Abstract
Sewage sludge residue (biosolids) was investigated for its potential as a long-term tailings cover. Biosolids may prevent oxygen diffusion into underlying sulfide tailings through microbial aerobic biodegradation of organic matter. Biosolids were investigated at laboratory-, pilot-, and field-scale using analysis of total organic matter (TOM) mass reduction and O2, CO2, CH4 concentrations to quantify the biodegradation rate. A 156-day, open microcosm experiment, in which the loss of biosolids mass over time at differing temperatures, mimicking ambient (20–22 °C), mesophilic (34 °C), and thermophilic (50 °C) conditions, indicated that TOM biodegradation was best in the mesophilic temperature range, with 14.8, 27.2, and 26.7 % mass depletion at ambient, mesophilic, and thermophilic conditions, respectively. The data was correlated to field-scale data that evaluated biodegradation rates via decreasing O2 and increasing CO2 concentrations. Field biodegradation rates were less than laboratory rates because lower mean annual temperatures (0.6–0.7 °C) diminished microbial activity. A calibrated model indicates that 20 % of a field application of biosolids will degrade within 2 years. However, the rate declines with time due to exhaustion of the most readily degradable organic fraction. If biodegradation cannot be maintained, the long-term effectiveness of biosolids as a covering material for mine tailings remains a concern.
Zusammenfassung
Klärschlämme wurden hinsichtlich ihrer Eignung als langfristige Haldenabdeckung untersucht. Durch mikrobiellen aeroben Abbau der organischen Substanz können sie die Sauerstoffdiffusion in das darunterliegende sulfidhaltige Haldenmaterial verhindern. Es wurden Klärschlämme im Labor-, Pilot-, und Feldmaßstab untersucht. Zur Bestimmung der Abbaurate wurden die Reduktion der gesamten organischen Substanz sowie die Konzentration von O2, CO2 und CH4 analysiert. Ein 156-Tage Mikrokosmos-Experiment wurde unter verschiedenen Temperaturbedingungen: Raumtemperatur (20-22 °C), mesophilen (34 °C) und thermophilen (50 °C) Bedingungen durchgeführt. Der Abbau der organischen Substanz war unter mesophilen Bedingungen am höchsten. Der Abbau der organischen Substanz unter Raumtemperatur, mesophilen und thermophilen Bedingungen betrug entsprechend 14,8 %, 27,2 % bzw. 26,7 %. Die Laborbefunde wurden mit den Ergebnissen des Feldversuchs verglichen, in denen die Abbauraten aus dem O2-Verbrauch und dem CO2-Anstieg ermittelt wurden. Durch die niedrige mittlere Jahrestemperatur von 0,6 bis 0,7 °C war die mikrobielle Aktivität im Feldversuch geringer, so dass auch die Abbauraten geringer als im Labor waren. Eine Modellierung zeigte, dass 20 % der Klärschlammabdeckung im Feld innerhalb von zwei Jahren abgebaut wird. Durch Zehrung der leicht abbaubaren organischen Substanz verringert sich die Abbaurate mit der Zeit. Wenn die biologische Abbaubarkeit nicht erhalten wird, bleibt die Langzeitwirkung der Klärschlämme als Abdeckmaterial für Bergbauhalden problematisch.
Resumen
Los residuos de lodos de aguas residuales (biosólidos) se estudiaron por su posible potencial para ser usados como cobertores de colas. Los biosólidos pueden prevenir la difusión de oxígeno dentro de las colas sulfuradas a través de su uso en la biodegradación aeróbica de la materia orgánica. Los biosólidos fueron estudiados a nivel de laboratorio, a escala piloto y a escala de campo, usando análisis de la reducción de masa del material orgánico total (TOM) y las concentraciones de O2, CO2 y CH4 para cuantificar la velocidad de biodegradación. En un experimento en microcosmos abierto realizado durante 156 días, en el cual se analizó la pérdida de biosólidos en función del tiempo a diferentes temperaturas, condiciones ambiente (20-22 °C), mesofílicas (34 °C) y termofílicas (50 °C), indicaron que la biodegradación de TOM fue mejor en el rango de temperatura mesofílica con una disminución de masa de 14,8, 27,2 y 26.7 % respectivamente para las tres condiciones. Los datos fueron correlacionados con datos a escala de campo que evaluaron las velocidades de biodegradación a través del decrecimiento de la concentración de O2 y el crecimiento de la concentración de CO2. Las velocidades de biodegradación en campo fueron menores que las velocidades de laboratorio debido a que las menores temperaturas promedios anuales (0,6-0,7 °C) disminuyeron la actividad microbiana. Un modelo calibrado indicó que el 20 % de una aplicación a campo de biosólidos sería degradada dentro de los dos años. No obstante, la velocidad declina con el tiempo debido al agotamiento de la fracción orgánica más fácilmente degradable. Si la biodegradación no puede ser mantenida, la efectividad de los biosólidos como material de cobertura para colas de mina seguirá siendo una preocupación.
