Abstract
A review of the validity of morphological traits defining the scorpionfish genus Parascorpaena Bleeker 1876 resulted in the re-identification of Parascorpaena bandanensis (Bleeker 1851) as Sebastapistes strongia (Cuvier 1829), reducing the number of valid species of Parascorpaena from nine to eight. A modified definition of Parascorpaena, based on morphological characters observed among the valid species, includes: usually complete lateral line, continuing onto the caudal-fin base; second to fifth or sixth pectoral-fin rays branched; usually two or three suborbital spines; body covered with cycloid scales; lower jaw slightly shorter than upper jaw; palatine teeth present; villiform teeth on upper jaw; distinct posterior lacrimal ridge instead of a developed spine; simple anterior and posterior lacrimal spines without additional spinous points; and a posterior lacrimal spine (PLS) oriented strongly forward, with smaller specimens exhibiting ventral orientation with anterior curvature, and those smaller than 20 mm standard length with the PLS oriented postero-ventrally. A concatenated phylogenetic tree constructed using mitochondrial cytochrome oxidase I (COI), 16S ribosomal ribonucleic acid (16S rRNA), and recombination activating gene 1 (RAG1) markers, demonstrated the monophyletic nature of Parascorpaena. In addition, molecular analysis placed Parascorpaena closer to a group represented by Sebastapistes mauritiana (Cuvier 1829) within the paraphyletic Sebastapistes Gill in Streets 1877.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The scorpionfish genus Parascorpaena Bleeker 1876, features palatine teeth, erect spines on the head and suborbital region, an occipital depression, a scaly thoracic and preventral region, as observed in the type species Scorpaena picta Cuvier in Cuvier and Valenciennes 1829. Bleeker (1876) also reclassified Scorpaena bandanensis Bleeker 1851 to Parascorpaena. Subsequently, Herre (1952) provided additional characteristics of Parascorpaena, including: oblong, compressed body; cutaneous filaments and appendages sometimes present on head and body; villiform teeth on both jaws, vomer and palatines; either ctenoid or cycloid scales; 36–60 scale rows in longitudinal series; scales absent on dorsal and anal fins; head naked (except behind eyes and on upper half of opercle), with spiny crests above; spines on preorbital; 2 opercular spines; 3–5 preopercular spines; dorsum of head more or less deeply grooved; 8–11 dorsal-fin soft rays; 3 anal-fin spines with 5 or 6 soft rays; branched dorsal-, anal-, and caudal-fin rays; non-elongated pectoral rays, upper rays branched or all rays unbranched, no free rays; and 7 branchiostegals. Smith (1957) further refined the definition of Parascorpaena based on a combination of characters, including: absence of chin barbels; fewer than 20 pectoral-fin rays; an anteriorly hooked lower hind preorbital spine; cycloid scales at most feebly crenulate; lateral line extending to caudal-fin base; 12 dorsal-fin spines connected by membrane; teeth absent on vomer but present on palatines; and fewer than 14 (total) gill rakers on lower part of outer gill arch. Based on these distinctive features, he then re-assigned Scorpaena aurita Rüppell 1838 to Parascorpaena and described a new species, namely Parascorpaena maculipinnis Smith 1957. Later, Dor (1984) re-assigned Scorpaena mossambica Peters 1855 to Parascorpaena without explanation.
Eschmeyer’s (1986) concept of Parascorpaena, to which he re-assigned Scorpaena mcadamsi Fowler 1938, included new characters plus a combination of some described by Bleeker (1876), Herre (1952), and Smith (1957), including a moderately compressed body, well-developed head spines, lacrimal with 2 spines over maxilla, the long posterior spine outwardly curved and anteriorly hooked (less so in young), 8–10 dorsal-fin soft rays, 14–18 pectoral-fin rays, and 35–45 scale rows in longitudinal series. Following that generic diagnosis, Motomura et al. (2011a) confirmed the taxonomic validity of Parascorpaena armata (Sauvage 1873) and Parascorpaena moultoni (Whitley 1961), clarifying the distribution range of P. armata in the Pacific Ocean, and restricting P. mossambica solely to the Indian Ocean. Furthermore, Motomura et al. (2011a) distinguished P. moultoni from P. mcadamsi, previously thought to be synonymous, based on the number of suborbital spines: two in P. moultoni, three in P. mcadamsi. The most recent addition to Parascorpaena is Parascorpaena poseidon Chou and Liao 2022, described from collections in Taiwan, resulting in a total of nine valid species of the genus Parascorpaena to date.
Although Mandrytsa (2001) categorized Parascorpaena as a subgenus of Scorpaena Linnaeus 1758, subsequent research recognized Parascorpaena as a distinct genus (Fricke 2005; Motomura et al. 2005, 2011a, 2015; Motomura 2009; Allen and Erdmann 2012; Larson et al. 2013; Delrien-Trottin et al. 2015; Mohapatra et al. 2015; Fricke et al. 2015, 2018; Koeda et al. 2016; Nelson et al. 2016; Golani and Fricke 2018; Kwik and Lim 2020; Emel’yanova and Pavlov 2021; Wibowo and Motomura 2021; Chou and Liao 2022; Poss and Motomura 2022; Mochizuki et al. 2023; Mochizuki and Motomura 2024). While the relationship of the genus Parascorpaena to other genera in the overall family Scorpaenidae has been explored (Ishida 1994; Lautredou et al. 2013), monophyly of the former has at no time been demonstrated.
