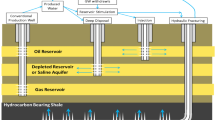
Abstract
In the face of climate change, less carbon intensive fuels are being sought. Natural gas has been perceived as a transition fuel, producing less CO2 when burned than coal, but it is not a renewable resource. Hydrocarbon-rich shale formations contain natural gas, natural gas condensate, and oil production potential, and the extraction of these compounds has allowed the USA to become one of the largest global producers of natural gas. Horizontal drilling and hydraulic fracturing are used to extract the shale gas, but hydraulic fracturing of one well typically requires between 7 and 19 million L of water. One option being explored is the use of treated mine water as an alternative to freshwater. The Marcellus and Utica shale formations underlie much of the eastern USA, and the Utica Shale is being pursued for its high natural gas potential in eastern Ohio. Permits for wells are being approved, but concerns about the water source for hydraulic fracturing fluid are increasing. We analyzed the carbon footprints of three different water sources: treated mine water from Corning, Ohio, freshwater from the Ohio River, and freshwater from Seneca Lake near the well site. CO2 emissions for each source were calculated during pumping, transportation, and treatment of the water for a one-time well use and annual use. The primary productivity increase that occurred after removal of mine discharge or reduction due to extraction from freshwater sources was also calculated. Annually, using treated mine water would emit 110,000 t of CO2-e (CO2 equivalent) if trucked to a treatment plant or 90,000 t of CO2-e if treated on-site, while using water from the Ohio River would emit 2,000 t of CO2-e, and using water from Seneca Lake would emit 4,500 t of CO2-e, annually. Of course, decreasing the amount of unpolluted freshwater used has other environmental benefits.
Zusammenfassung
Im Angesicht des Klimawandels werden Brennstoffe geringerer Kohlenstoffintensität gesucht. Erdgas wurde als Übergangsbrennstoff wahrgenommen, weil es weniger CO2 produziert als Kohle, aber es ist keine erneuerbare Ressource. Kohlenwasserstoffreiche Tonsteinformationen enthalten Erdgas, Erdgaskondensate und das Potential, Erdöl zu produzieren. Die Extraktion dieser Stoffe hat es erlaubt, daß die USA zu einem der größten globalen Produzenten von Erdgas wurde. Horizontalbohrungen und hydraulisches Fracken werden zur Förderung von Schiefergas genutzt, aber hydraulisches Fracken einer Bohrung benötigt typischerweise zwischen 7 und 19 Millionen L Wasser. Eine untersuchte Option ist die Verwendung von behandeltem Bergbauwasser als eine Alternative zu Süßwasser. Die Marcellus und Utica Tonformationen unterlagern weite Teile der östlichen USA,und der Utica Tonstein wird im östlichen Ohio wegen seines hohen Erdgaspotentiales aufgesucht. Bewilligungen für Bohrungen werden erteilt, aber Sorgen über die Herkunft des Wassers für das hydraulische Frackfluid nehmen zu. Wir haben den CO2-Fußabdruck von drei unterschiedlichen Wasserquellen untersucht: behandeltes Bergbauwasser von Corning, Ohio, Süßwasser aus dem Ohio Fluß, und Süßwasser aus dem Seneca See in der Nähe des Bohrplatzes. CO2-Emissionen für jede Quelle wurden für das Pumpen, den Transport und für die Wasseraufbereitung für den Fall einer einmaligen Nutzung oder einer mehrfachen Verwendung über ein Jahr berechnet. Die Zunahme primärer Produktivität, welche durch die Beseitigung des Bergbauwasseraustrittes entstand oder die Reduktion infolge der Entnahme aus Süßwasserquellen wurde ebenfalls berechnet. Für den Fall des Lastwagentransportes zu einer Wasseraufbereitung würde die Verwendung behandelten Bergbauwassers eine jährliche Emission von 110.000 t CO2-e (CO2-Äquivalent) bewirken, bei einer Behandlung vor Ort jedoch nur 90.000 t CO2-e, während die Nutzung von Wasser aus dem Ohio Fluß jährlich in 2.000 t CO2-e, und von Wasser aus dem Seneca See 4.500 t CO2-e resultierte. Selbstverständlich hätte die Verringerung der Nutzung sauberen Süßwassers andere Umweltvorteile.
