Abstract
An aortic aneurysm is a permanent and localized dilatation of the aorta resulting from an irreversible loss of structural integrity of the aortic wall. The infrarenal segment of the abdominal aorta is the most common site of aneurysms; however, they are also common in the ascending and descending thoracic aorta. Many cases remain undetected because thoracic aortic aneurysms (TAAs) are usually asymptomatic until complications such as aortic dissection or rupture occurs. Clinical estimates of rupture potential and dissection risk, and thus interventional planning for TAAs, are currently based primarily on the maximum diameter and growth rate. The growth rate is calculated from maximum diameter measurements at two subsequent time points; however, this measure cannot reflect the complex changes of vessel wall morphology and local areas of weakening that underline the strong regional heterogeneity of TAA. Due to the high risks associated with both open and endovascular repair, an intervention is only justified if the risk for aortic rupture or dissection exceeds the interventional risks. Consequently, TAAs clinical management remains a challenge, and new methods are needed to better identify patients for elective repair. We reviewed the pathophysiology of TAAs and the role of mechanical stresses and mathematical growth models in TAA management; as a proof of concept, we applied a multiscale biomechanical analysis to a case study of TAA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
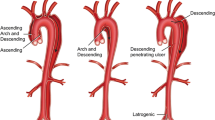
An aortic aneurysm is defined as a permanent and localized dilatation of the aorta resulting from an irreversible loss of structural integrity of the aortic wall. The infrarenal segment of the abdominal aorta is a common site for the development of aneurysms; however, aneurysms also form in the ascending, arch, and descending portions of the thoracic aorta. Most thoracic aortic aneurysms (TAAs) are found in the aortic root and/or ascending aorta (60 %) followed by the descending thoracic aorta (40 %), the aortic arch (10 %), and the thoraco-abdominal aorta (10 %), with some of them involving more than one aortic segment (Isselbacher 2005).
The prevalence and incidence of aneurysms of the thoracic aorta is more challenging to assess than for the abdominal aorta due to poorer access to screening data (Sakalihasan et al. 2011). Many cases remain undetected because TAAs are usually asymptomatic and indolent until complications such as aortic dissection or rupture occurs. Moreover, misdiagnoses of deaths due to aortic occurrences (erroneously attributed for example to myocardial infarction) contribute to underestimating the incidence of this disease (Elefteriades and Farkas 2010; Elefteriades et al. 2007). The incidence of TAAs seems to have risen over the past 20 years (Clouse et al. 1998, 2004; Acosta et al. 2006; Olsson et al. 2006). It is still not clear whether this increased incidence is due to an improved detection rate with a more prevalent use of CT-scanning or to an actual increase in the number of cases as suggested lately (Olsson et al. 2006; Elefteriades and Rizzo 2008).
TAAs clinical management remains a challenge in elective as well as emergency cases, with mortality for aortic rupture ranging from 94 to 100 % (Bickerstaff et al. 1982; Johansson et al. 1995).
Clinical decisions on treatment strategies for TAA are currently based on measurable indices that can be obtained from diagnostic images or patient data, such as aortic diameter and growth rate. However, mechanics-based indices, such as wall stresses and wall strains, are undoubtedly related to the events or rupture and dissection. Moreover, factors that are difficult to assess, such as the underlying causes of the aneurysm and the local state of weakening of the wall, also affect an individual aneurysm’s growth and its propensity for dissection or rupture (Braverman et al. 2011). In spite of advances in surgical care for TAA (including the use of endovascular techniques), surgical risks remain high, and therefore, the decision for intervention must be based on the balance between surgical risk and hazard of aortic rupture or dissection.
We reviewed the pathophysiology of TAAs and the role of mechanical stresses and mathematical growth models as possible tools to improve clinical management of aortic aneurysms. Then, we adopted a previously reported multiscale framework for modelling aortic tissue to a case study of TAA (Martufi and Gasser 2011, 2012a, b).
2 Aetiology and pathophysiology of TAAs
It is widely accepted that TAAs are the end result of a multifactorial process with genetic, environmental, and physiological factors (Hackman et al. 2008). Elevation of the activity of the proteolytic enzymes (MMPs) and down-regulation of the inhibitory enzymes (TIMPs) (Koullias et al. 2004a, b) shift the physiological balance towards an increased proteolytic activity and an irreversible pathological weakening of the aortic wall with consequent vessel dilatation and aneurysm formation.
Ascending aortic aneurysms Aneurysms of the aortic root and ascending aorta are most often related to cystic medial degeneration. The most prevalent histological features of this degenerative process are as follows: fragmentation of elastic fibres, dropout of smooth muscle cells (SMCs), mucoid degeneration, and accumulation of proteoglycans (PGs) within cystic spaces of cell depletion. This gradual degenerative process leads to focal weakening of the aortic wall, which in turn is thought to result in aneurysm formation. Cystic medial degeneration is a nonspecific degenerative condition of the media that has been traced to hypertension, and, to some extent, to the ageing process itself. When these changes within the muscular layers of the aorta occur in young patients, they can be associated with a vascular genetic disorder, such as Marfan syndrome (Isselbacher 2005). The decreased amount of elastin in the aortic wall and the loss of the elastic fibres’ highly organized structure are responsible for the observed abnormal aortic properties in patients with Marfan syndrome. Elastic fibres contents and structural organization play vital mechanical and biological roles in arteries. The loss of elastic fibres’ integrity is thus detrimental to arterial function and leads to progressive stiffening of the tissue and vessel dilatation (Jeremy et al. 1994).
The presence of an inherited genetic defect that affects the aortic wall has also been described in patients with TAAs who do not have apparent connective tissue disorders (Coady et al. 1999; Albornoz et al. 2006). Although these cases of TAAs may be sporadic, they are often present in multiple members of the same family and are referred to as familial TAA syndrome.
Bicuspid aortic valve (BAV) is associated with ascending TAAs and with an increased risk of acute dissections regardless of the presence or absence of significant hemodynamic valve dysfunction (Nistri et al. 1999; Nkomo et al. 2003; Pachulski et al. 1991). BAV patients showed an intrinsic abnormality of the aortic wall with congenital aortic fragility responsible for cystic medial degeneration in the wall of the ascending aorta (Fedak et al. 2003; Yasuda et al. 2003; Sa et al. 1999). A congenital inadequate production of fibrillin-1 may result in both the BAV and the aortic wall fragility (Fedak et al. 2003; Huntington et al. 1997). Moreover, inflammatory cells infiltration and SMC apoptosis may contribute to the additional weakening of the aortic walls of aneurysms associated with BAVs (Schmid et al. 2003).
Descending thoracic aneurysms More than one-fourth of the patients with abdominal aortic aneurysms (AAAs) also harbours a TAA, mostly located in the descending aorta (Larsson et al. 2011). This observation seems to confirm the hypothesis that aneurysmal pathology divides itself into two different entities separated at the arterial ligament that is attached to the inferior surface of the aortic arch: above the ligament is one pathology and below the ligament another one (Albornoz et al. 2006; Elefteriades 2008).