Currently, the genus Parascorpaena is distinguished from other related genera by having two lacrimal spines on the ventral margin, with the posterior lacrimal spine (PLS) oriented anteriorly, and cycloid scales (Smith 1957; Eschmeyer 1986; Poss 1999; Motomura et al. 2005, 2009; Poss and Motomura 2022). However, P. bandanensis has ctenoid scales and a postero-ventral PLS (Bleeker 1851; Herre 1952; de Beaufort and Briggs 1962; Allen and Erdmann 2012; this study). Additionally, anterior orientation of the PLS is a defining trait in Parascorpaena, yet it may not be evident in smaller specimens, often resulting in misplacement within Scorpaena or Sebastapistes Gill in Streets 1877. Such inconsistency in these diagnostic traits has necessitated a review of the key generic characters. Consequently, the definition of Parascorpaena has been modified to include the morphological features of all species herein considered valid. In addition, the monophyly were confirmed on the basis of molecular evidence.
Materials and methods
Standard length (SL) was measured to the nearest 0.1 mm with digital calipers. Head spine terminology follows Motomura et al. (2009) and Wibowo and Motomura (2021). Institutional codes follow Sabaj (2020). Curatorial procedures for KAUM specimens followed Motomura and Ishihara (2013).
DNA extractions were carried out using preserved muscle tissue subsamples in 99.5% ethanol, following the manufacturer’s protocols specified for the Wizard Genomic DNA Purification Kit (Promega Inc.). Polymerase chain reaction (PCR) amplifications were performed for the three different gene markers: two mitochondrial gene markers—cytochrome oxidase subunit I (COI), 16S ribosomal ribonucleic acid (16S rRNA), and a nuclear DNA locus of the recombination activating gene 1 (RAG1). The primers used in this study were based on Ward et al. (2005) and Ivanova et al. (2007) for COI, Palumbi et al. (1991) for 16S rRNA, and López et al. (2004) and Chen et al. (2007) for RAG1, as listed in Electronic Supplementary Material Table S1 (ESM Table S1).
The thermocycler conditions for the amplification of the three gene markers were as follows: for the COI gene marker, pre-denaturation at 94°C for 30 seconds (s), 30 cycles of denaturation at 94°C for 30 s, annealing at 48°C for 30 s, extension at 72°C for 30 s, and a final extension step at 65°C for 10 minutes (mins); 16S rRNA amplification followed a similar pattern with pre-denaturation at 94°C for 3 mins, 30 cycles of denaturation at 94°C for 45 s, annealing at 50°C for 45 s, extension at 72°C for 2.5 mins, and final extension at 72°C for 5 mins; for RAG1, 30 cycles of denaturation at 94°C for 35 s, annealing at 56°C for 30 s, and an extension at 72°C for 1 min 15 s. The PCR products were purified using Sephadex G-50 Fine (Cytiva). Subsequently, automated sequencing was performed bidirectionally, using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), and was analyzed on a model 3730xl DNA Analyzer (Applied Biosystems). The sequences obtained from 18 species were aligned using Clustal X (Thompson et al. 1994) and deposited in GenBank. The corresponding accession numbers are provided in Table 1, with the exception of the sequences pertaining to P. mossambica complex. MEGA X software (Kumar et al. 2018) was employed for optimal evolutionary modeling (Nei and Kumar 2000) and subsequent analyses, including generating a maximum likelihood tree using the Kimura 2-parameter model (Kimura 1980) with 1,000 bootstrap replications (Felsenstein 1985).
Comparative material: Caracanthus maculatus (Gray 1831): KAUM–I. 169070, 22.8 mm SL, Nazumado, Okago, Hachijo-jima Island, Izu Islands, Tokyo, Japan, 33°08.42′N, 139°44.19′E, 5–20 m. Neochirus brachypterus (Cuvier in Cuvier and Valenciennes 1829): KAUM-I. 167316, 112.9 mm SL, east of Kozakiyama, Kasasa, Minami-satsuma, Kagoshima, Japan, 31°25.44′N, 130°10.25′E, 36 m. Parascorpaena aurita: KAUM–I. 7642, 109.8 mm SL, off Ginowan, Okinawa-jima Island, Okinawa, Japan, 26°15–20′N, 127°42–45′E, 10–15 m; KAUM–I. 7646, 118.0 mm SL, off Isyado, Nakagusuku, Nakagami, Okinawa-jima Island, Okinawa, Japan, 26°16′03″N, 127°48′30″E, 2.5 m; KAUM–I. 79238, 95.9 mm SL, Kasari Bay, Kasari, Amami-oshima Island, Amami Islands, Kagoshima, Japan, 28°30′N, 129°39′E, 17 m; KAUM–I. 122877, 41.6 mm SL, KAUM–I. 161814, 44.2 mm SL, off Bandokorobana National Park, Beppu, Ei, Minami-kyushu, Kagoshima, Japan, 31°14′50″N, 130°26′00″E, 0.3–0.6 m; ROM 74350 (1 of 4), 19.7 mm SL, Hon Chong (Chong Island), Nha Trang Bay, Vietnam, 12°16′27″N, 109°12′8″E. Parascorpaena mcadamsi: AMS I. 33746-087 (1 of 2), 21.0 mm SL, southeast side