Resumen
En vista del cambio climático, los combustibles en base carbón tienen menor demanda. Gas natural está siendo percibido como un combustible de transición, produciendo menos CO2 en la combustión que el carbón, pero no es una fuente renovable. Formaciones de esquistos bituminosos ricos en hidrocarburos, gas natural condensado, potencial de producción de petróleo y la extracción de estos compuestos ha permitido que EEUU se convirtiera en uno de los mayores productores globales de gas natural. Las perforaciones horizontales y la fractura hidráulica están siendo usados para extraer el gas de los esquistos bituminosos, pero la fractura hidráulica de un pozo requiere entre 7 y 19 millones L de agua. Una de las opciones que está siendo explorada es el uso de agua de mina tratada como una alternativa frente al agua dulce. Las formaciones de esquistos bituminosos Marcellus y Utica están en el subsuelo de buena parte de EEUU oriental y el esquisto Utica es requerido por su alto potencial en gas natural en Ohio oriental. Los permisos para pozos están siendo aprobados pero la preocupación sobre la fuente de agua para fractura hidráulica se está incrementando. Analizamos las huellas de carbón de tres fuentes de agua diferentes: agua de mina tratada de Corning, Ohio, agua dulce del Río Ohio y agua dulce del Lago Seneca cerca del sitio del pozo. Las emisiones de CO2 para cada fuente fueron calculadas durante el bombeo, transporte y tratamiento de agua para el uso del pozo una vez y para el uso anual. Se calculó también la producción primaria que ocurrió después de la remoción de la descarga de mina o la reducción debido a la extracción de fuentes de agua dulce. Anualmente, usando agua de mina tratada se emitirían 110.000 t de CO2-e (CO2 equivalente) si se transportara hasta una planta de tratamiento o 90.000 t de CO2-e si se tratara en el sitio, mientras que usando agua del Río Ohio se emitirían 2.000 t de CO2-e y usando agua del Lago Seneca se emitirían 4.500 t de CO2-e, anualmente. Por supuesto, el descenso de la cantidad de agua dulce contaminada usada tiene otros beneficios ambientales.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming and climate change have been attributed to anthropogenic causes, with greenhouse gas emissions as the driving factor; especially from power production (Tett et al. 1999). Attempts at employing cleaner energy sources and tighter regulations on emissions aim to limit global warming (EPA 2009). Carbon emissions from power plants and other coal-fired industries must be controlled under the U.S. government’s Climate Action Plan (CAP) (Obama 2013). After the CAP was issued, the U.S. EPA delivered its proposal for carbon pollution standards for new power plants in order to limit the carbon that future power plants may emit (EPA 2013). The CAP also calls for increasing the use of cleaner energy sources, such as natural gas.
One method of analyzing the environmental impact of different activities on climate change is carbon footprinting. The term “carbon footprint” stems from “ecological footprint,” an expression coined by Wackernagel and Rees (1962) that analyzes resource consumption and waste accumulation of a human population or activity, measured in land area. Carbon footprinting may be used to evaluate the net carbon emissions caused by a certain activity (Weidema et al. 2008). Wiedmann and Minx (2008) define carbon footprints as “a measure of the exclusive total amount of carbon dioxide emissions that is directly and indirectly caused by an activity.” Practical details of a carbon footprint were given in the definition by Wright et al. (2011), which states that a carbon footprint is “a measure of the total amount of carbon dioxide and methane emissions…considering all relevant sources, sinks, and storage within the spatial and temporal boundary of the population, system or activity of interest.” Carbon footprints have been used to evaluate the impact of a variety of activities, such as schools, wastewater treatment plants, and plastics manufacturers (Dormer et al. 2013; GAP 2006; Gustavsson and Tumlin 2013) and is a useful method for comparing practices in terms of carbon emissions because it measures the amount of greenhouse gases produced with a carbon dioxide equivalent (CO2-e). The CO2-e is related to the global warming impact of the activity being measured (Konieczny et al. 2013). The CO2-e of each carbon source or sink is summed to determine the overall carbon footprint of an activity.
High fossil fuel consumption has exacerbated climate change and has fueled a high demand for cleaner energy (IPCC 2007). Although natural gas emits carbon during combustion (Eq. 1), it releases less carbon per unit energy than coal at the point of combustion (Eq. 2), and is therefore seen as a “bridge” fuel towards renewable energy (Eaton 2013; Jenner and Lamadrid 2012; Moniz et al. 2011). Coal produces approximately 38 times more energy per mole than natural gas (Eqs. 1 and 2), but, per unit energy, coal produces approximately seven times the CO2 that natural gas does (EIA 2014).
