consent for the use of their samples, according to the Declaration of Helsinki.
Immunohistochemical staining and evaluation
Immunohistochemical (IHC) staining was performed according to the manufacturer’s protocol, to assess the expression of lymphocyte markers and PD-1 on infiltrating lymphocytes and the expression of PD-L1 on synovial lining cells. Briefly, FFPE tissues of 3-µm thickness were deparaffinized, heated in a microwave for antigen retrieval, and incubated with 3% H2O2 for endogenous peroxidase blocking. The primary antibodies used for IHC were as follows: PD-1 (mouse monoclonal, clone: NAT105, ab52587, Abcam) at 1:100, PD-L1 (rabbit monoclonal, clone: E1L3N®, 13684, Cell Signaling Technology) and Negative Control Rabbit Immunoglobulin Fraction (rabbit polyclonal, X0903, Dako) for isotype control at 1:200, CD3 (mouse monoclonal, clone: F7.2.38, M7254, Dako) at 1:50 for total T cell marker, CD20 (mouse monoclonal, clone: L26, IR604, Dako) at 1:5 for B cells, CD56 (mouse monoclonal, clone: 1B6, NCL-L-CD56-1B6, Leica) at 1:100 for natural killer (NK) cells, and TIA-1 (mouse monoclonal, clone: 2G9A10F5, IM2550, Beckman Coulter) at 1:200 for cytotoxic marker of cytotoxic T-lymphocytes (CTL) or NK cells. The secondary antibody used was the Dako REAL EnVision System (Dako). The immunoreaction was visualized by using diaminobenzidine for 5 min.
Infiltrating lymphocytes were defined as PD-1 positive when the cell membrane was stained. The number of positive cells per 100 lymphocytes in three fields was manually calculated by using an optical microscope at 400-fold magnification. Synovial lining cells were defined as PD-L1 positive when the cytoplasm or cell membrane was stained. As for CD3, CD20, CD56, and TIA-1, three fields were observed at 400-fold magnification, and the number of positive lymphocytes per field was evaluated.
Statistical analysis
The JMP version 12 software package (SAS Institute, Tokyo, Japan) was used for statistical analyses. The Mann–Whitney U test was used for the comparison between two independent groups, and the Kruskal–Wallis test was used for independent multiple groups. A P value less than 0.05 was interpreted as statistically significant.
Results
Clinicopathological characteristics of patients
Table 1 shows patient characteristics. This study included a total of seven male (13.7%) and 44 female (86.3%) RA patients; their mean age was 61.1 years (range, 27–85 years). Rheumatoid factor (RF) was positive in 45 cases (88.2%) and negative in six cases (11.8%); C-reactive protein (CRP) was 0–8.8 mg/dL (mean, 2.1 mg/dL). Regarding treatment regimens, 39 cases (76.5%) were using disease-modifying antirheumatic drugs (DMARDs), which comprised 11 cases (21.6%) using MTX, and 28 cases (54.9%) using DMARDs other than MTX; the remaining 12 cases (23.5%) were using corticosteroid only. There was no case using biological agents. The distribution of Krenn’s synovitis scores was as follows: Five cases (9.8%) had a score of 4; five cases (9.8%) scored 5; ten cases (19.6%) scored 6; 15 cases (29.4%) scored 7; 12 cases (29.4%) scored 8; and four cases (7.8%) scored 9.
Features of lymphocytes infiltrating the RA synovium
In RA tissue, infiltrating lymphocytes were mostly CD3-positive T cells (Fig. 1a). CD20-positive B cells were mainly observed in lymphoid follicles, and the number of those infiltrating the synovium was small (Fig. 1b). CD56-positive NK cells were rarely seen (Fig. 1c). Some of the infiltrating lymphocytes were TIA-1 positive (Fig. 1d). In addition, the number of CD3-positive T cells correlated strongly with the number of TIA-1-positive lymphocytes (Fig. 2: R2 = 0.9046, P < 0.0001).
Immunohistochemical staining of rheumatoid arthritis (RA) synovial tissues for each lymphocyte marker. a Lymphocytes infiltrating the RA synovium were mostly CD3-positive T cells. b In RA, CD20-positive B cells were mainly found in lymphoid follicles, and the number of those infiltrating the synovium was small. c CD56-positive NK cells were rarely found in RA synovium. d Some lymphocytes infiltrating the RA synovium were TIA-1 positive
PD-1 and PD-L1 expression in RA and OA synovium
PD-1 expression on infiltrating lymphocytes was observed in 34/51 RA cases (66.7%) and 0/22 OA cases (0%); representative specimens are shown in Fig. 3a, b, respectively. The distribution is shown in Fig. 4a. The number of PD-1-positive lymphocytes was significantly larger in RA than in OA (P < 0.0001). PD-L1 expression on synovial lining cells was confirmed in 37/51 RA cases (72.5%) and 3/22 OA cases (13.6%); representative tissues are shown in Fig. 3c, d, respectively. The distribution is shown in Fig. 4b. PD-L1 expression was also significantly higher in RA than in OA (P < 0.0001).