Atherosclerosis is the predominant underlying cause for the majority of descending TAAs. These types of aneurysms typically develop just distally to the origin of the left subclavian artery with pathogenesis and risk factors that are similar to those for AAAs (Isselbacher 2005), including wall degeneration and extracellular matrix (ECM) adverse remodelling. The ECM provides an essential supporting scaffold that bestows the aortic wall with its structural and functional properties. The ECM mainly contains structural proteins such as elastin, collagen, and PGs (Carey 1991), and the three-dimensional organization of these components is essential to accomplish proper physiological function. Consequently, localized degradation of connective tissue proteins, principally elastin, triggers initial aortic wall dilation (Dobrin et al. 1984; Anidjar et al. 1990). Collagen turnover and irreversible pathological remodelling of the ECM promote enlargement of the vessel and local wall weakening, which eventually lead to aortic rupture.
3 Interventional planning of TAAs
Clinical estimates of rupture potential and dissection risk are based primarily on geometric factors and growth rate. The size of the aneurysm is a universally recognized predictor of rupture risk. Thus, interventional planning for TAAs relies mostly on the maximum diameter of the vessel (Elefteriades and Farkas 2010). Based on the longitudinal data from the Yale group, the risk of aortic rupture or dissection increased dramatically at a maximum diameter of 6.0 cm for ascending aneurysms and 7.0 cm for descending thoracic aneurysms. This led to the current guidelines for surgical intervention on TAAs of 5.5 cm for ascending aneurysms and 6.5 cm for descending aortic aneurysm. (Elefteriades 2008; Coady et al. 1997). However, at least half of all type A dissections do occur at a diameter \(<\)5.0 cm (Pape et al. 2007). At the same time, many patients with an ascending aortic aneurysm under 5.0 cm will have no complications. Further information is needed to drill down on which patients with “small” thoracic aortic aneurysms are at risk for complications and which patients with large thoracic aortic aneurysms are not.
The intervention criterion for patients with Marfan syndrome, with a positive family history, or with BAV has been set to lower levels, as there is some evidence that these patients have a higher rupture risk (Elefteriades 2008; Coady et al. 1997).
Growth rate has also been consistently shown to be a critical parameter in predicting aortic rupture (Griepp et al. 1999; Lobato and Puech-Leao 1998). Fast aneurysm expansion was found to be a risk factor for TAA rupture, independently from TAA size (Lobato and Puech-Leao 1998). The mean growth rate for all TAAs is 0.12 cm/year (Davies et al. 2002) with the descending aorta growing slightly faster than the ascending one. Even if baseline diameter is a “gold standard” predictor of TAA growth, a substantial individual variation in aneurysm expansion rates exists (Dapunt et al. 1994), thus making it complex to prospectively predict patient-specific enlargements.
Aneurysm expansion is defined in common clinical practice as the change in maximum aortic diameter over time, and this measure is normally obtained measuring the maximum diameter of the aorta at two points in time. However, monitoring methods that are related to only one cross-section provide only limited information about aneurysm growth. Areas of fast growth can be missed, axial growth cannot be quantified, and shape changes of potential interest for endovascular therapy-related decisions cannot be captured (Martufi et al. 2013).
4 Biomechanical modelling of TAAs
Biomechanical factors, such as structural and fluid-mechanical stresses, influence the biological activity (Xu et al. 2010; Nchimi et al. 2013) and play fundamental roles in the genesis and development of vascular diseases (Humphrey 2002; Xu et al. 2001; Lehoux and Tedgui 1998). These mechanical stresses are transmitted from the macroscopic to the cellular levels of a vascular tissue and influence the tissue’s mechanobiology, namely the tendency of the cells in a specific tissue to respond to any changes in the mechanical and chemical environment. The ensuing changes in turn affect and impair the specialized function of the tissue within its organ. Computer simulations and mathematical modelling can provide the mean to estimate and quantify these biomechanical factors and have the potential to contribute to more effective diagnosis and treatment for vascular diseases. From a strictly mechanical prospective, rupture and dissection can be regarded as a mechanical failure of the aortic wall occurring when the mechanical stress exceeds the local failure strength of the structurally compromised wall. One main objective for biomechanical modelling is to assess the risk of rupture and dissection of an individual aneurysm to provide the surgeons with indications for intervention and improved selection criteria for elective repairs that are tailored to each individual patient. Improvements in risk assessment will result in preventing aneurysm-related mortality without unnecessarily increasing the number of repair interventions (and associated surgical complications).
Biomechanical modelling has been extensively used to evaluate aortic wall stresses (Thubrikar et al. 1999; Borghi et al. 2008; Nathan et al. 2011; Shang et al. 2013; Beller et al. 2004; Khanafer and Berguer 2009; Tse et al. 2011). Starting from the stress maps, computationally derived indices such as peak wall stress (PWS; the maximum stress in the wall) and peak wall rupture risk (PWRR; the maximum stress/strength ratio in the wall) have been proposed for abdominal aortic aneurysms (Fillinger et al. 2003; Venkatasubramaniam et al. 2004; Gasser et al. 2010; Vande Geest et al. 2006). These indices integrate information from the wall stress with clinically known rupture risk factors and were shown to achieve a more reliable risk prediction than maximum diameter alone in AAAs.
Computing stress-derived parameters involves a nonlinear biomechanical analysis, which requires an accurate three-dimensional reconstruction of the aneurysm geometry, an appropriate constitutive laws for the aneurysmal tissue, and the in vivo loading and boundary conditions. To this end, the biomechanics of AAA has been studied extensively whereas TAA biomechanics has not been fully analysed. TAAs and AAAs can arise from different underlying causes and present very distinct pathophysiology. However, the common pathway of clinical progression for both pathologies involves proteolytic degradation of the ECM and biomechanical failure.
Biomechanical predictions rely on an accurate constitutive description of the aneurysmal tissue (Martufi and Gasser 2013; Rodriguez et al. 2009). To this end, there are two basic approaches, the “black box” phenomenological approach and the structurally derived approach. Phenomenological models cannot allocate stress and strains to the different histological constituents in the vascular wall, and therefore do not provide an interface for integrating evolution equations for individual tissue constituents. Consequently, they are not suited to study vascular wall remodelling directly (Thubrikar et al. 1999; Nathan et al. 2011; Shang et al. 2013; Beller et al. 2004; Okamoto et al. 2002; Vorp et al. 2003; Sokolis et al. 2012; Takamizawa and Hayashi 1987; Raghavan and Vorp 2000; Vaishnav et al. 1972; Fung et al. 1979; Humphrey 1995; Delfino et al. 1997; Rodriguez et al. 2008). In contrast, structurally derived constitutive models overcome this limitation and make it possible to first to consider the biological process at different length scales and then to integrate both histological and mechanical information in the mathematical model of the arterial wall (Gasser et al. 2012; Gasser 2011; Lanir 1983; Wuyts et al. 1995; Zulliger et al. 2004; Martufi and Gasser 2011; Ferruzzi et al. 2011; Bellini et al. 2012; Gasser et al. 2006; Holzapfel et al. 2000, 2002).