The USA was projected to surpass Saudi Arabia and Russia and become the largest producer of hydrocarbon fuels (petroleum and natural gas) by the end of 2013 (Breul and Doman 2013). Much of this growth comes from a large increase in production from shale plays. In order to exploit the vast quantities of natural gas and natural gas condensates within shale formations, horizontal drilling with hydraulic fracturing (fracking) is used. This process pumps high volumes of fluid at high pressure to fracture the target shale formation, props them open with sand or another proppant, which allow natural gas and natural gas condensates to flow back to the surface when pressure is released. Each well typically requires between 7 and 19 million L of water, usually pumped from a freshwater source (Ground Water Protection Council and ALL Consulting 2009). Studies have examined the relationship between energy use and water resources (Gleick 1994; Harte 1983) and the Pennsylvania Dept of Environmental Protection has proposed the use of mine-influenced water for the base fluid for hydraulic fracturing (PDEP 2013). This proposition is partly due to the over 5,000 km of Pennsylvania streams that are impacted by mine water (Cravotta 2008). The policy has garnered support from some environmental organizations, as well as the oil and gas industry (PDEP 2013), while there are still reservations from other groups citing water diversion between watersheds as a primary drawback (Downing 2014).
As conventional sources of natural gas are decreasing, unconventional sources such as shale gas have been expanding in the USA (Kerr 2010). From August 2009 to August 2013, drilling land rigs actively exploring for or developing oil or natural gas increased from 949 to 2,084, with much of this increase coming from the development of shale gas (Baker Hughes 2009, 2013). The Marcellus and Utica shale plays are two of the basins experiencing increased extraction; both occur in Ohio, where the greatest potential is associated with the Utica Shale (OEPA 2011). As of Nov. 2013, the Ohio Dept of Natural Resources (ODNR) had issued 988 horizontal permits with 606 horizontal wells drilled and 184 producing (ODNR 2013). Figure 1 shows the distribution of these permits in eastern Ohio. Companies such as BHP Billiton Ltd and Chesapeake Energy Corporation have invested in and are drilling for natural gas from shale in Ohio (Downing 2013; TERI Press 2013). Figure 2 shows gas production from the Utica Shale in eastern Ohio for calendar year 2012 (ODNR 2013). While surface water bodies, including the Ohio River and lakes managed by the Muskingum River Conservation District, including Seneca Lake, have been used as source water for hydraulic fracturing in Ohio, Ohio may follow Pennsylvania’s lead on the reuse of mine water. Mine water needs to be treated before being reused for hydraulic fracturing for several main reasons: (a) to remove iron and other metals that may scale or foul the well casing or occlude the fractured shale, (b) to buffer acid that may corrode the well casing, (c) to remove sulfate that may react with barium in the formation water to precipitate barite and occlude the fractured shale, and (d) to remove sulfate that may be metabolized by sulfate-reducing bacteria, releasing hydrogen sulfide. Current water sources (i.e. the Ohio River and Muskingum River Conservation District lakes) are used without pre-treatment.
Although there is both Marcellus and Utica shale in Ohio, the Utica Shale formation has more natural gas and natural gas condensate potential because the western edge of the Marcellus Shale is thinner (de Witt et al. 1993). A 61 km pipeline is under construction, which will further expand development of natural gas from the Utica Shale in Ohio (Dunnahoe 2013). It is predicted that by 2015, shale wells will produce 82 % of Ohio’s natural gas (Institute for Energy Research 2013). Carroll County is in eastern Ohio and has been the epicenter of shale gas development in Ohio, with many wells operated by various companies. As of Nov. 16, 2013, the ODNR had issued 347 total permits for horizontal wells in 14 different townships in Carroll County. Of those permits, 143 horizontal wells have been drilled, five are being drilled, 96 are permitted, and 103 wells are producing (Fig. 1; ODNR 2013). This paper focuses on water sources for fracking wells in Carroll County as a case study, due to the county‘s strong potential for shale gas (Hill and Kinahan 2013). We compare the carbon footprint for annual and one-time use for fracking for three sources: treated mine water, freshwater from the Ohio River, and freshwater from Seneca Lake.
Methods
The carbon footprint for each of the three scenarios has been calculated. In each scenario, it was assumed that the destination for water for hydraulic fracturing is Carrollton, Ohio in Carroll County. The three scenarios are: (a) pumping mine water from a high flow mine discharge in Corning, Ohio, and treating that water before using it for hydraulic fracturing, (b) pumping water from the Ohio River near Carrollton, Ohio, before using the water for hydraulic fracturing, and (c) pumping water from Seneca Lake near Senecaville, Ohio, before using the water for hydraulic fracturing. Figure 3 shows each of the locations and the transportation routes used in each scenario. The scenarios, assumptions, and emissions factors are detailed below.
Scenarios
Three different scenarios were analyzed in order to compare their carbon footprint. The first scenario used water from a high flow AMD discharge in Corning, Ohio (Fig. 3). The Corning mine is the closest abandoned mine discharge to the drilling activity and its high flow of AMD has significant negative impacts on the biology of Sunday Creek (Bowman 2012). The average chemistry and flow rate of the Corning discharge is shown in Table 1. Due to the high metal and sulfate content of the mine water, it must be treated before being used as the base for hydraulic fracturing fluid. Treatment was assessed in two sub-scenarios: (a) water is treated on-site and (b) the water is transported to the closest industrial wastewater treatment facility in Mannington, West Virginia. In both cases, the water is transported after treatment to a well pad near Carrollton, Ohio.