of Boot Reef, Coral Sea, 10°2′43″S, 144°41′52″E; KAUM–I. 115044, 43.7 mm SL, south of Kiriishi Port, Suwanose-jima Island, Tokara Islands, Kagoshima, Japan, 29°36′34″N, 129°42′50″E, 15–18 m. Parascorpaena mossambica complex: KAUM–I. 72115, 53.8 mm SL, off north of Sanekumisaki, Setouchi, Amami-oshima Island, Amami Islands, Kagoshima, Japan, 28°11′N, 129°11′E, 10–24 m; KAUM–I. 89146, 80.2 mm SL, tidepool east of Dafu Port, Xiaoliuqiu, Liuqiu, Taiwan, 22°20′14″N, 120°22′41″E, 0.3 m; KAUM–I. 153105, 62.3 mm SL, Okidomari Port, Okinoerabu-jima Island, Amami Islands, Kagoshima, Japan, 27°23′48″N, 128°33′13″E, 2–4 m. Parascorpaena moultoni: KAUM–I. 72114, 60.9 mm SL, north of Sanekumisaki, Setouchi, Amami-oshima Island, Amami Islands, Kagoshima, Japan, 28°11′43″N, 129°11′32″E, 10–24 m. Parascorpaena picta: KAUM–I. 20964, 121.4 mm SL, north end of Supply Bay, Townshead Island, Australia, 22°12.14′S, 150°28.32′E, 0–0.2 m. Scorpaena brevispina Motomura and Senou 2008: KPM-NI 16667, holotype, 116.1 mm SL, off Futo, Ito, east coast of Izu Peninsula, Shizuoka Prefecture, Japan, 34°52′N, 139°08′E, 45 m depth; KPM-NI 65197, 70.7 mm SL, Sagami Bay, Kanagawa Prefecture, Japan. Scorpaena jacksoniensis Steindachner 1866: KAUM–I. 35883, 111.7 mm SL, 100 m off Fairlight Beach, Sydney Harbour, NSW, Australia, 33°48′04″S, 151°16′29″E, 2–8 m. Scorpaena neglecta Temminck and Schlegel 1843: KAUM–I. 86597, 76. 3 mm SL, East China Sea, 31°24′43″N, 127°32′00″E, 128–129 m; KAUM–I. 157984, 241.6 mm SL, off Shima-kishi, Itoshima, Fukuoka, Japan, 33°34′N, 130°07′E. Scorpaena pepo Motomura et al. 2007: KAUM–I. 55849, 178.5 mm SL, between Tanega-shima and Yaku-shima Islands, Osumi Islands, Kagoshima, Japan, >100 m. Scorpaenopsis diabolus (Cuvier 1829): KAUM–I. 134822, 169.8 mm SL, Irie Beach, Nakasato, Kikai-jima Island, Amami Islands, Kagoshima, Japan, 28°21′07″N, 129°55′08″E, 0.1–1.0 m. Scorpaenopsis neglecta Heckel 1837: KAUM–I. 175622, 152.2 mm SL, Genkainada Sea, Fukuoka, Japan. Scorpaenopsis papuensis (Cuvier in Cuvier and Valenciennes 1829): KAUM–I. 160605, 52.9 mm SL, Isso, Yaku-shima Island, Osumi Islands, Kagoshima, Japan, 30°27′33″N, 130°28′43″E, 5–15 m. Scorpaenopsis vittapinna Randall and Eschmeyer 2002: KAUM–I. 176539, 37.9 mm SL, Doren, Setouchi, Kakeroma-jima Island, Amami Islands, Kagoshima, Japan, 28°06′45″N, 129°20′44″E, 3–15 m. Sebastapistes fowleri (Pietschmann 1934): KAUM–I. 58426, 31.6 mm SL, off Tomori, southwest coast of Yoron-jima Island, Amami Islands, Kagoshima, Japan, 27°01′N, 128°24′E, 12–28 m. Sebastapistes mauritiana (Cuvier in Cuvier and Valenciennes 1829): USNM 423398, 41.3 mm SL, Austral Islands, Tubuai, French Polynesia. Sebastapistes strongia (Cuvier in Cuvier and Valenciennes 1829): KAUM–I. 111242, 48.1 mm SL, off southeast Dongsha Island, Dongsha Atoll, Taiwan, 20°40′07″N, 116°46′01″E, 8–10 m; SMNS 10632, holotype of Scorpaena bandanensis (Fig. 1), 52.6 mm SL, Banda Neira, Banda Islands, Indonesia. Sebastapistes taeniophrys (Fowler 1943): USNM 99522, holotype of Scorpaena taeniophrys, 19.3 mm SL, Cammahala Bay, Luzon, Philippines. Sebastapistes tinkhami (Fowler 1946): KAUM–I. 57664, 28.8 mm SL, Kaitsuzaki, Setouchi, Amami Islands, Kagoshima, Japan, 28°06′34″N, 129°22′34″E, 2.0–18.0 m.
The following abbreviations are used in this paper: ALS (anterior lacrimal spine), PLS (posterior lacrimal spine), ALR (anterior lacrimal ridge), PLR (posterior lacrimal ridge), ADLS (antero-dorsal lacrimal spine), LLS (lateral lacrimal spine), AALS (additional spine along anterior lacrimal spine), and SR (first suborbital ridge). Specimens depicted in Figs. 2, 3, 4 were stained using cyanine blue to enhance visualization of particular features.
Size-dependent variations on the posterior lacrimal spine (PLS) in Parascorpaena, described as strongly hooked anteriorly (a–b), oriented ventrally with anterior curvature (c), and oriented postero-ventrally (d). a Parascorpaena aurita (KAUM–I. 7646), 118.0 mm SL; b P. picta (KAUM–I. 20964), 121.4 mm SL; c P. mcadamsi (AMS I. 33746-087), 21.0 mm SL); d P. aurita (ROM 74350), 19.7 mm SL. Scale bar: 1 mm
Spines and ridges along the lacrimal bone of Parascorpaena aurita (a KAUM–I. 7642) and Scorpaena jacksoniensis (b KAUM–I. 35883). Abbreviations: ALR anterior lacrimal ridge, PLR posterior lacrimal ridge, ADLS antero-dorsal lacrimal spine, LLS lateral lacrimal spine, ALS anterior lacrimal spine, AALS additional spine along anterior lacrimal spine, PLS posterior lacrimal spine, SR first suborbital ridge
Parascorpaena Bleeker 1876
Parascorpaena Bleeker 1876: 295 (type species: Scorpaena picta Cuvier 1829; type by original designation).