摘要
研究了生物固体(污泥残渣)用作尾矿长期盖层材料的潜力。生物固体能够通过有机物微生物有氧降解作用阻止氧气向下扩散进入硫化物尾矿。采用小型实验、中型实验和现场实验方法研究了生物固体总有机质(TOM)质量的衰减以及生物固体内O2、CO2和CH4的浓度变化,从而实现生物固体生物降解速率量化研究。户外小型实验时间165天,控制实验温度为环境温度(20−22°C)、嗜温温度(34°C)和嗜热温度(50°C);实验结果显示,生物固体质量在环境温度、嗜温温度和嗜热温度条件下随时间减少幅度分别为14.8 %、27.2 %和 26.7 %,嗜温温度是总有机质生物降解的最佳温度条件。该结果与通过减少O2含量、增大CO2浓度控制生物降解速率的现场实验结果一致。现场实验生物降解速率比小型实验生物降解速率低,这是由于现场实验年平均气温略低(0.6−0.7°C)减弱微生物活性造成的。校正模型预测结果显示,生物固体将在实际铺设两年内损失20 %,生物降解速率将随最易降解有机质耗尽而降低。如果不能继续维持生物固体生物降解作用,生物固体作为长期尾矿盖层材料的作用令人担忧。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tailings are the waste residue material generated via the processing of metal sulfide ores. They may contain abundant concentrations of gangue sulfide-bearing minerals such as pyrite and pyrrhotite. If left uncovered, tailings may oxidize, causing acid rock drainage (ARD) (Lottermoser 2010). The dissolution of pyrite can produce a highly acidic, sulfate- and metal-rich solution (Gerhardt et al. 2004; Gray 1997; INAP 2009). In Sweden, 59 Mt of mined waste rock and tailings originates from the mining industry annually, of which sulfide tailings are the most common type of waste (Avfall Sverige 2011; Statistics Sweden 2006). Tailings remediation and ARD mitigation is thus crucial and an array of methods are used to deal with the waste (Egiebor and Oni 2007; Johnson and Hallberg 2005). Diverse preventative remediation and treatment techniques have been developed to reduce the interaction of atmospheric oxygen and water with the underlying tailings. Current technologies are focused on engineered dry covers, underwater storage, and the use of organic reactive barriers (Höglund and Herberg 2004; Nehdi and Tariq 2007).
Engineered composite dry covers are stable and unreactive materials; inert glacial till and clay-rich till are conventionally used as protective- and sealing-layer materials, respectively, in Sweden. However, large volumes are required to treat the extensive areas of tailing impoundments, and the excavation of these materials also causes environmental impacts. Large volumes of industrial by-products and residues are generated annually (Demirbas 2011; Kan 2009), and so replacing natural materials with industrial waste residues could solve two environmental issues (Pérez-López et al. 2011). In Sweden, a total of 98 Mt of industrial waste residues were collected in 2008, of which 76 % was landfilled (Swedish Waste Management 2011). Waste materials from the paper industry and digested sewage sludge biosolids from biogas plants have been evaluated as alternative cover materials for landfills and mine waste (Höglund and Herberg 2004; Mäkitalo 2012; Strasser et al. 1995). These materials have been tested as sealing layers for sulfide tailings to prevent oxidation and atmospheric weathering (Hallberg et al. 2005; Peppas et al. 2000) and as vegetation substrate materials over tailings (Neuschütz and Greger 2010; Tordoff et al. 2000).
The use of organic reactive barriers (ORB) to mitigate oxygen penetration into an underlying tailings deposit is a novel technique employed in engineered dry cover remedial techniques. While clay and till covers attempt to create a physical barrier to prevent oxygen entry, aerobic biodegradation attempts to create a microbial community that consumes the oxygen before it reaches tailings. However, if the microbial community becomes anaerobic, the aerobic biodegradation method will not work. Additionally, if the integrity of the material is modified, then the capability of the cover to function over time, both physically and chemically, decreases.
Biosolids contain a large fraction of total organic matter (TOM), which is susceptible to aerobic (Eqs. 1–2) and anaerobic biodegradation (Peppas et al. 2000). TOM decomposition rates are normally highest in the first few years after application. Fifty per cent of the organic fraction of a surface biosolids application applied onto sulfide tailings in Sweden was depleted after 5 years by aerobic degradation (Forsberg and Ledin 2006), whereas only 5–10 % was removed from a buried sub-surface layer after 16 years, which was specifically attributed to anaerobic degradation processes (Ahlberg 2006).
Though aerobic degradation in a biosolids layer can mitigate oxygen diffusion into underlying tailings, the integrity of the material and the capability of the cover to function, both physically and chemically, decreases over time. Increased degradation may prevent biosolids from being a useful long-term sulfide tailings remediation solution. The process of aerobic digestion is generally focused on the degradation of starch fractions as a dominant component of organic matter (>60 % TS, Jia et al. 2014). However, it should be noted that sewage sludge biosolids have a complex composition of organic matter, that includes humic and fluvic substances, and other more recalcitrant organic matter like cellulose, lignin, tars (Jia et al. 2014); therefore, actual degradation will vary from the starch degradation equation. The degradation of basic carbohydrates proceeds as follows (Wang et al. 2008):
If microbial growth is considered, the equation can be expressed as follows:
where a, b, c, d, e, and f represent mole numbers (Maier et al. 2000).