The second scenario uses freshwater from the Ohio River for the hydraulic fracturing fluid. East Liverpool, Ohio, is the closest location on the Ohio River to Carrollton, Ohio. The water would be transported from East Liverpool to Carrollton. The third scenario examines freshwater from Seneca Lake near Senecaville, Ohio, as a source of water for hydraulic fracturing and its transport to a well near Carrollton.
Pumping Rates and Emissions
Carbon dioxide emitted during the pumping process is the same for each of the scenarios because the same volume of water is pumped. The flow rate of the mine discharge (71 L/s) and the maximum pumping rate of the water pump (42 L/s) were not limiting factors. The carbon footprint of pumping was analyzed using a Tsurumi EPT2 100-DD diesel water pump with a horsepower rating of 24.5. The CO2 emission factor of a diesel water pump is 522 g CO2-e/hp-hr (RSK Environmental Ltd 2002). The time needed to pump 19 million L with the Tsurumi pump is 125 h. The carbon footprint of pumping was calculated using the equation shown in Eq. 3.
Transportation
While trucking is an expensive transportation method compared to rail or pipeline, water pipelines are not well established in the Utica shale play, so trucking has been used for comparison. The carbon footprint of the transportation involved in each scenario was calculated using the shortest routes via state and interstate roads (Fig. 3). These calculations used a CO2 emission factor of 1.0725 kg CO2-e/km for a heavy-duty truck (EPA’s Center for Corporate Climate Leadership 2011). The maximum load that a heavy duty truck can hold is 40,000 L, and therefore 473 trips would be needed to transport 19 million L of water. For the scenario in which mine water is trucked to an existing treatment plant, the water is transported from the discharge site in Corning, Ohio, to the treatment facility in Mannington, West Virginia, then to a well near Carrollton, Ohio. The total distance involved in transport is 431 km, which was then multiplied by the emission factor. For the scenario in which mine water is treated on-site before being trucked to Carrollton, Ohio, the total distance traveled is 190 km. The transportation involved in the second and third scenarios is merely the trucking from the freshwater site to a well near Carrollton. For the second scenario, the emission factor is multiplied by 126 km, the distance between Senecaville and Carrollton. For the third scenario, the emission factor is multiplied by 52.5 km, the distance from East Liverpool to Carrollton. The calculation used is shown in Eq. 4.
Water Treatment
The carbon footprint of the treatment of the mine discharge was calculated using standard factors assuming a slaked lime plant including an equalization basin, a lime storage and feed system, a flash mix tank, an aeration tank, and a settling basin with sludge removal, following Stone et al. (2012). Neutralized mine water was then introduced into a fixed-bed anaerobic bioreactor dosed with ethanol for sulfate reduction (e.g. Kaksonen and Puhakka 2007; Neculita et al. 2007). The average acidity loading was calculated for the Corning AMD discharge (305 kg/day, see Table 1). The mass of hydrated lime needed to completely buffer the acidity was calculated based on an alkali requirement of 0.74 kg Ca(OH)2/kg acidity and a 90 % neutralization efficiency (GARD 2010), as shown in Eq. 5. The CO2 emissions during treatment were calculated as the sum (Eq. 6) of the emissions for: (1) the production of hydrated lime (Eq. 7), (2) the transportation of hydrated lime (Eq. 8), and (3) the energy used at the treatment plant (Eq. 9). Hydrated lime emits 0.74 kg CO2/kg in the high temperature process that converts limestone to lime and approximately 0.4 kg CO2/kg from the energy required (Ecofys 2009). It was assumed that hydrated lime was transported from Greer Lime in Riverton, WV to either the AMD treatment plant located in Mannington, WV, a distance of 208 km, one-way, or to Corning, Ohio, a distance of 381 km, one way. A single truck load holds approximately 22.5 t of material. The CO2 emissions from enough round-trips to supply hydrated lime to buffer the AMD were calculated using the same emissions factor as the water transport (1.0725 kg CO2-e/km). On average, a slaked lime facility uses 911 kWh/kg acidity buffered (Stone et al. 2012) and the average CO2 emission rate for WV electricity is 0.90 kg CO2/kWh, while the emission rate for Ohio is 0.80 kg CO2/kWh (EPA 2007). A fixed-bed anaerobic bioreactor would be dosed with 0.24 mL ethanol/L mine water or 871 L ethanol/day to achieve a removal rate of 625 mg/L SO4 based on stoichiometry (e.g. Kaksonen and Puhakka 2007). Average U.S. ethanol from a corn feedstock emits 68.6 g CO2-e/MJ or 1.448 kg CO2-e/L (CEPA 2009). Emissions for the ethanol from field to tank (CEPA 2009) are shown in Eq. 10. Since most bioreactors also support methanogenesis, it was assumed that 25 % of the ethanol was converted to methane. The emissions calculations shown in Eq. 11 is based on stoichiometric ratios of 2 mol ethanol: 3 mol methane and 2 mol ethanol: 1 mol CO2 (Tatton et al. 1989) and greenhouse gas equivalency of 25 kg CO2-e to 1 kg CH4 (IPCC 2007). Emissions from pumping are assumed to follow the Eq. 3 relationship.