Revised diagnosis. A genus of the family Scorpaenidae, characterized by the following combination of characters: usually complete lateral line, pored lateral-line scales continuing onto caudal-fin base (incomplete, continuing forward caudal-peduncle area in small specimens of P. moultoni); usually two or three suborbital spines; body covered with cycloid scales; usually second to fifth or sixth pectoral-fin rays branched (unbranched in small specimens); lower jaw slightly shorter than upper jaw; villiform teeth on upper jaw, canine-like teeth absent; palatine teeth present; posterior lacrimal ridge present; anterior and posterior lacrimal spines simple, without additional spinous points, PLS oriented anteriorly (small specimens <20 mm SL with PLS oriented postero-ventrally).
Description. Body size ranging from small to moderately large, characterized by deep, moderately compressed morphology. Head moderate in size; head length usually less than half of SL. Snout fairly blunt; dorsal profile steep. Eyes large; diameter usually slightly longer than snout length. Mouth large; maxilla extending just below or slightly beyond posterior margin of eye; four prominent pairs of mandibular pores on dentary, first pore positioned just behind tip of lower jaw, second pore along anterior lacrimal spine, third pore along posterior lacrimal spine and fourth pore behind posterior lacrimal spine (before posterior end of maxilla). Teeth on upper jaw villiform; teeth on lower jaw varying among species, from entirely villiform to canine-like on frontal area (e.g., P. mcadamsi); teeth on palatines small, villiform, in bands; teeth on vomerine villiform, in V-shaped patch. Body entirely covered with cycloid scales (rarely with weak ctenii); body scales extending to dorsal- and anal-fin soft ray membranes in some species (e.g., P. aurita); head mostly naked, a few scales behind posterior margin of eye and along posterior end of operculum, just behind opercular spines; dentary smooth, naked.
Dorsal-fin spines usually 12 (rarely 13) connected to soft rays; fourth or fifth spine longest; sixth to 11th spine gradually decreasing in size; 12th spine elongated, followed by usually 9 (rarely 8 or 10) branched soft rays; second dorsal-fin soft ray usually longest; seventh to tenth dorsal spines bearing black blotch in males in some species (e.g., P. mcadamsi). Anal-fin spines three, second spine longest; usually five branched soft rays. Pectoral-fin rays varying among species, ranging from usually 15 or 16 to 17 or 18; first (uppermost) ray simple, unbranched; second to fifth or sixth ray branched; lower rays thickened, unbranched; tips of fins variable, either just reaching or not reaching anal-fin spine base. Pelvic-fin length variable, not or just reaching anal-fin spine base.
Head spination. Nasal spines positioned bilaterally on nasal ridge, extending slightly beyond its height. Preocular spine relatively thick with broad base, located anteriorly within orbital region. Interorbital ridge originating either from mid-eye, anterior mid-eye, or along preocular-spine base, extending beyond posterior eye margin; rear end of ridge variable, forming a broad or faint loop, or distinct loop absent. Occipital pit variable, ranging from relatively deep to shallow, or not distinct. Supraocular and postocular spines situated above orbital region, close to one another. Supraocular spines occasionally possessing tentacle of variable size, from small (smaller than eye diameter; e.g., P. mcadamsi) to long (longer than eye diameter; e.g., P. mossambica). Tympanic spines simple, just behind postocular spine, usually separated by distance greater than that between spines on parietal and nuchal. Parietal and nuchal spines simple, close to one another; parietal spine originating posterior to origin of pterotic spine; nuchal spine originating just behind parietal spine. Sphenotic spine just behind posterior margin of eye, varying intraspecifically – sometimes singular, commonly in unevenly-sized pairs, and rarely in triplicate or lump-like. Pterotic spine simple, attached to skin, situated just behind sphenotic spine. Upper and lower post-temporal spines well-developed, uneven in size; upper spine shorter than and positioned just above lower spine; lower spine situated between pterotic and supracleithral spines. Lacrimal bone dorsally with two distinct ridges; ALR located before anterior eye margin, longer than PLR, located just behind origin of first suborbital ridge (just below ventral eye margin). ALS and PLS along ventral region of lacrimal bone, ALS prominently antrorse with tip not extending beyond lower lip; PLS notably hooked anteriorly in larger specimens, ventrally orientated with forward curvature in smaller specimens, postero-ventrally orientated without forward curvature in specimens <20 mm SL. Suborbital ridges variable, comprising a single or two distinct ridges, including two or three suborbital spines. Preopercular spines five; first and second along posterior margin of maxilla, covered with thick skin, usually with small tentacles; third to fifth exposed, progressively longer, fifth longest with small anterior supplemental preopercular spine. Opercular spines just behind pre-opercular margin; upper opercular spine slightly longer than lower opercular spine. Supracleithral spine single, short; located between upper and lower post-temporal spines (but closer to latter). Cleithral spine simple, base covered with operculum; spinous point not extending beyond most posterior tip of operculum. Postorbital spine usually absent; if present, lump-like, lacking a spinous point. Median interorbital ridge, coronal spine, ridge on lateral surface of maxilla, antero-dorsal lacrimal spines, lateral lacrimal spine, and additional spines on anterior lacrimal spine and posterior lacrimal spine all absent.
Taxonomic status of Scorpaena bandanensis. Scorpaena bandanensis was originally described by Bleeker (1851) based on a single specimen collected from Banda Neira, Banda Islands, Indonesia. Weber (1913) noted the resemblance between Scorpaena bandanensis and smaller specimens of P. picta, suggesting that they might be hybrids due to the shared characteristic of undivided pectoral-fin rays. However, this study reveals that juveniles of Parascorpaena naturally exhibit undivided pectoral-fin rays, indicating that the hybrid hypothesis is incorrect. Fowler (1939) originally proposed the genus Oligoscorpaena, designating Scorpaena bandanensis as its type species. However, Fricke et al. (2024) has been synonymized Oligoscorpaena with the genus Sebastapistes, although they have regarded Scorpaena bandanensis as a valid species of Parascorpaena. Currently, Scorpaena bandanensis is recognized as Parascorpaena bandanensis (Herre 1952; de Beaufort and Briggs 1962; Randall and Lim 2000; Fricke 2005; Allen and Erdmann 2012; Fricke et al. 2015; Kwik and Lim 2020).