Optimal aerobic digestion of sewage sludge biosolids in a wastewater treatment plant is normally performed under mesophilic- (30–37 °C) and thermophilic (45–60 °C) conditions (Bruce and Fisher 1984). Under ambient temperatures, aerobic digestion is relatively slower, yet is faster than anaerobic digestion. The process requires abundant oxygen as a reactant, and generates CO2 as an end product. In a parallel study, the authors investigated anaerobic biodegradation of the biosolids in laboratory tests, and found that ca. 27.8 % of the TOM in the biosolids was degraded after 230 days (Jia et al. 2014).
The objectives of the present study were: (1) to find out the degradability of TOM in biosolids under aerobic conditions, as indicated by TOM mass depletion and gas concentrations, and; (2) to evaluate the effect of different temperature ranges on the biodegradation kinetics of the biosolids and the extent of total biodegradation. Although temperature is not the single controlling parameter of biodegradation rates (moisture content, water saturation, and microbial communities and abundance also affect rates), temperature is a major controlling parameter that can easily be quantitatively evaluated. The overall aim of this paper was to use the obtained biodegradation rate to predict how long the material would mitigate oxygen transport to underlying sulfide tailings. This will facilitate the applicability of ORB’s for use in full-scale engineered dry cover designs in sulfide mine tailings remediation.
Materials and Methods
Laboratory-scale open-microcosm, pilot-scale, and field-scale experiments were conducted to quantify how different temperature ranges would affect microbial-mediated biodegradation and to assess potential scale-up effects. The open microcosms were initiated after an initial closed-microcosm experiment, which attempted to evaluate aerobic biodegradation rates by molar measurement of released gas potential (Eqs. 1–2), failed to maintain aerobic processes; anaerobic reactions were detected after 60 days, as indicated by the release of CH4 (Supplemental Fig. 1; supplemental files accompany the on-line version of the paper, and can be downloaded for free by all subscribers and IMWA members). The open microcosms were set up using a similar design (Fig. 1), but aerobic biodegradation rates were evaluated by directly measuring TOM mass reductions. The results were used to quantify biodegradation rates in the field- and pilot-scale tests using mass balance equations. Table 1 displays a list of the experiments conducted, temperature ranges, and key results.
Field and Pilot-Scale Experiments
Kristineberg Mine Field-Scale Biosolids
Field trials were conducted at a remediated sulfide tailings facility, at the Kristineberg Mine, in northern Sweden. The impoundment consisted of a pre-existing engineered dry cover that had been applied 13 years prior to this study. It comprised a 1.5 m deep glacial till protective layer, underlain by a 0.3 m low permeability till sealing layer. Due to a lack of vegetation on the impoundment, 12 Kt of biosolids were applied to a depth of 0.2 m using a rotating spreader. The biosolids came from a wastewater treatment plant in Stockholm. The biosolids had been anaerobically digested and had been stored outdoors at the mine site for 9 months prior to application. It was seeded and rapid grass establishment occurred within 2 years. Biosolids samples were collected on two occasions for their TOM (LOI) content: 3 spot samples after application in July 2009, and 2 spot samples in October 2011. The differences in the sample collection dates reflects the difference in the age of the biosolids in order for a TOM mass balance to be performed. The samples were placed into an oxygen-free argon-filled container immediately after retrieval, frozen, and then analysed within 24 h.
Boliden Mine Field-Scale Biosolids
Field trials were also conducted at an uncovered sulfide tailings impoundment at the Boliden Mine, in northern Sweden. Two plots, consisting of fresh biosolids and 1-year weathered biosolids, were applied with a rotating spreader onto fresh sulfide-mine tailings to a depth of 0.2 m. The fresh biosolids sample was collected on the day of application. The 1-year weathered biosolids was retrieved using a hand-held trowel, 1 year after application. Both samples were placed into an oxygen-free argon-filled container, frozen, and analysed within 24 h. The biosolids were sourced from the same locations within a year apart. They consisted of an 80:20 vol% ratio mix of two biosolids sourced from a wastewater treatment plant, and a biogas plant from the nearby town of Skellefteå. The wastewater treatment plant had digested the biosolids at 38 °C for 15 days, whereas the biogas plant had digested it at 53 °C for 50 days. Both biosolids had been dewatered to a 22 % dry weight before mixing in the field. Two spot samples of each were retrieved using an oxygen-free argon-filled container, frozen, and analyzed for their TOM (LOI) contents within 24 h.