Immunohistochemical staining of rheumatoid arthritis (RA) and osteoarthritis (OA) synovial tissues for programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1). a Infiltration of PD-1-positive cells was observed in the RA synovium. b Infiltration of PD-1-positive cells was not seen in the OA synovium. c PD-L1 expression on synovial lining cells in RA. d PD-L1 was rarely expressed on synovial lining cells in OA
Programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) expression in rheumatoid arthritis (RA) and osteoarthritis (OA) synovium. a The number of PD-1-positive cells was significantly larger in the RA group than in the OA group (P < 0.0001). b PD-L1 expression on synovial lining cells was significantly higher in the RA group than the OA group (P < 0.0001). c There was no significant difference between RA treatment groups in the number of PD-1-positive cells (P = 0.2824). d There was no significant difference between RA treatment groups in the PD-L1 expression on synovial lining cells (P = 0.2368)
In RA synovium, PD-1-positive lymphocytes were detected in 24/39 cases (61.5%) treated with DMARDs, which comprised 8/11 cases (72.7%) treated with MTX and 16/28 cases (57.1%) treated with non-MTX-DMARDs; PD-1-positive lymphocytes were detected in 10/12 cases (83.3%) treated with corticosteroid only. On the other hand, PD-L1 was expressed on synovial lining cells in 29/39 DMARDs-treated cases (74.4%), comprising 8/11 MTX cases (72.7%) and 21/28 non-MTX-DMARDs-treated cases (75.0%); PD-L1 was expressed on synovial lining cells in 8/12 cases (66.7%) treated with corticosteroid only. The distribution is shown in Fig. 4c, d, respectively; there was no significant difference in the number of PD-1-positive lymphocytes (P = 0.2824) and PD-L1 expression on synovial lining cells (P = 0.2368) when divided into each group.
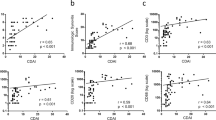
Relationship between the number of PD-1-positive lymphocytes and the state of disease in RA synovium
We analyzed the associations between the number of PD-1-positive lymphocytes and the markers related to the state of disease such as RF state, the number of CD3-positive T cells, CRP, and Krenn’s synovitis score in RA synovium. The number of PD-1-positive lymphocytes was not associated with RF state (Fig. 5a: P = 0.1803). The correlation between the number of PD-1-positive lymphocytes and the number of CD3-positive T cells was very mild (Fig. 5b: R2 = 0.1011, P = 0.0230). On the other hand, there was no correlation between the number of PD-1-positive lymphocytes and CRP (Fig. 5c: R2 = 0.0147, P = 0.3970), or Krenn’s synovitis score (Fig. 5d: R2 = 0.0183, P = 0.344).
Relationship between the number of programmed cell death 1 (PD-1)-positive cells and rheumatoid factor (RF), the number of CD3-positive T cells, C-reactive protein (CRP), and Krenn’s synovitis score in rheumatoid arthritis (RA) synovium. a The number of PD-1-positive lymphocytes was not associated with RF state (P = 0.1803). b The correlation between the number of PD-1-positive cells and the number of CD3-positive T cells was mild (R2 = 0.1011, P = 0.0230). c There was no correlation between the number of PD-1-positive cells and CRP (R2 = 0.0147, P = 0.3970). d There was no correlation between the number of PD-1-positive cells and Krenn’s synovitis score (R2 = 0.0183, P = 0.3444)
Relationship between PD-L1 expression on synovial lining cells and the state of disease in RA synovium
We also analyzed the associations between PD-L1 expression on synovial lining cells and RF state, the number of CD3-positive T cells, CRP, and Krenn’s synovitis score in RA synovium. PD-L1 expression was significantly associated with RF state (Fig. 6a: P = 0.0454) and correlated with the number of CD3-positive T cells (Fig. 6b: R2 = 0.5571, P < 0.0001), CRP (Fig. 6c: R2 = 0.4060, P < 0.0001), and Krenn’s synovitis score (Fig. 6d: R2 = 0.7785, P < 0.0001).