The other important distinction to be made is between risk predictions based on passive constitutive models and those made taking into account the inherent active property of all biological tissues that grow and remodel in response to the stimuli their cells are sensing. Stress analyses performed with constitutive models where tissue growth and remodelling have been suppressed (passive models) can only cover a limited time period in which the biological process does not change the mechanical properties of the tissue (Thubrikar et al. 1999; Nathan et al. 2011; Shang et al. 2013; Beller et al. 2004; Okamoto et al. 2002; Vorp et al. 2003; Sokolis et al. 2012; Takamizawa and Hayashi 1987; Raghavan and Vorp 2000; Vaishnav et al. 1972; Fung et al. 1979; Humphrey 1995; Delfino et al. 1997; Rodriguez et al. 2008; Gasser 2011; Lanir 1983; Wuyts et al. 1995; Zulliger et al. 2004; Martufi and Gasser 2011; Ferruzzi et al. 2011; Bellini et al. 2012; Gasser et al. 2006; Holzapfel et al. 2000, 2002). When medium- and long-term predictions need to be made, the inherent ability of biological tissues to grow and remodel and dynamically adjust when changes in mechanical loading occur needs to be properly addressed (Martufi and Gasser 2013; Humphrey and Holzapfel 2012).
5 Structurally derived model of TAA
We adopted a previously reported multiscale framework for modelling aortic tissue (Martufi and Gasser 2011, 2012a, b) and adapted it to a patient-specific case of TAA. The method was applied to study one case of ascending TAA with one baseline scan and one follow-up scan prior to surgery. Passive constitutive properties of the vascular tissue were estimated from biaxial tensile test data on a surgically removed specimen (following approved institutional ethics protocol and patient’s consent), while the remodelling properties were derived from the clinical follow-up study. Finally, the tissue macroscopic response is analysed, and the applicability of the proposed approach for investigating clinically relevant problems in TAA management is demonstrated.
5.1 Histomechanical modelling of TAA wall
The ECM rather than being solely an architectural framework for the surrounding cells is an active structure that controls the mechanical environment to which vascular tissue is exposed. Mechanical forces and deformations guide the development of the tissue structure, which eventually leads to a mechanically optimized structure. Homeostasis (or the physiological preservation of this structure) relies on a delicate (coupled) balance between continual degradation and synthesis of tissue constituents. Of the two main load-bearing components of the ECM, elastin, although extremely stable (turnover rate of the lifetime of humans), is nonetheless degraded by numerous MMPs and its degradation together with the disruption of elastin-SMC interactions is a direct stimulus for cellular activity (Bäck et al. 2013). Collagen, on the other hand, exists in the tissue in a constant state of deposition and degradation (Humphrey 1999). Mature fibroblasts perceive changes in the mechanical loading and adjust the expression and synthesis of collagen molecules in order to account for the changes in the micro-mechanical environment. Collagen fibrils are assembled into suprafibrillar structures (collagen fibres) that, in turn, define the tissue’s macroscopic mechanical behaviour (Wess 2008). Consequently, including the development of the hierarchical collagen structures when modelling aneurysm mechanics is a crucial step needed to understand the mechanical properties of the vascular wall and predict physiological realistic mechanical loading states (Martufi and Gasser 2013).
Passive model Vascular tissue was regarded as a fibrous collagenous tissue, where fibres of collagen reinforce an otherwise isotropic matrix material. At low strains, collagen fibres are mechanically inactive, and the non-collagenous matrix material determines the vascular wall’s properties. Collagen fibres were assembled from bundles of proteoglycans (PG) cross-linked collagen fibrils (CFPG-complex) with different undulations. For simplicity, the alignment of the collagen fibres with respect to their referential orientations \(\mathbf{N}\) was defined with an isotropic orientation density function \(\rho \left( \mathbf{N}\right) =\rho _0\), which adjusted over time according to the local stretch field (see below § Collagen turnover model).
The constitution of the CFPG complex was defined by a virtually linear stress-strain response and a triangular probability density function (PDF) that characterizes the undulation of collagen fibrils, within a collagen fibre. The triangular PDF defines the relative amount of engaged collagen fibrils when exposing the collagen fibre to a stretch \(\lambda \) (Martufi and Gasser 2011). Consequently, the limits \(\lambda _{\min }\) and \(\lambda _{\max }\) of the triangular PDF denote fibre stretches at which the first and last collagen fibrils become straightened out and start to bear tension load. Assuming an incompressible collagen fibre, these assumptions yielded a piece-wise analytical expression for the collagen fibre’s Cauchy stress as detailed elsewhere (Martufi and Gasser 2011).
Collagen turnover model Collagen turnover in the vascular wall is accomplished by modelling fibroblast cells that are connected to collagen fibrils and spread throughout the entire collagen network. It is assumed that fibroblast cells sense the state of strain and deposit pre-stretched collagen fibrils in an effort to maintain a homeostatic environment where, at physiological loading, just 10 % of collagen fibrils are engaged. The orientation-dependent mechanical stimulus \(\zeta (\mathbf{N})\) defined as the ratio between the present stretch \(\lambda \left( \mathbf{N} \right) \) and the homeostatic physiological stretch \(\lambda _\mathrm{ph}\) served as a mechanical stimulus for the collagen turnover. In details, when the existing collagen is stretched more than the newly formed collagen \((\zeta > 1)\), collagen turnover is amplified to increase the total collagen density in the tissue, in such a way that a higher amount of collagen guarantees homeostasis. Similarly, for \(\zeta < 1\), the collagen turnover is reduced in order to reach homeostasis through a net loss of collagen. Finally, it is assumed that collagen fibrils are removed from the existing collagen structure with a purely time-based process without changing their undulation characteristics, i.e. only the collagen density is reduced. In contrast, newly formed collagen fibrils are integrated at a certain distribution of pre-stretches that causes the collagen density and the undulation characteristics to change. The structural concept adopted implicitly defines the pre-stretch of collagen fibrils via the limits of the triangular distribution, i.e. by defining the undulation limits of the newly deposited fibrils (\(\lambda _{\min }^\mathrm{NEW}\) and \(\lambda _{\max }^\mathrm{NEW}\)) as detailed elsewhere (Martufi and Gasser 2012a, b).
5.2 Parameter estimation
The passive model integrates two mechanical parameters \(\mu \) and \(k\), and three structural parameters \((\lambda _{\min },\,\lambda _{\max },\,\rho \left( \mathbf{N}\right) )\) with clear physical meaning. The mechanical parameter \(\mu \) quantifies the matrix shear modulus and describes the elastin-driven low stress response of the tissue. The mechanical parameter \(k\) gives a measure of the stiffness of the CFPG complex and characterizes the collagen-driven high strain response. The structural parameter \(\rho \left( \mathbf{N} \right) \) defines the tissue anisotropy, and the values of \(\lambda _{\min }\) and \(\lambda _{\max }\) characterize the degrees of waviness of the collagen fibrils and the transition between low and high stress response.
The collagen turnover model requires an estimation of the turnover parameter \(\eta \), which defines the time scale for reaching homeostatic conditions; the undulation limits of the newly deposited fibrils \(\lambda _{\min }^\mathrm{NEW}\) and \(\lambda _{\max }^\mathrm{NEW} = \lambda _{\min }^\mathrm{NEW} + \varDelta \lambda ^\mathrm{NEW}\); the homeostatic collagen fibre stretch \(\lambda _\mathrm{ph} = \lambda _{\min }^\mathrm{NEW} +0.224 \varDelta \lambda ^\mathrm{NEW}\); and the maximum collagen production rate \(\dot{\rho }_\mathrm{MAX}\), which was introduced to represent the limited capability of a fibroblast cell to produce collagen.