Kristineberg Pilot-Scale Biosolids
A 0.25 m thick biosolids sealing layer was applied onto fresh unoxidized sulfide-mine tailings (48 % FeS2, 4.8 % Fe1−xS) in a pilot-scale test cell at the Georange environmental test site at the Kristineberg Mine, northern Sweden (Fig. 2). The cell measured 5 × 5 m2 by 3 m deep and was lined with an inert HDPE liner that resisted acid attack from the materials contained therein. The biosolids layer was covered by a 0.3 m thick porous drainage layer, and a protective layer of locally derived glacial till (1.2 m), and was open at the top to atmospheric conditions. The anaerobically digested biosolids used was from a nearby wastewater treatment plant in Lycksele.
Gas samples (O2, CH4, and CO2) were collected 586–795 days after application from quartzite-filled geotextile balls located 0.05 m above the biosolids sealing layer in the porous drainage layer (Fig. 2). It was not possible to collect gas samples within the biosolids layer due to clogging. The porous drainage layer above the biosolids layer provided an indication of released gas products due to aerobic biodegradation. Samples were extracted using a Maihak S710 gas analyzer following procedures described by Hallberg et al. (2005). The gas samples were analyzed biweekly from spring to autumn. Sampling intervals between autumn and spring were larger due to sample retrieval not being possible during the winter months due to sub-zero temperatures. CH4 and CO2 were calibrated with specific gas concentrations prior to the sampling. The precision of the instrument was better than 2 % of the analyzed value according to the manufacturer. Aerobic degradation processes were calculated using an interpretation of data from Nason et al. (2013), which showed a negative correlation between the concentrations of O2 and CO2 and no correlation between these gases and CH4, which indicated that solely aerobic degradation processes were occurring.
The gas concentrations (%) were converted into mmol gas using the Ideal Gas Law. Pressure was assumed constant at atmospheric levels, and the temperature (Kelvin) was measured biweekly using a thermistor located 0.05 m from the geotextile ball. Concentrations were converted per unit gram of biosolids, as in the laboratory experiments, for a direct unit comparison. A known gas volume was calculated using the porosity of the top 0.05 m biosolids layer and the overlying 1.2 m protective layer. The total mass of biosolids was calculated by multiplying volume (depth × area) (0.05 m × 25 m2) and arbitrary density of 721 kg m−3 (Glover 1989).
Chemical Analysis
The samples were dried at 105 °C for 24 h for the dry weight determination, and loss of ignition (LOI) was performed by taking an aliquot of the sample and heating it to 1000 °C. Precision of the method was generally better than 5 %. All samples were sent to the accredited ALS commercial laboratory in Luleå, Sweden for analysis.
Mass Balance Calculation
Mass balance calculations of TOM loss (% TS) were performed for the field applications of biosolids from Kristineberg and Boliden. The field systems represent open experiments with respect to escaping gas concentrations and surface weathering of the biosolids. Quantification of TOM redistribution within the solid fraction of the biosolids was derived by normalizing to a fixed element (Ti) that was assumed to be immobile during weathering. Mass balance calculations were performed at each sample interval using a modification of Gresens’ equation of metasomatic alteration (Grant 1986). The original concentration (CO) and the final concentration (CF) of the TOM (e) are expressed as ratios to the normalizing element, Ti (n).
Laboratory-Scale Microcosm Experiment
The biosolids used for the laboratory-scale microcosm experiments were from the Skellefteå Biogas plant situated in northern Sweden. After sampling, the biosolids (with moisture) was thoroughly homogenized with a shovel, and was stored at 4 °C before use. The experiments were started within 3 months of the sampling date. Prior to the experiment, all of the 500 mL glass infusion flask microcosms, together with secured 32 mm airtight rubber stoppers and aluminum rings, were sterilized at 120 °C for 4 h. The biosolids was homogenized manually and the TS of the biosolids was determined.
Open Microcosm Experiment
Three experiments were carried out at different incubation temperatures representing ambient room temperature (20–22 °C), mesophilic (34 °C), and thermophilic (50 °C) conditions (Fig. 1) to provide an indication of degradation rates facilitated by aerobic processes.
Fresh biosolids corresponding to a 10 g dry weight (d.w.) were cut into small pieces (<5 mm) with a flat stainless steel shovel before being transferred into 500 mL glass infusion flask microcosms. The flask was capped with a thin geotextile layer and fastened with a hollow aluminum ring that allowed ambient air to diffuse freely in and out without water penetration. The geotextile was washed with 5 % HCl and Milli-Q™ water and air dried. For the 34 and 50 °C batches, the flasks were put into a thermostat- equipped oven. The temperature was monitored with an external thermometer and the variation was found to be within ±1 °C.
Moisture loss for the samples was compensated for with a syringe by adding Milli-Q™ filtered and deionized water twice a week, every second day and every day, for the 20–22, 34, and 50 °C batches, respectively. By using a weighing balance (±0.01 g), water was added to maintain the weight of the samples at a constant level (the same as at day 0) throughout the experimental period, although it is acknowledged that water loss might not be the only source of weight loss, e.g. some volatile or organic matter could have degraded into CO2 or CH4. The flasks were then hand-shaken vigorously to achieve an even distribution. To eliminate the chance of aggregation in the 34 and 50 °C batches, the biosolids was cut into smaller pieces with a flat stainless steel shovel, as necessary. The tiny loss of fresh mass due to the cutting process was accounted for in the mass balance calculation. Samples were set up in multiple parallel experiments. A total of six samples for each batch in triplicate were included, which resulted in 54 samples for the three batches.