Relationship between programmed cell death 1 (PD-L1) expression on rheumatoid arthritis (RA) synovial lining cells and rheumatoid factor (RF), the number of CD3-positive T cells, C-reactive protein (CRP), and Krenn’s synovitis score in RA synovium. a A higher PD-L1 expression on synovial lining cells was significantly associated with RF positive state (P = 0.0454). b PD-L1 expression on synovial lining cells was significantly correlated with the number of CD3-positive T cells (R2 = 0.5571, P < 0.0001). c PD-L1 expression on synovial lining cells was significantly correlated with CRP (R2 = 0.4060, P < 0.0001). d PD-L1 expression on synovial lining cells was strongly correlated with Krenn’s synovitis score (R2 = 0.7785, P < 0.0001)
Discussion
Most autoimmune diseases result from dysfunction in a complicated immune tolerance system; the PD-1 pathway is one facet of this system [24]. PD-1-deficient mice developed autoimmune arthritis [10, 18]; PD-1-positive T cells are thought to be involved in the control of autoimmune arthritis. It was reported that PD-1 suppressed T cell proliferation and that blockade of PD-1 enhanced antiviral immunity [25]. Recently, it has been demonstrated that various malignant tumor cells expressing PD-L1 and/or PD-L2, the ligands for PD-1, escape from the host immunity by rejecting CD8+ cytotoxic T cells [26]. From the above, the function of PD-1 is considered to suppress the immune response and regulate the peripheral tolerance.
It could be possible that PD-1 expression on infiltrating lymphocytes inversely correlates with disease state because PD-1 negatively regulates autoimmunity. In our study, PD-1-positive lymphocytes increased in RA synovial tissues. However, no clear relationship was found between the number of PD-1-positive lymphocytes and RF status, CRP, and Krenn’s synovitis score; the correlation between the number of PD-1-positive lymphocytes and the number of CD3-positive T cells was very mild. In RA, PD-1-positive T cells have been reported to increase in plasma [19], synovial fluid [17, 18], and synovial tissue [18]. Taken together, it is suggested that even though PD-1-positive lymphocytes increase in synovium to suppress inflammation, the effects of this PD-1 immune tolerance mechanism may not be sufficient in RA. The linkage of single-nucleotide polymorphisms (SNPs) of the PD-1 gene to RA susceptibility [27] is also consistent with the possibility of PD-1 dysfunction in RA. In addition, the increased number of PD-1-positive lymphocytes may reflect the exhaustion of T cells due to chronic inflammation [28]. Yet, our results were not consistent with the previous study that demonstrated a decrease of PD-1 expression in peripheral T cells, implying various roles of PD-1 in RA [20]. This discrepancy might be due to the difference of the patient population such as race or duration of disease.
On the other hand, a higher PD-L1 expression on synovial lining cells was significantly associated with RF positive state, a larger number of infiltrating CD3-positive T cells, a higher CRP, and a higher Krenn’s synovitis score in RA. These results suggest that PD-L1 expression on synovial lining cells reflects the disease state. PD-L1 plays an important role in the immune escape mechanism of many malignant tumors [15]. In recent years, inhibition therapy of malignant tumors that targets the PD-1/PD-L1 pathway with anti-PD-1 antibody has made remarkable progress [29]. Thus, PD-L1 contributes to peripheral tolerance by interacting with PD-1.
However, the perpetuation of synovitis in the presence of an immunosuppressive system such as PD-L1-positive synovial lining cells is paradoxical. One possible explanation is that the negative adjustment in immunity effected by PD-1/PD-L1 or a similar system may be an inadequate counterweight to strong immune stimulation. In patients with RA and SS, the concentrations of the inhibitory cytokine IL-10 were elevated, suggesting that negative adjustment may not work sufficiently [30,31,32]. Another possible explanation is the ectopic expression of PD-L1. PD-L1 is expressed not only on antigen-presenting cells (APC) but also on nonhematological cells, which are nonprofessional APC, induced by IFN-γ stimulation [13, 16, 33]. PD-L1 expression on nonprofessional APC has also been observed in salivary epithelial cells in SS, and not just in malignant tumors. In addition, PD-L1 was expressed on human submandibular gland cells by IFN-γ stimulation. Furthermore, the expression of PD-L1 on SS patients’ salivary epithelial cells was correlated with the degree of lymphocyte infiltration to the salivary glands [16]. The elevation of IFN-γ has been reported in RA synovium [34]; it is possible that PD-L1 expression on synovial lining cells may be induced by IFN-γ stimulation due to upregulation of infiltrating lymphocytes.
A recent study has confirmed the expression of PD-L1, PD-L2, B7-H3, and B7-H4 in the RA synovium [3]. In addition, administration of PD-L1.FC (fusion protein to activate PD-1) elevated PD-1 activity and inhibited T cell proliferation, resulting in the reduction of arthritis, in a mouse model [18]. The PD-1/PD-L1 pathway is therefore a promising therapeutic target in RA. Our results were consistent with those of previous studies.
There are at least two limitations to our study. First, the number of patients in every treatment group was relatively small. There are many pharmacotherapies for RA, and they utilize a variety of mechanisms. In addition, differences between individual patients are large. Therefore, further studies with larger samples in every treatment group are required. Secondly, PD-1 and PD-L1 expression levels were evaluated by IHC only; further analyses such as gene abnormality and transcriptional systems are needed to clarify the biological roles of PD-1/PD-L1 in RA.