A constant collagen fibre density \(\rho _0 = 1/4\pi \,\hbox {sr}^{-1}\) was prescribed for the passive model (time-independent) (Martufi and Gasser 2011, 2012a, b). Results of the biaxial tensile test on TAA tissue were used to estimate the passive material parameters \((\mu , k, \lambda _{\min },\,\lambda _{\max })\) and the width \(\varDelta \lambda ^\mathrm{NEW}\) of the triangular PDF that defined the newly formed collagen.
The expansion of the aneurysm between two consecutive computed tomography angiography (CTA) scans was measured, and patient-specific mean arterial pressure was computed from the patient chart. The data analysis revealed a maximum outer diameter of 36.7 mm, MAP of 75 mmHg, and a growth rate of 1.2 mm/year.
The turnover parameter \(\eta \) was set to two months, i.e. the half-life of vascular collagen (Nissen et al. 1978). \(\dot{\rho }_\mathrm{MAX}\) and \(\lambda _{\min }^\mathrm{NEW}\) were estimated from the response of a numerical relaxation test and the expansion of a simplified TAA tube model.
5.3 Biaxial tensile test
The above-outlined constitutive model for vascular tissue was implemented at the integration points of elements using a Q1P0 mixed Finite Element formulation (Simo and Taylor 1991). The material parameters \(\mu , k\) and the structural parameters \(\lambda _{\min },\,\lambda _{\max },\,\varDelta \lambda ^\mathrm{NEW}\) of the passive (time-independent) constitutive model were identified from equibiaxial tensile test data on a surgically removed specimen. The clear physical meaning of the model parameters allowed their straightforward identification by manual adjustments; see Fig. 1 and Table 1. It must be noted that an isotropic material constitutive law was used to fit the equibiaxial anisotropic response of the material. The anisotropy of the tissue is recovered after the initial remodelling step leading to the homeostatic condition (see Sect. 5.5).
Macroscopic constitutive response of the thoracic aortic aneurysm (TAA) wall under equibiaxial tension. Best fit finite element (FE) results (open circles) according to the multiscale constitutive model were compared to patient-specific data (lines). Material and structural parameters are given in Table 1
The structural parameters \(\lambda _{\min }\) and \(\lambda _{\max }\) identified from the experimental result, which define the lower and upper limits of the collagen undulation, were significantly higher in TAA tissue as compared to the values reported from identification performed on AAA tissues (Martufi and Gasser 2011; Gasser 2011; Gasser et al. 2012). Consequently, collagen in TAA appears to be remarkably more undulated. The higher values found for \(\lambda _{\min }\) and \(\lambda _{\max }\) are consistent with the observed high elastin content in the thoracic aorta, which tends to increase the recoil effect of elastin and initiate the recruitment of collagen at a higher macroscopic stretches (Dobrin and Mrkvicka 1994; Raghavan et al. 1996).
Collagen has a main impact on the mechanical properties of arterial tissue at high strain levels, and a direct correlation between collagen content and tissue stiffness and strength has become generally accepted (Roach and Burton 1957). It is noted here that the stiffness in the CFPG complex (determined by parameter \(k\)) was lower in the TAA tissue as compared to the values for AAAs (Martufi and Gasser 2011; Gasser 2011; Gasser et al. 2012), which may point towards the observed lower stiffness of the thoracic aorta due to lower collagen density (Bergel 1961; Langewouters et al. 1984).
5.4 Relaxation test
A uniaxial relaxation test obtained recording the stress at fixed displacement was performed on a single cubic tissue element. Because the spectrum of the collagen deposition stretch was assumed to be constant, the mechanical stimulus was also constant for the relaxation test, where the macroscopic stretch is time-independent.
Without constraining the collagen production rate (i.e. \({\dot{\rho }}_{\max }^+ =\infty \)), collagen would be constantly produced, such that the stress increases continuously until infinity. Consequently, we used the relaxation test to define the limit of the collagen production rate \(\dot{\rho }_{\max }^{+}\). To this end, a strain of 14 per cent (Gasbarro et al. 2007) was applied, and a steady-state target stress of 64 kPa was reached with \(\dot{\rho }_{\max }^{+} = 3.8 \times 10^{-5}\,(\hbox {sr day})^{-1}\). Here, sr stands for steradian and denotes the unit of the solid angle. The target stress was computed as the circumferential stress for a healthy thoracic aorta of 29 mm in diameter (Mao et al. 2008) with a wall thickness of 2.1 mm and loaded with a MAP of 75 mmHg. The undulation parameters \(\lambda _{\min }^\mathrm{NEW} = 0.84\) and \(\lambda _{\max }^\mathrm{NEW} = 1.205\) were used, where Sect. 5.5 motivates this choice.
5.5 Thoracic aortic aneurysm tube model
The simplified tube model was loaded with a quasi-static pressure of 75 mmHg (10 kPa), corresponding to the MAP for the patient, and had an outer diameter of 36.7 mm and wall thickness of 2.1 mm. Symmetry conditions were applied, and 540 Q1P0 mixed hexahedral finite elements were used to represent a quarter of the model. Bending effects from inhomogeneous stress across the wall were captured by three finite elements across the thickness.
The stress field with the passive constitutive description was computed first, and a constant collagen fibre structure \((\rho = 0.0796\,\, \hbox {sr}^{-1},\,\lambda _{\min } = 1.135,\,\lambda _{\max } = 1.5,k = 7.5\, \hbox {MPa},)\) was prescribed and served as the initial structure for collagen turnover. Due to its highly nonlinear character, the passive formulation predicted a strong stress gradient across the vessel wall; see Fig. 3a. Such stress gradients are thought to be non-physiological, and residual stress in the load-free configuration is able to reduce the gradients across the wall thickness. Activating collagen turnover \((\lambda _{\min }^\mathrm{NEW} = 0.84\) and \(\lambda _{\max }^\mathrm{NEW} = 1.205)\) in the model redistributed the stress (Fig. 3b) and homogenized the wall stress distribution across the thickness, suggesting that the homeostatic solution recovers the residual stresses in the load-free state.
Increasing the undulation of collagen fibrils, i.e. decreasing their pre-stretch at deposition, triggered aneurysm growth, where the measured average expansion rate was used for parameter identification. Specifically, the TAA tube model matched the clinical follow-up data for \(\lambda _{\min }^\mathrm{NEW} =0.856\) and \(\lambda _{\max }^\mathrm{NEW} = 1.221\), i.e. the diameter increased by 1.2 mm/year (Fig. 2). While the macroscopic stress did not change considerably (Fig. 3c), depositing newly formed collagen at a higher undulation caused not only growth of the TAA but also failed to reach homeostasis, leading to a weak stress gradient across the wall.