Primary Productivity
Primary productivity of the stream impacted by the Corning discharge would increase due to removal of the mine water. Many studies have compared primary productivity between waters impacted by mining and unimpacted waters (Gyure et al. 1987; Levings et al. 2005; Morgan 1985; Nixdorf et al. 2003; Niyogi et al. 2002; Parkhill and Gulliver 2002). Bauers (2003) compared primary productivity rates in streams in or near Corning’s watershed (in the Sunday Creek, Monday Creek, and Federal Creek watersheds). The results from this study were used when calculating the carbon sink created by removing the mine discharge as the difference in carbon uptake by the stream with mine discharge compared to the stream with a reduced flow of mine discharge. The productivity rate of impacted sites, presented in Bauers (2003), was multiplied by the average flow rate downstream from the discharge site (Qpre) to find the carbon uptake without removal of discharge. To find the carbon uptake with the removal of mine discharge for use in hydraulic fracturing, the productivity rate of reference sites (Bauers 2003) was multiplied by the difference between the mean flow rate of the sample location downstream and the pumping rate of the water pump (Qpost). The calculation is shown in Eq. 12.
Primary productivity would be reduced by extracting water from the Ohio River; the reduction in primary productivity due to extraction from the Ohio River was calculated using Eq. 13.
While primary productivity of Seneca Lake may fluctuate due to extraction of water for hydraulic fracturing, at the extraction rate in this case study, the lake level would be reduced by approximately 10 cm, while the winter lake drawdown is approximately 3 m (U.S. Army Corps of Engineers 2014). The existing variability would outweigh the change in primary productivity due to extraction due to hydraulic fracturing at the stated pumping rate; therefore, the primary production change in the Seneca Lake scenario has been disregarded.
Results
The three different sources of water used as the base of hydraulic fracturing fluid were compared by analyzing the carbon footprint of each scenario. Carbon dioxide emitted during all steps of each scenario was calculated for this comparison and is shown in Table 2.
Carbon footprints were calculated for both a one-time fracking of a well and for a constant supply for continuous use for fracking various wells for 1 year. By calculating both of these, the difference in CO2 emissions was better observed for likely constant operating conditions. Pumping 19 million L of water takes 125 h (5.2 days). Therefore, the pumping, transportation, and treatment processes would occur a maximum of 70 times annually (Table 3).
The carbon footprint during the process of using mine discharge as source water was calculated (Table 2); single well and annual totals are shown in Table 3. Carbon dioxide emitted during treatment was the largest source, of which 97.7 % was due to energy used during treatment, 2.1 % was from the sulfate reducing bioreactor, 0.1 % was due to emissions during lime production, and 0.001 % was due to transportation of the lime in both treatment scenarios. Annually, pumping for treatment at an existing treatment plant would emit 111,904 kg, transport would emit 15,305,015 kg, and treatment would emit 93,777,950 kg, while for on-site treatment, emissions due to pumping would remain the same, while annual emissions due to transportation would decrease to 6,746,990 kg and emissions due to treatment would decrease to 83,024,130 kg annually. Biologic CO2 uptake of the impacted sites without removal of mine discharge is 22.6 kg CO2/day, or ≈8,233 kg CO2/year. Carbon uptake using data from reference sites is 32.9 kg CO2/day, or ≈12,010 kg CO2/year. The difference in carbon uptake is 10.3 kg CO2/day, equivalent to an annual difference of 3,760 kg of CO2. If water were sourced for only one well, biologic carbon uptake would not change because the ecosystem would not have sufficient time to recover. If extraction of mine water were constant, the stream system would be allowed to partially recover and the carbon uptake would increase.
The carbon footprint for freshwater use from the Ohio River accounted for pumping, transportation and reduction in primary productivity due to water removal. The same primary productivity factor was used as was used for the Corning case studies for comparability, but rather than a change in primary productivity rate and flow volume, only a change in flow volume was used in the calculation. The reduction in primary productivity amounted to 11.2 kg/day or 4093 kg/year. Like the Corning scenarios, the change in primary productivity was only included in the annual calculation.