In summary, this study investigated PD-1/PD-L1 expression on RA synovial tissue and found that PD-L1 expression on synovial lining cells was associated with the disease state. Our results suggest the possibility that PD-1/PD-L1 axis may be involved in the pathogenesis of RA.
References
Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10.
Itonaga I, Fujikawa Y, Sabokbar A, Murray DW, Athanasou NA. Rheumatoid arthritis synovial macrophage-osteoclast differentiation is osteoprotegerin ligand-dependent. J Pathol. 2000;192:97–104.
Guo G, Shang Y, Zhu G, Bao X, Xu S, Chen Y. The expression and distribution of immunomodulatory proteins B7-H1, B7-DC, B7-H3, and B7-H4 in rheumatoid synovium. Clin Rheumatol. 2012;31:271–81.
Yoshida S, Higuchi F, Ishibashi Y, et al. Downregulation of RCAS1 and upregulation of cytotoxic T cells affects synovial proliferation and apoptosis in rheumatoid arthritis. J Rheumatol. 2008;35:1716–22.
Griffiths GM, Alpert S, Lambert E, McGuire J, Weissman IL. Perforin and granzyme A expression identifying cytolytic lymphocytes in rheumatoid arthritis. Proc Natl Acad Sci USA. 1992;89:549–53.
Belfrage H, Bhiladvala P, Hedlund G, Dohlsten M, Kalland T. Combined activation of murine lymphocytes with staphylococcal enterotoxin and interleukin-2 results in additive cytotoxic activity. Cancer Immunol Immunother. 1994;38:265–71.
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95.
Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–72.
Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98:13866–71.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51.
Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22.
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9.
Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8.
Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Kobayashi M, Kawano S, Hatachi S, et al. Enhanced expression of programmed death-1 (PD-1)/PD-L1 in salivary glands of patients with Sjögren’s syndrome. J Rheumatol. 2005;32:2156–63.
Wan B, Nie H, Liu A, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–50.
Raptopoulou AP, Bertsias G, Makrygiannakis D, et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 2010;62:1870–80.
Greisen SR, Rasmussen TK, Stengaard-Pedersen K, et al. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol. 2014;43:101–8.
Li S, Liao W, Chen M, et al. Expression of programmed death-1 (PD-1) on CD4+ and CD8+ T cells in rheumatoid arthritis. Inflammation. 2014;37:116–21.
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Krenn V, Morawietz L, Burmester GR, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–64.
Yoshida S, Arakawa F, Higuchi F, et al. Gene expression analysis of rheumatoid arthritis synovial lining regions by cDNA microarray combined with laser microdissection: up-regulation of inflammation-associated STAT1, IRF1, CXCL9, CXCL10, and CCL5. Scand J Rheumatol. 2012;41:170–9.
Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 2013;12:1091–100.
Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–7.
Lin SC, Yen JH, Tsai JJ, et al. Association of a programmed death 1 gene polymorphism with the development of rheumatoid arthritis, but not systemic lupus erythematosus. Arthritis Rheum. 2004;50:770–5.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30.
Cush JJ, Splawski JB, Thomas R, et al. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:96–104.
Anaya JM, Correa PA, Herrera M, Eskdale J, Gallagher G. Interleukin 10 (IL-10) influences autoimmune response in primary Sjögren’s syndrome and is linked to IL-10 gene polymorphism. J Rheumatol. 2002;29:1874–6.
Bertorello R, Cordone MP, Contini P, et al. Increased levels of interleukin-10 in saliva of Sjögren’s syndrome patients. Correlation with disease activity. Clin Exp Med. 2004;4:148–51.
Brookes SM, Price EJ, Venables PJ, Maini RN. Interferon-gamma and epithelial cell activation in Sjögren’s syndrome. Br J Rheumatol. 1995;34:226–31.
Dolhain RJ, ter Haar NT, Hoefakker S, et al. Increased expression of interferon (IFN)-gamma together with IFN-gamma receptor in the rheumatoid synovial membrane compared with synovium of patients with osteoarthritis. Br J Rheumatol. 1996;35:24–32.
Acknowledgments
We offer our special thanks to Mayumi Miura, Kanoko Miyazaki, Yuki Morotomi, Chie Kuroki, Kaoruko Nagatomo, Kensaku Sato, and Kazutaka Nakashima for their technical support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Matsuda, K., Miyoshi, H., Hiraoka, K. et al. Clinicopathological value of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) expression in synovium of patients with rheumatoid arthritis. Clin Exp Med 18, 487–494 (2018). https://doi.org/10.1007/s10238-018-0515-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-018-0515-4