The genus Parascorpaena is widely recognized for the combination of cycloid scales and anteriorly hooked PLS (Eschmeyer 1986; Poss 1999; Motomura et al. 2005, 2009; Poss and Motomura 2022), with the exception of P. bandanensis, which possess ctenoid scales (de Beaufort and Briggs 1962; Allen and Erdmann 2012). However, examination of the holotype of P. bandanensis (Fig. 1), measuring 52.6 mm SL, revealed that the PLS was oriented postero-ventrally. In fact, the overall description, based on scale type and PLS orientation, aligns P. bandanensis more closely with the genus Sebastapistes than with its presumed congeners. Furthermore, the holotype lacked an occipital pit and coronal spines, and had only two simple spines along the ventral margin of the lacrimal (ALS anteriorly oriented and PLS postero-ventrally oriented), 15 pectoral-fin rays, and three suborbital spines, which aligned P. bandanensis more closely with the characteristics of Sebastapistes strongia. Distinguishing characters, such as the presence of ctenoid scales, two simple spines on the ventral margin of the lacrimal, a posteroventrally oriented posterior lacrimal spine, usually 15 pectoral rays, two or three suborbital spines, and lack of an occipital pit, are consistent with the diagnosis of Sebastapistes strongia given by Poss (1999), Motomura (2009), Allen and Erdmann (2012), Motomura et al. (2011a, 2014), and Poss and Motomura (2022). Consequently, the present study concluded unequivocally that cycloid scales were a characteristic of all species of Parascorpaena, and that P. bandanensis should be treated as a junior synonym of Sebastapistes strongia.
Remarks. The review across all valid species of morphological traits that traditionally distinguished Parascorpaena from other genera has led to a refined definition of the former, based upon morphology. Descriptions of certain morphological characters and head spines, shared by all species, unless specifically stated as restricted to a particular species, are also included here.
Chou and Liao (2022) provided a detailed description of the suborbital spines of P. poseidon, noting the absence of a suborbital ridge. However, their figure 2, which illustrated preserved specimens, shows a distinct ridge evident along the first suborbital spine. It is therefore suggested that P. poseidon may exhibit distinct suborbital ridges, contrary to the description of Chou and Liao (2022). This characteristic has been corroborated in a subsequent redescription of P. poseidon, which delineated two distinct suborbital ridges (Mochizuki and Motomura 2024).
Although, some specimens of Parascorpaena have 13 dorsal spines, that number is considered an abnormality due to its rarity, a characteristic shared with Hoplosebastes Schmidt 1929, Scorpaenodes Bleeker 1857, and Thysanichthys Jordan and Starks 1904 (Jordan and Starks 1904; Eschmeyer 1969; Ishida 1994; Poss 1999; Nakabo 2002; Poss and Motomura 2022; Roy et al. 2022). However, Parascorpaena is easily distinguishable from the other three genera, based on scale type, cycloid scales covering the former compared with ctenoid scales in the others (Jordan and Starks 1904; Eschmeyer 1969; Poss and Motomura 2022; Roy et al. 2022). Additionally, all species of Parascorpaena have palatine teeth, which are absent in Hoplosebastes and Scorpaenodes (Eschmeyer 1969; Ishida 1994; Poss 1999; Poss and Motomura 2022; Roy et al. 2022).
Most species within the genera Parascorpaena (excluding P. maculipinnis and P. poseidon) and Sebastapistes (excluding Sebastapistes coniorta Jenkins 1903, Sebastapistes galactacma Jenkins 1903, and Sebastapistes perplexa Motomura Motomura, Aizawa and Endo 2014) were originally classified under the genus Scorpaena. Shared similarities of the three genera include 12 dorsal spines (rarely 13), three anal spines, some pectoral-fin rays branched, palatine teeth present, and some meristic counts overlapping, including pectoral-fin rays (15–18 in Parascorpaena vs. 13–21 in Scorpaena vs. 14–16 in Sebastapistes), and pored lateral-line scales (16–25 vs. 21–30 vs. 11–27) (Poss 1999; Wibowo and Motomura 2021; Poss and Motomura 2022; Mochizuki et al. 2023). In a cladistic analysis by Ishida (1994), Parascorpaena and Scorpaena were defined in one clade by three derived characters, including absence of a swimbladder, 1 + 2 hypurals, and fused scapula and uppermost radial. However, no derived trait was found that separated the two genera from each other. Because Sebastapistes was not included in the comparison, the myological and osteological relationship within the three genera remained unclear.
The close morphological similarities between Scorpaena and Sebastapistes have been clarified by Wibowo and Motomura (2021), with particular focus on Indo-Pacific species. Following their findings, Scorpaena is distinguished from Sebastapistes by the consistent combined traits of an occipital pit and three suborbital spines (vs. either lacks an occipital pit with 1–3 suborbital spines or has an occipital pit with only two suborbital spines in the latter). Moreover, they characterized Sebastapistes by the absence of a lateral lacrimal spine, a condition shared with Parascorpaena. However, the presence of spinous point on the lateral region of the lacrimal bone in some specimens of Sebastapistes necessitates further examination of this characteristic across the genus.
Nevertheless, Parascorpaena, defined by the combination of cycloid scales and anteriorly oriented PLS (Fig. 2a–c), is distinguished from both Scorpaena (Fig. 2d–f) and Sebastapistes (Fig. 2g–i) which have ctenoid scales and the PLS oriented ventrally (Fig. 2d) or postero-ventrally (Fig. 2g). It should be noted that some specimens of Scorpaena also have a postero-ventrally oriented PLS (Wibowo and Motomura 2021), indicating that this condition is not exclusively limited to species of Sebastapistes (Poss and Motomura 2022).