The carbon footprint of using freshwater from Seneca Lake was calculated based only on pumping and transportation; primary production was not included since the decrease in water level due to extraction for hydraulic fracturing is a small fraction of the lake’s typical drawdown.
Discussion
The sources and volume of uncontaminated freshwater are limited (Postel 1992). With this growing concern, more focus is being placed on the water use in hydraulic fracturing (Nicot and Scanlon 2012). Of the three possible sources of water for hydraulic fracturing, treated mine water had the largest carbon footprint. There was considerable CO2 emitted due to the extensive multi-step treatment of the discharge and the transportation to and from the industrial treatment plant. The emissions were reduced by on-site treatment by just less than 20 %. Of course, if the mine water discharge was closer to the drilling site, as they likely would be at some sites, this aspect would become less significant.
The millions of liters required for hydraulic fracturing fluid for one well is multiplied when considering the number of wells in use and the number of times these wells must be stimulated (fracked). When most of the operators are using freshwater as the source for the fluid, many ecosystems may be significantly altered, especially in areas that are already water scarce (Dillon 2011), since there is less available habitat for aquatic organisms, such as various invertebrates and plants. Along with reducing the volume of freshwater, decreased stream flow can affect an ecosystem by threatening survival of organisms that thrive in streams with a strong flow. Reduced baseflow also reduces the potential of a stream to dilute pollutants and contaminants that enter the stream (Ferrar 2010). This concern has led regulators and companies to consider mine water as a source of water for hydraulic fracturing (PDEP 2013). New treatment technologies may reduce the impact of mine water reuse.
It is clear that the need for water for hydraulic fracturing is a concern. Based on this analysis, water reuse from mine drainage is carbon intensive; however, other factors go into the selection of water sources for hydraulic fracturing. The scenario used here considers an existing abandoned mine discharge causing impaired aquatic biology that is closer to the epicenter of shale drilling in Ohio than many other high flow mine discharges in Ohio (Bowman 2012; Bowman et al. 2013); however, Corning is still 190 km from Carrollton. Other scenarios may include pumping from underground mine pools or receiving water from nearby active mining operations. If mine water did not need intensive treatment or just minimal treatment on-site (either before or after transportation) were possible, mine water reuse for hydraulic fracturing would have a carbon footprint closer to that of sourcing freshwater from large water bodies.
Conclusions
The carbon footprints of freshwater sources were significantly smaller than using treated mine water. Annually, using treated mine water would emit 110,000 t of CO2-e (CO2 equivalent) if trucked to an existing treatment plant or 90,000 t of CO2-e if treated on-site, while using freshwater from the Ohio River would emit 2,000 t of CO2-e, and using freshwater from Seneca Lake would emit 4,500 t of CO2-e, annually. The CO2 emitted during the pumping process was the same for each source, but the distance traveled using freshwater was much smaller than that for the transportation of the mine discharge. The process of wastewater treatment for the mine water was also a large contribution to the carbon footprint of mine water reuse, since the freshwater sources require no treatment before use in hydraulic fracturing. However, greater emphasis is being placed on finding alternate sources of water for hydraulic fracturing to conserve available freshwater, especially in water-scarce regions.
References
Baker Hughes (2009) Baker Hughes Announces August 2009 Rig Counts. http://www.bakerhughes.com. Accessed 22 Nov 2013
Baker Hughes (2013) Baker Hughes Announces August 2013 Rig Counts. http://www.bakerhughes.com. Accessed 22 Nov 2013
Bauers CK (2003) Whole stream metabolism and detrital processing in streams impacted by acid mine drainage. Unpubl thesis, Ohio Univ, Athens, OH, USA
Bowman JR (2012) Comprehensive non-point source (NPS) monitoring report for acid mine drainage (AMD). Voinovich School of Leadership and Public Affairs, Ohio Univ, Athens
Bowman JR, Kruse NA, Migliore E, Gilliom R (2013) Regional baseline groundwater quality report for Athens, Belmont and surrounding counties. Voinovich School of Leadership and Public Affairs, Ohio Univ, Athens