In parallel to the above experiments, controls (n = 1) were set up to test the effects of high temperature and drought on TOM biodegradation rates. The treatment and scheduled sampling time for the control followed the same procedures, except that the biosolids was sterilized in an oven at 105 °C for 24 h. The material was ground to a particle size <1 mm with a ceramic mortar. Afterwards, 10 g d.w. of the material was put in a 500 mL glass infusion flask microcosm and Mill-Q™ water was added to compensate for water loss due to drying. Six controls were included in each batch, resulting in 18 control samples.
Dry Mass Determination
In each batch, the leftover dry mass was determined after 5, 10, 20, 40, 90, and 156 days. Each sample was repeated in triplicate, resulting in 18 samples in a single batch. At the end of each scheduled leftover dry mass determination, the samples were dried in a 105 °C oven for 24 h. After cooling at room temperature, the moisture in the samples was further removed using a desiccator, and the leftover dry mass was determined. It was assumed that TOM contents in both experiments were homogenous and had the same content of organic fractions distributed in all of the samples. Microbial communities were assumed to be distributed evenly in each microcosm.
Results and Discussion
Aerobic Degradation of Biosolids
Open Microcosm Experiment
Results for the open microcosms are presented in Fig. 3 and summarized together with the Kristineberg and Boliden field data in Fig. 3. After 156 days of incubation, 8.9, 16.3, and 16.0 % of the biosolids (d.w.) was broken down in the 20–22, 34, and 50 °C microcosms, respectively (Fig. 2) The major organic components in the biosolids were comprised of lignin (48 % TS) and carbohydrates (11.8 % TS), which account for ca. 60 % of the TS (Jia et al. 2014). If it is assumed that the biosolids were homogeneous, and that 60 % of the material was represented by the TOM, then 10 g d.w. biosolids is comprised of 6 g TOM. The decrease of total mass in the 20–22, 34, and 50 °C batches at day 156 corresponded to a TOM decrease of 14.8, 27.2, and 26.7 %, respectively, if one assumes that the microcosms were closed systems, relative to the loss of any constituents other than gases.
The decrease of TOM from the biosolids under aerobic conditions were similar to the decrease under anaerobic conditions in a previous parallel study. In that study (Jia et al. 2014), ca. 27.8 % of organic matter was estimated to be degraded after 230 days. Both aerobic and anaerobic degradation experiments show that a high proportion of the biosolids (72–73 % TS) were not degraded after 156–230 days, strongly indicating that a major fraction of the organic matter in the biosolids is recalcitrant to degradation. Also, warmer temperatures led to faster mass depletion over time, affirming that warmer temperatures increases microbial activity and biodegradation. Increased degradation kinetics at higher temperatures in the open-experiment was due to optimal aerobic digestion of biosolids, which occur in typical temperature ranges in mesophilic (30–37 °C) and thermophilic (45–60 °C) conditions (Bruce and Fisher 1984), although there did not seem to be a linear increase in degradation rates with temperature. The biosolid batches at 34 °C apparently underwent mesophilic processes, with the 50 °C batch undergoing thermophilic processes.
Regarding mass loss as a function of incubation time, a large variation was observed among the triplicates at 20–22 °C, especially for the day 20 measurements (Fig. 3a). This may reflect an inhomogeneous distribution of the populations of degrading organisms or variations in the biosolids. Alternative explanations to the high variability could be the effect of temperature on microbial communities or the possibility that they reside in hotspots. As a consequence, degradation kinetics differed a lot relative to incubation time. According to OECD (2006), the first 10 days of incubation may account for the highest rates of degradability of readily degradable organic fractions. However, the degradation of the biosolids in the open microcosm was as low as 3 % (corresponding to 5 % TOM) during this period. The data suggest that a high fraction of the organic matter in this specific biosolids were initially recalcitrant towards biodegradation or that the bacterial community took some time to adjust to the experimental environment.
Regarding the biodegradation kinetics, the experiments confirm that degradation is a function of temperature (Table 1). The respective decreases of 14.8, 27.2, and 26.7 % of TOM at ambient, mesophilic, and thermophilic temperatures illustrates that peak biodegradation occurs at mesophilic temperature ranges. This is important when understanding field temperature ranges, and the likelihood of increased organic matter depletion in warm climates.
An interesting phenomenon observed was that the mass of the control sample declined similarly to the mass of the fresh samples, although at a slower rate (Fig. 3). The control biosolids were subjected to 105 °C for 24 h before the experiment was initiated, with the hypothesis that microorganisms would be killed, and biodegradation minimized. However, the results may indicate that the conditions implemented to sterilize the biosolids were not sufficient, and that microbial degradation still persisted. This may imply that the microorganisms in the biosolids were resilient to an extreme temperature environment, which may simulate what might occur in drought conditions.