The anterior orientation of the PLS is one of the defining characters of the genus Parascorpaena, although not apparent in juvenile specimens, as noted by Eschmeyer (1986), Poss (1999), and Poss and Motomura (2022). However, the ventral or postero-ventral orientation of the PLS in some specimens has frequently lead to misplacement of species under Scorpaena or Sebastapistes. Consequently, a PLS orientation-status character is established here, following comparisons across different sizes of specimens of Parascorpaena.
Larger specimens of Parascorpaena (e.g., 118.0 mm SL and 121.4 mm SL as shown in Fig. 3a and 3b, respectively) possess a strongly anteriorly hooked PLS, whereas smaller specimens tend to have a ventrally-oriented PLS, although anteriorly curved (Fig. 3c). The smallest specimen available to this study with anterior curvature was 21.0 mm SL. Specimens of 20.0 mm SL or less had the PLS oriented postero-ventrally, as shown in Fig. 3d, indicating that specimens >20 mm SL with a ventrally- or postero-ventrally-directed PLS should not be placed under Parascorpaena, but referred to either of the genera Scorpaena or Sebastapistes.
The anteriorly-oriented posterior lacrimal spine observed in Parascorpaena is similar to that of Scorpaena canariensis (Sauvage 1878). Nevertheless, species of Parascorpaena are distinguished by cycloid scales, contrasting with the ctenoid scales found in Scorpaena canariensis, as mentioned in Motomura et al. (2005). Additionally, the geographic distribution of these taxa differs, with the genus Parascorpaena is predominantly distributed within the Indo-Pacific region, while Scorpaena canariensis has been exclusively documented within the Atlantic region.
Although scale type can further separate species of Parascorpaena (cycloid scales) from species of Scorpaena and Sebastapistes (ctenoid scales), some species of the latter two genera have cycloid scales, including Scorpaena cardinalis Solander and Richardson in Richardson 1842, Scorpaena jacksoniensis, Scorpaena orgila Eschmeyer and Allen 1971, Sebastapistes pascuensis (Eschmeyer and Allen 1971), and Sebastapistes galactacma (see Richardson 1842; Steindachner 1866; Jenkins 1903; Eschmeyer and Allen 1971; Poss 1999; Motomura et al. 2011b; Wibowo and Motomura 2021). Such inconsistency in scale type may result in misidentification of juvenile specimens, particularly as the defining anterior orientation of PLS in Parascorpaena is not distinct in smaller specimens.
Scorpaena cardinalis, Scorpaena jacksoniensis, and Scorpaena orgila are most closely similar to P. aurita, sharing a combination of characters, such as a well-defined occipital pit, pectoral-fin rays usually 17 (16–18), palatine teeth, and cycloid scales (Richardson 1842; Steindachner 1866; Eschmeyer and Allen 1971; Poss 1999; Motomura et al. 2011b; Wibowo and Motomura 2021). However, all species of Parascorpaena can be easily distinguished from the above species by the absence of a lateral lacrimal spine (vs. lateral lacrimal with two spinous points) and absence of additional ALS and PLS spines (vs. additional ALS and PLS spines present in Scorpaena cardinalis; additional ALS spine present in Scorpaena jacksoniensis) (Motomura et al. 2011b; Wibowo and Motomura 2021). Spines and ridges along the lacrimal bone in P. aurita and Scorpaena jacksoniensis are shown in Fig. 4, which illustrates that the ALS and PLS of all species of Parascorpaena (Fig. 4a) are simple, lacking additional spinous points in contrast to Scorpaena jacksoniensis (Fig. 4b) (Motomura et al. 2011b; Wibowo and Motomura 2021). Moreover, Scorpaena jacksoniensis has both an antero-dorsal lacrimal and lateral lacrimal spine (Motomura et al. 2011b; Wibowo and Motomura 2021), both being absent in Parascorpaena, which instead has a posterior lacrimal ridge positioned behind the first suborbital ridge.
Among the valid species of Parascorpaena, Sebastapistes pascuensis closely resembles P. moultoni, sharing morphological features including cycloid scales, palatine teeth, a shallow occipital pit, two suborbital spines, dorsal-fin rays usually XII, 9, anal-fin rays usually III, 5, and pectoral-fin rays usually 15 or 16. Some specimens in both species exhibit black spots on the dorsal spines (in P. moultoni males only). However, Sebastapistes pascuensis is distinguished by a brown to black spot on the opercle (Eschmeyer and Allen 1971), such being absent in P. moultoni. Live coloration further separates the two species, with Sebastapistes pascuensis being brown to greenish-brown (Eschmeyer and Allen 1971) and P. moultoni rosy-red (Whitley 1961; Mochizuki et al. 2023). On the other hand, a combination of characters, including an undefined occipital pit, three suborbital spines,15 or 16 pectoral-fin rays, coronal spines absent, and black spots present posteriorly on the dorsal spines (in P. mcadamsi males only) suggest that Sebastapistes galactacma is closest to P. mcadamsi. However, the latter has canine-like teeth on the lower jaw in males (vs. villiform teeth in Sebastapistes galactacma) (Jenkins 1903; Poss and Motomura 2022; Mochizuki et al. 2023).