Breul H, Doman L (2013) U.S. expected to be largest producer of petroleum and natural gas hydrocarbons in 2013. U.S. Energy Information Administration, Washington, DC
CEPA (California Environmental Protection Agency) (2009) Detailed California-modified GREET pathway for Corn Ethanol, Air Resources Board, Stationary Source Div, Sacramento, CA, USA. http://www.arb.ca.gov/fuels/lcfs/012009lcfs_cornetoh.pdf. Accessed 16 April 2014
Cravotta CA III (2008) Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 1: constituent quantities and correlations. Appl Geochem 23:166–202
de Witt WJ, Roen JB, Wallace LG (1993) Stratigraphy of Devonian black shales and associated rocks in the Appalachian basin. USGS Bulletin 1909-B, Washington, DC
Dillon M (2011) Water scarcity and hydraulic fracturing in Pennsylvania: examining Pennsylvania water law and water shortage issues presented by natural gas operations in the Marcellus shale. Temple Law Rev 84:201–246
Dormer A, Finn DP, Ward P, Cullen J (2013) Carbon footprint analysis in plastics manufacturing. J Clean Prod 51:133–141
Downing B (2013) Ohio Utica Shale: BHP Billiton to expand in U.S. shale oil, gas drilling. Akron Beacon J. http://www.ohio.com. Accessed 22 Nov 2013
Downing B (2014) 35 Pennsylvania eco-groups oppose SB 411 in letter. Akron Beacon J. http://www.ohio.com. Accessed 17 March 2014
Dunnahoe T (2013) Unconventional oil and gas report: Ohio’s Utica shale development has room to grow. Oil Gas J 111(10)
Eaton TT (2013) Science-based decision-making on complex issues: Marcellus shale gas hydrofracking and New York City water supply. Sci Total Environ 461–462:158–169
Ecofys (2009) Methodology for the free allocation of emission allowances in the EU ETS post 2012, Sector report for the lime industry. http://ec.europa.eu/clima/policies/ets/cap/allocation/docs/bm_study-lime_en.pdf. Accessed 1 Dec 2013
EIA (Energy Information Administration) (2014) Frequently asked questions: how much coal, natural gas, or petroleum is used to generate a kilowatt hour of energy? http://www.eia.gov/tools/faqs/faq.cfm?id=667&t=2. Accessed 17 March 2014
Environmental Protection Agency (2007) eGRID2006, version 2.1. http://www.epa.gov/cleanenergy/documents/egridzips/eGRID2006V2_1_Summary_Tables.pdf. Accessed 1 Dec 2013
Environmental Protection Agency (2009) Endangerment and cause or contribute findings for greenhouse gases, section 202(a) of the Clean Air Act; Final Rule. Federal Register 74: 66496–66546, Washington, DC
Ohio Environmental Protection Agency (OEPA) (2011) Drilling for natural gas in the Marcellus and Utica Shales: environmental regulatory basics. http://epa.ohio.gov/Portals/0/general%20pdfs/generalshale711.pdf. Accessed 23 July 2014
Environmental Protection Agency (2013) Standards of performance for greenhouse gas emissions from new stationary sources: electric utility generating units. Federal Register 79(5):1430–1519, Washington, DC
EPA’s Center for Corporate Climate Leadership (2011) Emission factors for greenhouse gas inventories. http://www.epa.gov/climateleadership/documents/emission-factors.pdf. Accessed 23 July 2014
Ferrar K (2010) Do the natural gas industry’s surface water withdrawals pose a health risk? Fracktracker Administrator. http://www.fractracker.org/2010/09/do-the-natural-gas-industry%E2%80%99s-surface-water-withdrawals-pose-a-health-risk/. Accessed 2 Dec 2013
Gleick PH (1994) Water and energy. Annu Rev Energ Environ 19:267–299
Global Acid Rock Drainage (GARD) Guide (2010) Drainage treatment. Ch 7.0. http://www.gardguide.com/index.php/Chapter_7. Accessed 1 Dec 2013
Global Action Plan (GAP), Stockholm Environment Institute, Eco-Logica Ltd (2006) UK Schools Carbon Footprint Scoping Study. Sustainable Development Commission, London
Ground Water Protection Council, ALL Consulting (2009) Modern Shale Gas development in the United States: a primer. Prepared for the US DOE, Office of Fossil Energy, and NETL, Pittsburgh, PA, USA
Gustavsson DJI, Tumlin S (2013) Carbon footprints of Scandinavian wastewater treatment plants. Water Sci Technol 68:887–893
Gyure RA, Konopka A, Brooks A, Doemel W (1987) Algal and bacterial activities in acidic (pH 3) strip mine lakes. Appl Environ Microbiol 53:2069–2076
Harte J (1983) Water constraints on energy development: a framework for analysis. J Am Water Resour As 19:51–57
Stone JJ, Hengen, T, Squillace, M (2012) Life cycle assessment analysis for active and passive acid mine drainage treatment. Presented, life cycle assessment XII conference, Tacoma, WA, USA