Maximum principal Cauchy stress computed with the thoracic aortic aneurysm (TAA) tube model. a Prediction that neglected collagen turnover (passive model). b Prediction that accounted for collagen turnover and led to homeostasis \((\lambda _{\min }^\mathrm{NEW} = 0.84;\,\lambda _{\max }^\mathrm{NEW} = 1.205)\). c Prediction that accounted for collagen turnover and predicted growth \((\lambda _{\min }^\mathrm{NEW} = 0.856;\,\lambda _{\max }^\mathrm{NEW} = 1.221; \quad \dot{\rho }_{\max }^{+} = 3.8 \times 10^{-5}\,\, (\hbox {sr day})^{-1})\)
5.6 Patient-specific finite element simulation
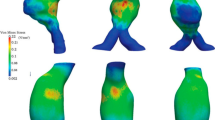
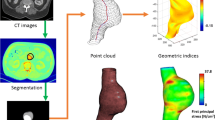
The three-dimensional patient-specific TAA geometry was reconstructed from a stack of CTA images imported into an image-processing package (ScanIP; Simpleware Ltd., Exeter, UK). A consistent smoothing protocol was applied to improve the reconstructed geometry without resorting to CAD tools, which contributed to preserve the natural details of the aneurysms. A mesh generating software (ScanFE;SimplewareLtd.,Exeter,UK) was used to discretize the reconstructed geometry into a preliminary grid of shells for FEM analysis. The mesh quality was then improved with a second mesh-processing package (Hypermesh; Altair Engineering, Inc. Troy, MI, USA), through which the arterial wall was discretized into 10203 hexahedral elements. A wall with a constant thickness of 2.1 mm, consistent with the experimental measures, was considered, and a single finite element across the wall thickness was used. A mean arterial blood pressure of 75 mmHg (10 kPa) was prescribed, and the distal ends of supra-aortic vessels and of descending aorta were fixed in all directions, while all nodal degrees of freedom except rotation were blocked for the nodes in the proximal aortic sinus. The stress field with the passive constitutive description was computed first. In line with the results from other passive TAA wall models (Nathan et al. 2011; Shang et al. 2013), the macroscopic stress was distributed non-uniformly over the wall (Fig. 4a). On top of the passive prediction, collagen turnover was activated \((\lambda _{\min }^\mathrm{NEW} = 0.84\) and \(\lambda _{\max }^\mathrm{NEW} = 1.205)\). In our model, the collagen adapts until the optimal microstructure configuration is reached, and the macroscopic stress converge to the homeostatic solution with an almost uniform stress distribution across the wall (Fig. 4b), suggesting that residual stresses in the reference state are accounted for once the homeostatic solution is generated.
The second step of the simulation was obtained by changing the undulation parameters of the collagen to simulate aneurysm growth (Fig. 4c). The quantitative values of the parameters \((\lambda _{\min }^\mathrm{NEW} =0.856\) and \(\lambda _{\max }^\mathrm{NEW} = 1.221)\) were optimized performing simulation on a simplified tube model as described in the previous paragraph. The physiological meaning of changing these parameters resides in assuming that fibroblasts are capable of laying collagen at a predetermined preferred stretch. It appears clear that since the aorta is dilated and enlarging, the preferred undulation of the collagen would be increasing slightly, as predicted by the simulations on the simplified tube model.
6 Summary and conclusions
Aortic aneurysms are the end result of a multifactorial process that culminates in irreversible pathological remodelling of the aortic wall connective tissue (Fedak et al. 2003; Davies 1998; Tang et al. 2005; Botta and Elefteriades 2006). The overall result is a gradual imbalance between synthesis and degradation of tissue constituents (Koullias et al. 2004a, b) leading to tissue proteolytic degradation (Koullias et al. 2004a, b) and alteration of the ECM. The increased proteolytic activity within the aortic wall is primarily attributed to elevated concentration and activity of MMPs. Although the exact mechanisms are still unknown, there is evidence that this biological activity is localized, such that spots of increased expression and activation of MMPs contribute to fast local aneurysm expansion (Sinha et al. 2006).
Current management of patients with TAA disease involves a multifaceted approach that includes evaluation for underlying genetic causes, screening of family members, modifications of patient’s lifestyle, and imaging surveillance to monitor growth. However, current surveillance data rely only on maximum diameter measurements at two subsequent points in time, which provide only limited information about the local growth of the aneurysm (Martufi et al. 2013). Specifically, complex changes of vessel wall morphology cannot be captured, and regions of fast diameter growth, as a consequence of local increase in biological activity, may be missed (Martufi et al. 2013).
There is an urgent need to explore and investigate the growth pattern of the entire aneurysm to highlight focal areas of growth and non-invasively locate spots of increased enzyme activation, which may be responsible for focal weakening and aneurysm rupture. If successfully derived, this local growth information will allow on one hand a critical evaluation of conventional procedures to measure aneurysm enlargement and, on the other hand, give detailed longitudinal data to validate biomechanical models that describe aneurysm growth (Martufi and Gasser 2012a, b).
Within this communication, we have provided one example of the use of a structurally inspired model to study growth of thoracic aneurysm. In our case study, the maximum diameter of the aneurysm, as measured from CT at two follow-ups, appeared to be growing at a rate of 1.2 mm/year, a typical growth rate for thoracic aneurysms (Davies et al. 2002). We applied a collagen-based growth framework that has been previously successfully adopted for abdominal aneurysms (Martufi and Gasser 2012a, b). The simulations performed included an initial simulation that remodels and reorients the CFPG complexes and serves as the basis for subsequent simulations of growth in the medium term (in this case 1 year). One of the clear advantages of using this active framework of tissue remodelling is the ability to take into account the presence of residual stresses in the load-free configuration. The use of passive constitutive models requires the prescription of residual stress in the load-free configuration in order to predict physiological stress states. However, for complex geometries like TAAs, the residual strain state is unknown and hypothetical assumptions are relied on (Polzer et al. 2013). In contrast, with constitutive descriptions that account for the vascular wall’s ability to adapt to the mechanical loading state (such as the one described herein), the development of the residual stress field is a natural consequence of the method. It must be noted that the residual stress is due to elastin and SMCs, in addition to collagen fibres (Alford et al. 2008). Consequently, improvements in the framework are needed to include the effects of elastin pre-stretch, as well as SMCs activation.
Comparing the three stress maps of Fig. 3, one appreciates the role of the collagen–proteoglycan complexes in sustaining and redistributing the load in an aneurysm. Lower stresses and a homogeneous stress across the wall thickness are obtained going from baseline to the homeostatic state of the aneurysm (Fig. 3b). From there, the model predicts growth at the maximum diameter level of 1.2 mm/year, in accordance with what was observed clinically.
There are several limiting assumptions in this study. First, remodelling is due solely to collagen–proteoglycan bundles that grow and change their orientation as a function of the local mechanics. The isotropic matrix in the model accounts for elastin and other ECM tissue constituents. No degradation of the elastic matrix is modelled; rather the amount of elastin as estimated at baseline (likely lower than what present in a healthy wall) is assumed to remain constant for the duration of the growth simulation. Rationale for this assumption is that elastin degradation is more involved in the initial stages of aneurysm formation than at later stages. Nonetheless, a modification of the model that accounts for further elastin degradation would be beneficial, in particular when long-term predictions are sought after. Second, smooth muscle cells are not directly modelled; as a consequence, no active contraction is considered for the tissue. This assumption is very reasonable for the AAA case where almost no SMCs are present in a physiological functional state in the later stages of the pathology (Lopez-Candales et al. 1997). The same has not been demonstrated to be true for thoracic aneurysm (Tang et al. 2005), and therefore, this limitation needs to be addressed. Third, the thoracic tissue demonstrated anisotropy in its material characteristics. For simplicity, we fitted the equibiaxial stress-strain curves with an isotropic constitutive law at baseline. The anisotropy in the tissue is recovered after the first step of the simulation due to collagen fibres remodelling. It should be further evaluated whether other tissue components contribute to anisotropy at baseline, and if this is the case, anisotropic constitutive laws at baseline should be derived.