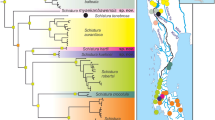
Monophyly. Monophyly of the genus Parascorpaena has at no time been investigated, and the sister taxon of the genus has remained uncertain. In this study, the molecular analysis focused on the four species of Parascorpaena, namely, P. aurita, P. mcadamsi, P. mossambica complex, and P. moultoni. Specimens within the P. mossambica complex are originally classified under P. mossambica; however, molecular analysis indicates that these specimens may actually represent three distinct species. Therefore, this study provisionally identifies these taxa as a complex, pending further confirmation. A dataset of 18 sequences was generated, including representatives from various genera such as Neochirus Chou et al. 2023, Scorpaena, Scorpaenopsis Heckel 1837, Sebastapistes, and Caracanthus Krøyer 1845 as the outgroup. Notably, the specimen initially identified as Dendrochirus brachypterus (Cuvier in Cuvier and Valenciennes 1829) has been reclassified under the proposed new genus Neochirus by Chou et al. (2023). The concatenated sequences utilized in the analysis comprised COI (471 bp), 16S rRNA (501 bp), and RAG1 (528 bp), resulting a total dataset length of 1,500 bp for each sequence. This molecular analysis provided a valuable insight into evolutionary history and taxonomic placement of Parascorpaena within the tribe Scorpaenini (sensu Nelson et al. 2016).
The phylogenetic tree (Fig. 5) constructed from the concatenated sequences revealed the genetic similarities within species of Parascorpaena, affirming the monophyletic nature of the genus. Bootstrap values on distinct clades emphasized the divergence of Parascorpaena from other genera. In addition, species representing Scorpaena and Scorpaenopsis were also found to be monophyletic. However, the paraphyletic nature of species representing Sebastapistes demonstrated a need for further study.
Essentially, despite the morphological similarity of Parascorpaena with both Sebastapistes and Scorpaena, including number of fin spines, palatine teeth, and overlapping meristics (e.g., pectoral-fin rays), as noted above, the molecular evidence suggests a closer affinity between Parascorpaena and Sebastapistes mauritiana. This implies that species of Parascorpaena are more closely related to the Sebastapistes I group, represented by S. mauritiana [and probably Sebastapistes ballieui (Sauvage in Vaillant and Sauvage 1875), a sister species of S. mauritiana], than to Scorpaena and the Sebastapistes II group, represented by S. fowleri and S. strongia.
Although further taxonomic study is needed for the genus Sebastapistes, as implied by the above findings, comparisons on size and distribution depth among three closely related genera were also noted. Species of Parascorpaena and Scorpaena range from small to large, Poss (1999) noting that P. picta can grow to 170.0 mm SL (largest recorded size in the genus), whereas P. mcadamsi reaches a maximum length of only 80.0 mm SL. Species of Scorpaena distributed in the Indo-Pacific region range from 472.5 mm SL in Scorpaena cardinalis (see Wibowo and Motomura 2021) to the smallest species, Scorpaena sororreginae Wibowo and Motomura 2021, at 43.6 mm SL. On the other hand, Sebastapistes are usually small, the largest recorded being Sebastapistes ballieui at 85.9 mm SL (Motomura et al. 2014), with the apparently smallest, Sebastapistes taeniophrys being ca. 35.0 mm SL (Poss and Motomura 2022). Both Parascorpaena (approximately 0–45 m) and Sebastapistes (<50 m) are limited to shallower waters (Poss 1999; Poss and Motomura 2022), whereas species of Scorpaena can be found at various depths, ranging from 0 to 600 m (Wibowo and Motomura 2021).
Mandrytsa (2001) classified both Parascorpaena and Sebastapistes as subgenera of the genus Scorpaena. However, both morphological and molecular evidence presented in this study strongly suggested that both of the former should be recognized as distinct genera. Morphological variations, including scale type, coloration features, and spines along the lacrimal bone, are evident among Parascorpaena, Scorpaena, and Sebastapistes, and the differentiation between closely related species (e.g., between P. aurita and Scorpaena jacksoniensis, and P. moultoni and Sebastapistes pascuensis) further underscores the distinctiveness of the genera. This conclusion was substantiated by the molecular data, revealing that Parascorpaena and Sebastapistes (I and II) each form distinct clades, which are clearly divergent from the clade of Scorpaena, contradicting the classification proposed by Mandrytsa (2001).
References
Allen GR, Erdmann MV (2012) Reef fishes of the East Indies. Vol. 1–3. Tropical Reef Research, Perth
Bleeker P (1851) Bijdrage tot de kennis der ichthyologische fauna van de Banda-eilanden. Nat Tijdschr Ned Ind 2:225–261
Bleeker P (1857) Bijdrage tot de kennis der ichthyologische fauna van de Sangi-eilanden. Nat Tijdschr Ned Ind 13:369–380
Bleeker P (1876) Genera familiae Scorpaenoideorum conspectus analyticus. Versl Akad Amst (Ser 2) 9:294–300
Chen W-J, Ruiz-Carus R, Orti G (2007) Relationships among four genera of mojarras (Teleostei: Perciformes: Gerreidae) from the western Atlantic and their tentative placement among percomorph fishes. J Fish Biol (Suppl B) 70:202–218
Chou T-K, Liao T-Y (2022) A new species of Parascorpaena Bleeker, 1876 (Teleostei: Scorpaenidae) from Taiwan. Zool Stud 61:9
Chou T-K, Liu M-Y, Liao T-Y (2023) Systematics of lionfishes (Scorpaenidae: Pteroini) using molecular and morphological data. Front Mar Sci 10:1109655