Hill EW, Kinahan KL (2013) Ohio Utica Shale Gas Monitor. Maxine Goodman Levin College of Urban Affairs Cleveland State Univ, Cleveland, OH, USA. http://www.urban.csuohio.edu/publications/hill/OhioUticaShaleGasMonitor_Aug_20_2013.pdf. Accessed 1 Dec 2013
Institute for Energy Research (2013) Ohio: an energy and economic analysis. http://instituteforenergyresearch.org/analysis/ohio-an-energy-and-economic-analysis/. Accessed 23 July 2014
IPCC (2007) Climate change 2007: synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, Switzerland
Jenner S, Lamadrid AJ (2012) Shale gas vs. coal: policy implications from environmental impact comparisons of shale gas, conventional gas, and coal on air, water and land in the United States. Energy Policy 53:442–453
Kaksonen AH, Puhakka JA (2007) Sulfate reduction based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng Life Sci 7(6):541–564
Kerr R (2010) Natural gas from shale bursts onto the scene. Science 328:1624–1626
Konieczny P, Dobrucka R, Mroczek E (2013) Using carbon footprint analysis to evaluate environmental issues of food transportation. Sci J Logist 9:3–10
Levings CD, Varela DE, Mehlenbacher NM, Barry KL, Piercey GE, Guo M, Harrison PJ (2005) Effect of an acid mine drainage effluent on phytoplankton biomass and primary production at Britannia Beach, Howe Sound, British Columbia. Mar Pollut Bull 50:1585–1594
Moniz EJ, Jacoby HD, Meggs AJM (2011) The future of natural gas: an interdisciplinary MIT study. Massachusetts Institute of Technology, Cambridge
Morgan MD (1985) Photosynthetically elevated pH in acid water with high nutrient content and its significance for the zooplankton community. Hydrobiologia 128:239–247
Neculita CM, Zagury GJ, Bussiere B (2007) Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: critical review and research needs. J Environ Qual 36:1–16
Nicot JP, Scanlon BR (2012) Water use for shale-gas production in Texas, U.S. Environ Sci Technol 46:3580–3586
Nixdorf B, Krumbeck H, Jander J, Beulker C (2003) Comparison of bacterial and phytoplankton productivity in extremely acidic mining lakes and eutrophic hard water lakes. Acta Oceologica 24:S282–S288
Niyogi DK, Lewis WMJ, McKnight DM (2002) Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 5:554–567
Obama B (2013) The President’s climate action plan. White House Executive Office of the President, Washington, DC
Ohio Department of Natural Resources (2013) Utica/Point Pleasant Shale Wells: Week of 11/10/2013-11/16/2013. Div of Oil and Gas Resources Mgmt, Columbus
Ohio Watershed Data (2013) Sunday Creek surface water data. www.watersheddata.com. Accessed 2 Nov 2013
Parkhill KL, Gulliver JS (2002) Effect of inorganic sediment on whole-stream productivity. Hydrobiologia 472:5–17
PDEP (PA Dept of Environmental Protection) (2013) White paper: utilization of Mine Influenced Water for Natural Gas Extraction Activities. Harrisburg, PA, USA. http://www.bipc.com/files/media/misc/6c8353f92ee6a9c7b973a78c1edd462f.pdf
Postel S (1992) Last oasis: facing water scarcity. WW Norton & Company Ltd, London
Press TERI (2013) Ohio’s resurgent natural gas industry spends millions to set up shop. Energy First 1:10
RSK Environmental Ltd (2002) Atmospheric emissions inventories-methods and assumptions. South Caucasus Pipeline Expansion Project Environmental and Social Impact Assessment, Helsby
Tatton MJ, Archer DB, Powell GE, Parker ML (1989) Methanogenesis from ethanol by defined mixed continuous cultures. Appl Environ Microbiol 55(2):440–445
Tett SFB, Stott PA, Allen MR, Ingram WJ, Mitchell JFB (1999) Causes of twentieth-century temperature change near the Earth’s surface. Nature 399:569–572
U.S. Army Corps of Engineers (2014) Senecaville Lake. http://www.lrh-wc.usace.army.mil/wc/sesns.htm. Accessed 18 March 2014
Wackernagel M, Rees W (1962) Our ecological footprint: reducing human impact on the Earth. New Society Publ, Gabriola Island
Weidema BP, Thrane M, Christensen P, Schmidt J, Lokke S (2008) Carbon footprint: a catalyst for life cycle assessment. J Ind Ecol 12:3–6
Wiedmann T, Minx J (2008) A definition of ‘carbon footprint’. In: Pertsova CC (ed) Ecological economics research trends. Nova Science, Hauppauge, pp 1–11
Wright LA, Kemp S, Williams I (2011) ‘Carbon footprinting’: towards a universally accepted definition. Carbon Manage 2:61–72
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macy, T.R., Kruse, N.A. & Stuart, B.J. Carbon Footprint Analysis of Source Water for Hydraulic Fracturing: A Case Study of Mine Water Versus Freshwater. Mine Water Environ 34, 20–30 (2015). https://doi.org/10.1007/s10230-014-0291-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-014-0291-7