Finally, while deposition and reorientation of collagen fibres in our model depend on the local state of deformation and stress, an intermediate step (the simplified tube model) was necessary in order to calibrate the material parameters to the observed global growth patterns. We are currently evaluating non-invasive indices that could be used to modulate material parameters locally, without the need for a prior calibration.
Quantitative measurements of regional deformations or local strain could serve as an index to infer the local state of the wall and could be used as a gauge to change the structural parameters in our growth framework. Deformation (strain) and strain rate measurements are already extensively used to evaluate ventricular ischaemia (Leung and Ng 2010), heart failure (Mak et al. 2012), and cardiac hypertrophy (Shih et al. 2011). Previous studies have showed significant changes in the material properties as well as in the collagen content and orientation in aneurysmal tissue. In particular, the deformability of an aneurysm appears to be related to its strength (Di Martino et al. 2006); therefore, a local increase in deformation could help identifying areas where there is elevated activity of tissue constituents turnover so that changes in material parameters could come from non-invasive measures that are clinically feasible, instead of relying on prior calibration.
Although nonlinear continuum mechanics is directly applicable to biomechanical problems, developing computational models able to adequately address clinical cases remains a challenging task. Indeed, most models have employed classical phenomenological approaches (passive models) and have used advances in medical imaging only to define patient-specific geometries. Models that account for the vascular wall’s ability to adapt to the mechanical loading state (growth & remodelling models) are more suited to give a comprehensive and clinically relevant view of aneurysms pathophysiology and could further increase the accuracy of risk indices for rupture and complications.
This review focused on the structural aspects of aneurysm mechanics. Abnormal flow patterns and haemodynamic stresses on the diseased aortic wall are thought to play an important role in the development of TAAs; this notwithstanding, we chose to focus on the structural stresses, due to their involvement in the mechanical failure (rupture) of aneurysms. In conclusion, TAA disease is characterized by a strong regional heterogeneity within the thoracic segments in terms of biomechanical properties, atherosclerotic distribution, proteolytic activity, and cell signalling pathways. Therefore, maximum diameter measurements are ill suited to provide a reliable risk measure for rupture and complications because they overlook the highly heterogeneous nature of the aortic vessel.
The integration of biomechanical patient-specific variables, including local wall deformability, local stresses, and mechanical properties patterns, is expected to be a paradigm change in the management of these complex patients to improve diagnosis and tailor individual’s treatments.
References
Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, Zdanowski Z (2006) Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg 44:237–43
Albornoz G, Coady MA, Roberts M, Davies RR, Rizzo J, Elefteriades JA (2006) Familial thoracic aortic aneurysms and dissections—incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg 82(4):1400–1405
Alford PW, Humphrey JD, Taber LA (2008) Growth and remodeling in a thick-walled artery model: effects of spatial variations in wall constituents. Biomech Model Mechanobiol 7(4):245–262
Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB (1990) Elastase-induced experimental aneurysms in rats. Circulation 82(3):973–981
Bäck M, Gasser TC, Michel J-B, Caligiuri G (2013) Spotlight review: biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc Res. doi:10.1093/cvr/cvt040
Beller CJ, Labrosse MR, Thubrikar MJ, Robicsek F (2004) Role of aortic root motion in the pathogenesis of aortic dissection. Circulation 109(6):763–769
Bellini C, Di Martino ES, Federico S (2012) Mechanical behaviour of the human atria. Ann Biomed Eng (online)
Bergel DH (1961) The static elastic properties of the arterial wall. J Physiol 156:445–457
Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ, Cherry KJ, Joyce JW, Lie JT (1982) Thoracic aortic aneurysms: a population-based study. Surgery 92:1103–1108
Borghi A, Wood NB, Mohiaddin RH, Xu XY (2008) Fluid-solid interaction simulation of flow and stress pattern in thoracoabdominal aneurysms: a patient-specific study. J Fluids Struct 24(2):270–280
Botta D, Elefteriades JA (2006) Matrix metalloproteinases in thoracic aortic aneurysm disease. Int J Angiol 15:1–8
Braverman A, Thompson R, Sanchez L (2011) Diseases of the aorta. In: Bonow R, Mann D, Zipes D, Libby P (eds) Braunwald’s heart disease, 9th edn. Elsevier, Philadelphia, pp 1309–1337
Carey D (1991) Control of growth and differentiation of vascular cells by extracellular matrix proteins. Ann Rev Physiol 53:161–177
Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton J 3rd (1998) Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 280(22):1926–1929
Clouse WD, Hallett JW Jr, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ 3rd (2004) Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 79:176–80
Coady MA, Davies RR, Roberts M, Goldstein LJ, Rogalski MJ, Rizzo JA, Hammond GL, Kopf GS, Elefteriades JA (1999) Familial patterns of thoracic aortic aneurysms. Arch Surg 134:361–367
Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, Elefteriades JA (1997) What is the appropriate size criterion for resection of thoracic aortic aneurysm? J Thorac Cardiovasc Surg 113:476–491
Dapunt OE, Galla JD, Sadeghi AM, Lansman SL, Mezrow CK, de Asla RA, Quintana C, Wallenstein S, Ergin AM, Griepp RB (1994) The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 107:1323–1332
Davies MJ (1998) Aortic aneurysm formation: lessons from human studies and experimental models. Circulation 98:193–195
Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA (2002) Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 73:17–28
de Sa M, Moshkovitz Y, Butany J, David TE (1999) Histologic abnormalities of the ascending aorta and pulmonary trunk in patients with bicuspid aortic valve disease: clinical relevance to the Ross procedure. J Thorac Cardiovasc Surg 118:588–596
Delfino A, Stergiopulos N, Moore JE Jr, Meister JJ (1997) Residual strain effects on the stress field in a thick wall finite element model of the human carotid bifurcation. J Biomech 30(8):777–786
Di Martino ES, Bohra A, Vande Geest JP, Gupta NY, Makaroun MS, Vorp DA (2006) Biomechanical properties of ruptured versus non-ruptured Abdominal Aortic Aneurysm wall tissue. J Vasc Surg 43(3):570–576
Dobrin PB, Baker WH, Gley WC (1984) Elastolytic and collagenolytic studies of arteries. Implications for the mechanical properties of aneurysms. Arch Surg 119:405–409