Pilot-Scale Experiment
Aerobic degradation rates, as indicated via instant gas concentrations, are presented in Fig. 4a. The O2 and CO2 concentrations in the upper layer of the biosolids sealing layer are correlated due to aerobic degradation via atmospheric oxygen influx (Eq. 1) to the upper layer. The oxygen was transported to the sealing layer through the overlying protective layer, as it was more permeable (6 × 10−7 m s−1 in the till; Höglund and Herberg 2004) than the sealing layer, which allowed oxygen to diffuse freely to the biosolids (Nason et al. 2013). Figure 4b displays cumulative concentrations of all three gases. CH4 gas accumulation was minimal, indicating that aerobic degradation prevailed in the upper biosolids layer.
As degradation of the biosolids consumed O2, CO2 correspondingly increased, per Eq. 1. This is indicated in the data at days 664, 678 and 753 (Fig. 4a). Over time, the rate of cumulative gas concentration (mmol) per unit biosolids (g) was seen to decline (Fig. 4b). For comparison, the range of CO2 (0.00–0.03 mmol g−1) concentrations equates to 1 % of the total concentrations (Fig. 4b) in the microcosm data (0.0–2.0 mmol g−1), even though the experiment was conducted over a longer time period in the field.
The elevated CO2 concentrations released in the microcosm affirms the high reactivity of the fresh biosolids during the first 90 days. Over time, as represented in the pilot-scale experiment, even though oxygen is constantly recharged from the atmosphere, the released concentrations of CO2 declined. This likely resulted from exhaustion of the more readily reactive TOM in the biosolids layer due to aerobic degradation prior to the initiation of the experiment, since the biosolids had aged for 586 days.
Linking Laboratory Results to Prior Field Applications
The results in Fig. 5 indicate that the TOM lost via biodegradation in the field biosolids applications was less than in the laboratory biosolids. The Boliden field data indicated an approximate mass loss of 1 % d.w. in the first year after application. However, TOM decreased by 15.6 % which is similar to the 20–22 °C experiment. The TOM loss from the Kristineberg application, which represented 2.2 years of weathering, indicated that 6.1 and 22.2 % of the TS and TOM had been removed, respectively. The results indicate that the loss of TOM by aerobic degradation was slower in the field than in the laboratory.
The data indicates a relative mass addition to the biosolids in the field. This may be attributed to mixing of the biosolids with underlying layers, such as the glacial till and tailings. It is also likely that degrading vegetation on the biosolids may have recharged organic matter fractions in the material. Although the Boliden 1-year weathered biosolids field application did not have visible vegetation establishment, the Kristineberg 2.2-year weathered biosolids had substantial vegetation establishment due to it having been hydroseeded. Nason et al. (2014) analysed the Kristineberg field site used in this study, and attributed some mass contribution being from vegetation decay, but concluded that it was not significant. However, this addition of organic matter may have offset organic matter depletion in the field.
In the open microcosm experiment, the process was conducted under optimal conditions with respect to temperature. As the field experiments had mean annual temperatures of 0.6 and 0.7 °C for the Boliden and Kristineberg Mine sites, respectively (Axelsson et al. 1991; Lindvall and Eriksson 2003), the biodegradation rates of the biosolids were, as predicted, slower than the laboratory rates. Nevertheless, biodegradation rates at the mesophilic (34 °C) and thermophilic (50 °C) temperatures, presumably provide a perspective on potential biosolids biodegradation rates under accelerated field conditions or in regions of higher mean annual ambient temperatures.
Degradation kinetics can also be much slower in in situ field conditions, due to seasonal climatic differences and water content variation. In northern Sweden, where the field experiments were conducted, the ground surface is typically covered with snow for 5 months of the year (Axelsson et al. 1986). This lowers microbial activities and, hence, biodegradation rates. Additionally, thermistors located in the pilot-scale experiment in the subsurface biosolids sealing layer indicated that temperatures were below freezing throughout 4 months of the year (Shcherbakova 2006). This may have limited diffusion of oxygen to the subsurface environment and affected the microbial biodegradation kinetic rates. The resulting effects may have contributed to reducing the reactivity of the biosolids and to limiting the rate of biodegradation.
Snow melt and increased water saturation of the surface biosolids applied in spring may also have limited oxygen ingress into the material, potentially creating an anoxic environment, as has been found in previous studies in Sweden (Ahlberg 2006). Temperatures in the subsurface biosolids sealing layer in summer reached a maximum of 12 °C (Shcherbakova 2006). Consideration of the above climatic and hydraulic factors are additional reasons why degradation of the incubated laboratory microcosm biosolids was more rapid than in the field tests.
The predicted biodegradation rate of a field biosolids application is shown in the theoretical model (Fig. 6). Mass depletion (%) was calculated in comparison to the mass at day 0. The laboratory- and field-experimental data were used as reference points for the inferred TOM biodegradation rates. The model assumes biodegradation of only the more easily degradable TOM fractions, as calculated in a parallel study assessing anaerobic biodegradation rates (Jia et al. 2014). Biodegradation rates differ over time for the different field applications; rates were slower for the Kristineberg surface biosolids sealing layer than for the Boliden surface biosolids layer, largely because the Kristineberg biosolids were applied after being kept in an open storage container for 9 months.