Dobrin PB, Mrkvicka R (1994) Failure of elastin and collagen as possible critical connective tissue alterations underlying aneurysmal dilation. Cardiovasc Surg 2:484–488
Elefteriades JA, Barrett PW, Kopf GS (2007) Litigation in non-traumatic diseases? A tempest in the malpractice maelstrom. Cardiology 109:263–72
Elefteriades JA, Farkas EA (2010) Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 55:841–857
Elefteriades JA (2008) Thoracic aortic aneurysm: reading the enemy’s playbook. Yale J Biol Med 81:175–186
Elefteriades JA, Rizzo JA (2008) Epidemiology, prevalence, incidence, trends. In: Elefteriades JA (ed) Acute aortic disease. Informa Healthcare, New York, pp 89–98
Fedak PW, de Sa MP, Verma S, Nili N, Kazemian P, Butany J, Strauss BH, Weisel RD, David TE (2003) Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg 126(3):797–806
Ferruzzi J, Vorp DA, Humphrey JD (2011) On constitutive descriptors of the biaxial mechanical behaviour of human abdominal aorta and aneurysms. J R Soc Interface 8:435–450
Fillinger MF, Marra SP, Raghavan ML, Kennedy FE (2003) Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg 37:724–732
Fung YC, Fronek K, Patitucci P (1979) Pseudoelasticity of arteries and the choice of its mathematical expression. Am J Physiol Hearth C 237:H620–H621
Gasbarro MD, Shimada K, Di Martino ES (2007) Mechanics of abdominal aortic aneurysm. Eur J Comput Mech 16:337–363
Gasser TC, Auer M, Labruto F, Swedenborg J, Roy J (2010) Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur J Vasc Endovasc 40:176–185
Gasser TC, Gallinetti S, Xing X, Forsell C, Swedenborg J, Roy J (2012) Spatial orientation of collagen fibers in the abdominal aortic aneurysms wall and its relation to wall mechanics. Acta Biomater 8(8):3091–3103
Gasser TC, Ogden RW, Holzapfel GA (2006) Hyperelastic modelling of arterial layers with distributed collagen fiber orientations. J Roy Soc Interface 3:15–35
Gasser TC (2011) An irreversible constitutive model for fibrous soft biological tissue: a 3d microfiber approach with demonstrative application to abdominal aortic aneurysms. Acta Biomater 7(6):2457– 2466
Griepp RB, Ergin MA, Galla JD, Lansman SL, McCullough JN, Nguyen KN, Klein JJ, David Spielvogel D (1999) Natural history of descending thoracic and thoracoabdominal aneurysms. Ann Thorac Surg 67(6):1927–1930
Hackman AE, LeMaire SA, Thompson RW (2008) Long term suppressive therapy: clinical reality and future prospects. In: Elefteriades JA (ed) Acute aortic disease. Informa Healthcare, New York, pp 309–330
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elasticity 61:1–48
Holzapfel GA, Gasser TC, Stadler M (2002) A structural model for the viscoelastic behavior of arterial walls: continuum formulation and finite element analysis. Eur J Mech A Solids 21(3):441–463
Humphrey JD (1995) Mechanics of the arterial wall: review and directions. Crit Rev Biomed Eng 23(1–2):1–162
Humphrey JD (2002) Cardiovascular solid mechanics: cells, tissues, and organs. Springer, New York
Humphrey JD, Holzapfel GA (2012) Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech 45:805–814
Humphrey JD (1999) Remodelling of a collagenous tissue at fixed lengths. J Biomech Eng 121:591–597
Huntington K, Hunter AG, Chan KL (1997) A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol 30:1809–1812
Isselbacher EM (2005) Thoracic and abdominal aortic aneurysms. Circulation 111(6):816–28
Jeremy RW, Huang H, Hwa J, McCarron H, Hughes CF, Richards JG (1994) Relation between age, arterial distensibility, and aortic dilatation in the Marfan syndrome. Am J Cardiol 74:369–373
Johansson G, Markström U, Swedenborg J (1995) Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg 21:985–988
Khanafer K, Berguer R (2009) Fluid-structure interaction analysis of turbulent pulsatile flow within a layered aortic wall as related to aortic dissection. J Biomech 42(16):2642–2648
Koullias GJ, Korkolis DP, Ravichandran P, Psyrri A, Hatzaras I, Elefteriades JA (2004a) Tissue microarray detection of matrix metalloproteinases, in diseased tricuspid and bicuspid aortic valves with or without pathology of the ascending aorta. Eur J Cardiothor Surg 26(6):1098–1103
Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA (2004b) Increased tissue microarray MMP expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg 78(6):2106–2110
Langewouters GJ, Wesseling KH, Goedhard WJA (1984) The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech 17:425– 435
Lanir Y (1983) Constitutive equations for fibrous connective tissues. J Biomech 16(1):1–12
Larsson E, Vishnevskaya L, Kalin B, Granath F, Swedenborg J, Hultgren R (2011) High frequency of thoracic aneurysms in patients with abdominal aortic aneurysms. Ann Surg 253(1):180–184
Lehoux S, Tedgui A (1998) Signal transduction of mechanical stresses in the vascular wall. Hypertension 32(2):338–345
Leung DY, Ng AC (2010) Emerging clinical role of strain imaging in echocardiography. Heart Lung Circ 19(3):161–174
Lobato AC, Puech-Leao P (1998) Predictive factors for rupture of thoracoabdominal aortic aneurysm. J Vasc Surg 27:446–453
Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW (1997) Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol 150:993–1007
Mak S, Van Spall HG, Wainstein RV, Sasson Z (2012) Strain, strain rate and the force frequency relationship in patients with and without heart failure. J Am Soc Echocardiogr 25(3):341–348
Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, Flores FR, Gao YL, Budoff MJ (2008) Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol 15(7):827–34
Martufi G, Auer M, Roy J, Swedenborg J, Sakalihasan N, Panuccio G, Gasser TC (2013) Multidimensional growth measurements of abdominal aortic aneurysms. J Vasc Surg 58(3):748–755
Martufi G, Gasser TC (2011) A constitutive model for vascular tissue that integrates fibril, fiber and continuum levels with application to the isotropic and passive properties of the infrarenal aorta. J Biomech 44:2544–2550
Martufi G, Gasser TC (2012a) Histo-mechanical modeling of the wall of abdominal aorta aneurysms. In: Preprints MATHMOD 2012 Vienna—full paper volume, Inge Troch, Felix Breitenecker. ARGESIM Report No. S38
Martufi G, Gasser TC (2012b) Turnover of fibrillar collagen in soft biological tissue with application to the expansion of abdominal aortic aneurysms. J R Soc Interface 9(77):3366–3377
Martufi G, Gasser TC (2013) Review: the role of biomechanical modeling in the rupture risk assessment for abdominal aortic aneurysms. J Biomech Eng 135(2):021010
Nathan DP, Xu C, Gorman JH III, Fairman RM, Bavaria JE, Gorman RC, Chandran KB, Jackson BM (2011) Pathogenesis of acute aortic dissection: a finite element stress analysis. Ann Thorac Surg 91(2):458–463
Nchimi A, Cheramy-Bien JP, Gasser TC, Namur G, Gomez P, Seidel L, Albert A, Defraigne JO, Labropoulos N, Sakalihasan N (2013) Multifactorial relationship between 18f-fluoro-deoxy-glucose positron emission tomography signaling and biomechanical properties in unruptured aortic aneurysms. Circ Cardiovasc Imaging (on line)
Nissen R, Cardinale GJ, Udenfriend S (1978) Increased turnover of arterial collagen in hypertensive rats. Proc Natl Acad Sci USA 75:451–453
Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G (1999) Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 82:19–22
Nkomo VT, Enriquez-Sarano M, Ammash NM, Melton LJ 3rd, Bailey KR, Desjardins V, Horn RA, Tajik AJ (2003) Bicuspid aortic valve associated with aortic dilatation; a community-based study. Arterioscler Thromb Vasc Biol 23:351–356
Okamoto RJ, Wagenseil JE, DeLong WR, Peterson SJ, Kouchoukos NT, Sundt TM III (2002) Mechanical properties of dilated human ascending aorta. Ann Biomed Eng 30:624–635
Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F (2006) Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14 000 cases from 1987 to 2002. Circulation 114:2611–2618
Pachulski RT, Weinberg AL, Chan KL (1991) Aortic aneurysm in patients with functionally normal or minimally stenotic bicuspid aortic valve. Am J Cardiol 67(8):781–782
Pape LA, Tsai TT, Isselbacher EM, Oh JK, O’Gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S, Bossone E, Suzuki T, Cooper JV, Froehlich JB, Nienaber CA, Eagle KA (2007) Aortic diameter >or=5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 116:1120–1127
Polzer S, Bursa J, Gasser TC, Staffa R, Vlachovsky R (2013) A numerical implementation to predict residual strains from the homogeneous stress hypothesis with application to abdominal aortic aneurysms. Ann Biomed Eng 41(7):1516–1527
Raghavan ML, Vorp DA (2000) Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J Biomech 33:475–482
Raghavan ML, Webster MW, Vorp DA (1996) Ex vivo biomechanical behavior of abdominal aortic aneurysm: assessment using a new mathematical model. Ann Biomed Eng 24:573–582
Roach MR, Burton AC (1957) The reason for the shape of the distensibility curves of arteries. Can J Physiol Pharmacol 35:681–690
Rodriguez JF, Martufi G, Doblare’ M, Finol EA (2009) The effect of material model formulation in the stress analysis of abdominal aortic aneurysms. Ann Biomed Eng 37(11):2218–2221
Rodriguez JF, Ruiz C, Doblare’ M, Holzapfel GA (2008) Mechanical stresses in abdominal aortic aneurysms: influence of diameter, asymmetry, and material anisotropy. J Biomech Eng 130:021023
Sakalihasan N, Kuivaniemi H, Nusgens B, Durieux R, Defraigne JO (2011) Aneurysm: epidemiology aetiology and pathophysiology. In: McGloughlin T (ed) Biomechanics and mechanobiology of aneurysms (Studies in Mechanobiology, Tissue Engineering and Biomaterials), vol 7. Springer, Berlin, pp 1–33
Schmid FX, Bielenberg K, Schneider A, Haussler A, Keyser A, Birnbaum D (2003) Ascending aortic aneurysm associated with bicuspid and tricuspid aortic valve: involvement and clinical relevance of smooth muscle cell apoptosis and expression of cell death-initiating proteins. Eur J Cardiothoracic Surg 23:537–543
Shang EK, Nathan DP, Sprinkle SR, Vigmostad SC, Fairman RM, Bavaria JE, Gorman RC, Gorman JH III, Chandran KB, Jackson BM (2013) Peak wall stress predicts expansion rate in descending thoracic aortic aneurysms. Ann Thorac Surg 95:593–8
Shih J-Y, Tsai W-C, Huang Y-Y, Liu Y-W, Lin C-C, Huang Y-S, Tsai L-M, Lin L-J (2011) Assocation of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr 24(5):513–519
Simo JC, Taylor RL (1991) Quasi-incompressible finite elasticity in principal stretches. Continuum basis and numerical algorithms. Comput Methods Appl Mech Eng 85:273–310
Sinha I, Bethi S, Cronin P, Williams DM, Roelofs K, Ailawadi G, Henke PK, Eagleton MJ, Deeb GM, Patel HJ, Berguer R, Stanley JC, Upchurch GR Jr (2006) A biologic basis for asymmetric growth in descending thoracic aortic aneurysms: a role for matrix metalloproteinase 9 and 2. J Vasc Surg 43(2):342–348
Sokolis DP, Kritharis EP, Giagini AT, Lampropoulos KM, Papadodima SA, Iliopoulos DC (2012) Biomechanical response of ascending thoracic aortic aneurysms: association with structural remodeling. Comput Method Biomech 15(3):231–248
Takamizawa K, Hayashi K (1987) Strain energy density function and uniform strain hypothesis for arterial mechanics. J Biomech 20(1):7–17
Tang PCY, Coady MA, Lovoulos C, Dardik A, Aslan M, Elefteriades JA, Tellides G (2005) Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation 112:1098–1105
Thubrikar MJ, Agali P, Robicsek F (1999) Wall stress as a possible mechanism for the development of transverse intimal tears in aortic dissections. J Med Eng Technol 23(4):127–134
Tse KM, Chiu P, Lee HP, Ho P (2011) Investigation of hemodynamics in the development of dissecting aneurysm within patient-specific dissecting aneurismal aortas using computational fluid dynamics (CFD) simulations. J Biomech 44(5):827–836
Vaishnav RN, Young JT, Janicki JS, Patel JS (1972) Nonlinear anisotropic elastic properties of the canine aorta. Biophys J 12(8):1008–1027
Vande Geest JP, Di Martino ES, Bohra A, Makaroun MS, Vorp DA (2006) A biomechanics-based rupture potential index for abdominal aortic aneurysm risk assessment: demonstrative application. Ann NY Acad Sci 1085:11–21
Venkatasubramaniam AK, Fagan MJ, Mehta T, Mylankal KJ, Ray B, Kuhan G, Chetter IC, McCollum PT (2004) A comparative study of aortic wall stress using finite element analysis for ruptured and non-ruptured abdominal aortic aneurysms. Eur J Vasc Surg 28:168–176
Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP (2003) Effect of aneurysm on the tensile strength and biomechanical behaviour of the ascending thoracic aorta. Ann Thorac Surg 75:1210–1214
Wess TJ (2008) Collagen fibrillar structure and hierarchies. In: Fratzl P (ed) Collagen structure and mechanics. Springer, New York, pp 49–80
Wuyts FL, Vanhuyse VJ, Langewouters GJ, Decraemer WF, Raman ER, Buyle S (1995) Elastic properties of human aortas in relation to age and atherosclerosis: a structural model. Phys Med Biol 40:1577–1597
Xu C, Lee S, Singh TM, Sho E, Li X, Sho M, Masuda H, Zarins CK (2001) Molecular mechanisms of aortic wall remodeling in response to hypertension. J Vasc Surg 33(3):570–578
Xu XY, Borghi A, Nchimi A, Leung J, Gomez P, Cheng Z, Defraigne JO, Sakalihasan N (2010) High levels of 18f-fdg uptake in aortic aneurysm as wall are associated with high wall stress. Eur J Vasc Endovasc 39:295–301
Yasuda H, Nakatani S, Stugaard M, Tsujita-Kuroda Y, Bando K, Kobayashi J, Yamagishi M, Kitakaze M, Kitamura S, Miyatake K (2003) Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparison with tricuspid aortic valve. Circulation 108(Suppl 1):II291–II294
Zulliger MA, Fridez P, Hayashi K, Stergiopulos N (2004) A strain energy function for arteries accounting for wall composition and structure. J Biomech 37(7):989–1000
Acknowledgments
The authors gratefully acknowledge Mr. Raied Aburashed and Mr. Michael Layzell for the three-dimensional reconstruction of the aortic geometry. The work was supported in part by the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martufi, G., Gasser, T.C., Appoo, J.J. et al. Mechano-biology in the thoracic aortic aneurysm: a review and case study. Biomech Model Mechanobiol 13, 917–928 (2014). https://doi.org/10.1007/s10237-014-0557-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-014-0557-9