Although the present study shows that only the readily degradable fractions in the biosolids have been degraded, the recalcitrant fractions in the biosolids are still potentially biodegradable. For the mining industry, it is crucial that an engineered cover using biosolids be effective for a long period, e.g. <100 years. The results suggest that a large percentage of the biosolids mass remains after aerobic biodegradation has mostly ceased.
Using Biosolids for Sulfide-Mine Tailings Remediation
Biosolids may be applied as a surface vegetation substrate to promote vegetation establishment on sulfide-mine tailings, or onto pre-existing engineered dry covers (Nason et al. 2014; Neuschütz 2009). The Kristineberg experiment evaluated a 0.2 m deep biosolids application to promote plant establishment, which after seeding, was achieved after 2 years. The total mass volume depletion was 6.13 %, with ca. 20 % TOM depletion. The concern for surface layer thickness and TOM depletion is therefore not an issue in surface applications, as the established vegetation will decompose and replenish the lost TOM over time.
There is, however, concern for using biosolids as a sealing layer material, due to combined TOM depletion from aerobic and anaerobic biodegradation. Jia et al. (2014) concluded that 27.8 % of the TOM was degraded from biosolids due to anaerobic degradation processes within a 230 day experiment at 20–22 °C. Similarly, 14.8 % TOM was degraded by aerobic degradation processes at the same temperature in the present study in 156 days. The combination of degradation processes may be a significant limitation to the efficiency of the sealing layer to prevent oxygen diffusion to underlying sulfide-mine tailings over time. The thickness, lack of organic matter recharge by degrading plant material, and exhaustion of readily degradable organic matter, may all have reduced the organic reactivity of the barrier to oxygen, which may affect the integrity of the physical barrier sealing layer.
Conclusions
Open microcosm experiments that measured biosolids mass depletion as an indication of microbially mediated biodegradation showed that 14.8, 27.2, and 26.7 % TOM of sewage sludge biosolids was depleted at 20–22, 34, and 50 °C, respectively. Biodegradation was much slower in surface biosolids applications in the field in northern Sweden, as lower mean temperatures (0.6–0.7 °C) and climatic parameter differences such as a higher degree of saturation and freezing conditions decreased microbial activity. These results indicates that microbially mediated biodegradation rates do not increase above mesophilic temperature ranges. Modeling kinetic reaction rates against the data shows a rather stable trend, with ca. 20 % of the biosolids degraded over 2 years. This rate is not a concern in surface applications of biosolids when used as a vegetation substrate, where organic matter recharge due to established vegetation decay will continue to mitigate oxygen transport to underlying tailings. However, if used as a sealing layer barrier without organic matter recharge, combined aerobic and anaerobic degradation processes may hinder the function of the cover to prevent oxygen transport to the tailings over time.
References
Ahlberg G (2006) Ageing of sewage sludge—some physical and chemical properties in relation to landscaping. In: PhD thesis, Göteborg Univ, Sweden
Avfall Sverige AB (2011) Swedish waste management, Malmö. http://www.avfallsverige.se/fileadmin/uploads/Rapporter/Utveckling/Rapporter_2011/SAH_eng111219.pdf. Accessed Apr 2014
Axelsson C-L, Karlqvist L, Lintu Y, Olsson T (1986) Gruvindustrins restproduktupplag –fältundersökningar med vattenbalsansstudie i Kristineberg. Uppsala Geosystem AB [in Swedish with summary in English]
Axelsson C-L, Ekstav A, Holmén J, Jansson T (1991) Efterbehandling av sandmagasin I Kristineberg, Hydrogeologiska förutsättningar för åtgärdsplan: Lakvattenbalanser och vittringsbegränsande åtgärder. Report (in Swedish)
Bruce AM, Fisher WJ (1984) Sludge stabilisation-methods and measurement. In: Bruce A (ed) Sewage sludge stabilization and disinfection. Water Research Centre/Ellis Horwood Ltd, Chichester, pp 23–47
Demirbas A (2011) Waste management, waste resource facilities and waste conversion process. Energy Convers Manage 52:1280–1287
Egiebor NO, Oni B (2007) Acid rock drainage formation and treatment: a review. Asia Pac J Chem Eng 2:47–62
Forsberg LS, Ledin S (2006) Effects of sewage sludge on pH and plant availability of metals in oxidizing sulphide mine tailings. Sci Total Environ 358:21–35
Gerhardt A, de Bisthoven LJ, Soares AMVM (2004) Macroinvertebrate response to acid mine drainage; community metrics and on-line behavioural toxicity bioassay. Environ Pollut 130:263–274
Glover TJ (1989) Pocket reference, 4th edn. Southgate Publ, Crediton
Grant JA (1986) The isocon diagram—a simple solution to Gresens’ equation for metasomatic alteration. Econ Geol 81:1976–1982
Gray NF (1997) Environmental impact and remediation of acid mine drainage: a management problem. Environ Geol 30:62–71
Hallberg RO, Granhagen JR, Liljemark A (2005) A fly ash/biosludge dry cover for the mitigation of AMD at the Falun mine. Chem Erde Geochem 65:43–63
Höglund LO, Herberg R JR (eds)(2004) MiMi—performance assessment main report. MiMi 2003:3. The MISTRA-programme MiMi, mitigation of the environmental impact from mining waste. MiMi Print, Lulea
INAP (2009) Global acid rock drainage (GARD) guide. www.gardguide.com. Accessed Apr 2014
Jia Y, Nason P, Alakangas L, Maurice C, Öhlander B (2014) Degradation of digested sewage sludge residue under anaerobic conditions for mine tailings remediation. Environ Earth Sci 72:3642–3654
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Kan A (2009) General characteristics of waste management: a review. Energy Educ Sci Technol A 23:55–69
Lindvall M, Eriksson N (2003) Investigation of weathering properties of tailings sand from Boliden’s Aitik Copper Mine, Sweden—a summary of twelve years of investigations. In: Proceedings of 6th International Conf on Acid Rock Drainage, Cairns, Australia
Lottermoser BG (2010) Mine wastes: characterization, treatment, and environmental impacts, 3rd edn. Springer, Berlin
Maier RM, Pepper IL, Gerba CP (2000) Environmental microbiology. Academic Press, New York
Mäkitalo M (2012) Green liquor dregs as sealing layer material to cover sulphidic mine waste deposits. In: Licentiate thesis, Luleå Univ of Technology, Sweden
Nason P, Alakangas L, Öhlander B (2013) Using sewage sludge as a sealing layer to remediate sulphidic mine tailings: a pilot-scale experiment, northern Sweden. Environ Earth Sci 70:3093–3105
Nason P, Alakangas L, Öhlander B (2014) Impact of sewage sludge on groundwater quality at a formerly remediated tailings impoundment. Mine Water Environ 33:66–78
Nehdi M, Tariq A (2007) Stabilization of sulphidic mine tailings for prevention of metal release and acid drainage using cementitious material: a review. J Environ Eng Sci 6:423–436
Neuschütz C (2009) Phytostabilization of mine tailings covered with fly ash and sewage sludge. In: PhD thesis, Stockholm Univ, Sweden
Neuschütz C, Greger M (2010) Stabilization of mine tailings using fly ash and sewage sludge planted with Phalaris arundinacea L. Water Air Soil Pollut 207:357–367
OECD (2006) Test No. 310: Ready Biodegradability - CO2 in sealed vessels (Headspace Test), OECD Guidelines for the Testing of Chemicals. Section 3, OECD Publishing, Paris. doi.10.1787/9789264016316-en
Peppas A, Komnitsas K, Halikia I (2000) Use of organic covers for acid mine drainage control. Miner Eng 13:563–574
Pérez-López R, Quispe D, Castillo J, Nieto JM (2011) Acid neutralization by dissolution of alkaline paper mill wastes and implications for treatment of sulfide-mine drainage. Am Mineral 96:781–791
Shcherbakova E (2006) Geochemical and hydrological aspects of interactions between water and mine waste. In: Licentiate thesis, Luleå Univ of Technology, Sweden
Statistics Sweden (2006) Discharges to water and sewage sludge production in 2006. In: Publ in collaboration with the Swedish Environmental Protection Agency. www.scb.se/templates/Publikation__232141.asp. Accessed Apr 2014
Strasser H, Brunner H, Schinner F (1995) Leaching of iron and toxic heavy metals from anaerobically-digested sewage sludge. J Ind Microbiol 14:281–287
Tordoff GM, Baker AJM, Willis AJ (2000) Current approaches to the vegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
Wang H, Brown SL, Magesan GN, Slade AH, Quintern M, Clinton PW, Payn TW (2008) Technological options for the management of biosolids. Environ Sci Pollut Res 15:308–317
Acknowledgments
We thank the GEORANGE program and the Center of Advanced Mining and Metallurgy (CAMM) for financial support, Anton Lundkvist from Boliden Mineral AS, Sweden for field assistance during the biosolids sample collection, and Désirée Nordmark and Tommy Wikström for their assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
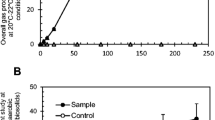
Cumulative gas production per unit weight biosolids (Experiment 1: failed closed-microcosm). A) Samples. B) Control. The oxygen increase here was due to O2 added at day 5, 10 and 20 for the purpose of keeping aerobic condition in the microcosm (PDF 121 kb)
Rights and permissions
About this article
Cite this article
Nason, P., Jia, Y., Maurice, C. et al. Biodegradation of Biosolids Under Aerobic Conditions: Implications for Cover Materials for Sulfide Mine Tailings Remediation. Mine Water Environ 35, 273–282 (2016). https://doi.org/10.1007/s10230-015-0339-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-015-0339-3