Cuvier G (1829) Le Règne Animal, distribué d’après son organisation, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Edition 2. Déterville, Paris
Cuvier G, Valenciennes A (1829) Histoire naturelle des poissons. Vol. 4. F. G. Levrault, Paris
de Beaufort LF, Briggs JC (1962) The fishes of the Indo-Australian Archipelago. Vol 11. Scleroparei, Hypostomides, Pediculati, Plectognathi, Opisthomi, Discocephali, Xenopterygii. E. J. Brill, Leiden
Delrien-Trottin E, Williams JT, Bacchet P, Kulbicki M, Mourier J, Galzin R, de Loma TL, Mou-Tham G, Siu G, Planes S (2015) Shore fishes of the Marquesas Islands, an updated checklist with new records and new percentage of endemic species. Check List 11:1–13
Dor M (1984) Checklist of the fishes of the Red Sea (CLOFRES). Israel Academy of Sciences and Humanities, Jerusalem
Emel’yanova NG, Pavlov DA (2021) Feature of oogenesis and spermatozoa ultrastructure in the fishes of the genera Parascorpaena and Scorpaenopsis (Scorpaenidae). J Ichthyol 61:669–679
Eschmeyer WN (1969) A systematic review of the scorpionfishes of the Atlantic Ocean (Pisces: Scorpaenidae). Occ Pap Calif Acad Sci 79:1–143
Eschmeyer WN (1986) Family no 149: Scorpaenidae. In: Smith MM, Heemstra PC (eds) Smiths' sea fishes. Springer-Verlag, New York, pp 463–478
Eschmeyer WN, Allen GR (1971) Three new species of scorpionfishes (family Scorpaenidae) from Easter Island. Proc Calif Acad Sci (Ser 4) 37:515–527
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fowler HW (1938) Descriptions of new fishes obtained by the United States Bureau of Fisheries steamer “Albatross”, chiefly in Philippine seas and adjacent waters. Proc U S Natl Mus 85:31–135
Fowler HW (1939) New subfamilies, genera and subgenera of fishes. Not Nat Acad Nat Sci Philadelphia 26:1–2
Fowler HW (1943) Contributions to the biology of the Philippine Archipelago and adjacent regions. Descriptions and figures of new fishes obtained in Philippine seas and adjacent waters by the United States Bureau of Fisheries steamer “Albatross”. Bull U S Natl Mus 100(14):i–iii + 53–91
Fowler HW (1946) A collection of fishes obtained in the Riu Kiu Islands by Captain Ernest R. Tinkham, A.U.S. Proc Acad Nat Sci Phila 98:123–218
Fricke R (2005) Types in the fish collection of the Staatliches Museum für Naturkunde in Stuttgart, described in 1845–2004. Stuttg Beitr Naturk A (Biol) 684:1–95
Fricke R, Eschmeyer WN, Van der Laan R (2024) Eschmeyer’s Catalog of Fishes: genera, species, references. https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Electronic version. Accessed 07 July 2024
Fricke R, Golani D, Appelbaum-Golani B (2015) New record of the Mozambique scorpionfish, Parascorpaena mossambica (Peters, 1855) (Actinopterygii: Scorpaeniformes: Scorpaenidae), from Israel, Gulf of Aqaba, Red Sea. Acta Ichthyol Piscat 45:423–425
Fricke R, Mahafina J, Behivoke F, Jaonalison H, Léopold M, Ponton D (2018) Annotated checklist of the fishes of Madagascar, southwestern Indian Ocean, with 158 new records. FishTaxa 3:1–432
Golani D, Fricke R (2018) Checklist of the Red Sea fishes with delineation of the Gulf of Suez, Gulf of Aqaba, endemism and Lessepsian migrants. Zootaxa 4509:1–215
Gray JE (1831) Description of a new genus of percoid fish, discovered by Mr. Samuel Stutchbury, in the Pacific sea, and now in the British Museum. Zool Misc 1:20
Heckel JJ (1837) Ichthyologische beiträge zu den familien der Cottoiden, Scorpaenoiden, Gobioiden und Cyprinoiden. Ann Wien Mus Naturgesch 2:143–164, pls 8–9
Herre AWCT (1952) A review of the scorpaenoid fishes of the Philippines and adjacent seas. Philipp J Sci 80:381–482
Ishida M (1994) Phylogeny of the suborder Scorpaenoidei (Pisces: Scorpaeniformes). Bull Nansei Natl Fish Res Inst 27:1–112
Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN (2007) Universal primer cocktails for fish DNA barcoding. Mol Ecol Notes 7:544–548
Jenkins OP (1903) Report on collections of fishes made in the Hawaiian Islands, with descriptions of new species. Bull U S Fish Com 22:417–511, pls 1–4
Jordan DS, Starks EC (1904) A review of the Scorpaenoid fishes of Japan. Proc U S Natl Mus 27: 91–175
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Koeda K, Hibino Y, Yoshida T, Kimura Y, Miki R, Kunishima T, Sasaki D, Furukawa T, Sakurai M, Eguchi K, Suzuki H, Inaba T, Uejo T, Tanaka S, Fujisawa M, Wada H, Uchiyama T (2016) Annotated checklist of fishes of Yonaguni-jima island, the westernmost island in Japan. The Kagoshima University Museum, Kagoshima
Krøyer HN (1845) Ichthyologiske bidrag. Nat Tidsskr Kjøbenhavn 1:639–649
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kwik JTB, Lim KKP (2020) Scorpionfishes (Teleostei: Scorpaenoidei) of Singapore. Nat Singapore 13:11–26
Larson HK, Williams RS, Hammer MP (2013) An annotated checklist of the fishes of the Northern Territory, Australia. Zootaxa 3696:1−293
Lautredou AC, Motomura H, Gallut C, Ozouf-Costaz C, Cruaud C, Lecointre G, Dettai A (2013) New nuclear markers and exploration of the relationships among Serraniformes (Acanthomorpha, Teleostei): the importance of working at multiple scales. Mol Phylogenet Evol 67:140–155
Linnaeus C (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Laurentii Salvii, Holmiae
López JA, Chen WJ, Orti G (2004) Esociform phylogeny. Copeia 2004:449–464
Mandrytsa SA (2001) Seismosensory system and classification of scorpionfishes (Scorpaenijormes: Scorpanoidei). Perm State Univ Press, Perm [In Russian]
Mochizuki K, Motomura H (2024) Distributional range extension of the shallow water scorpionfish Parascorpaena poseidon (Perciformes: Scorpaenidae), with a revised diagnosis of the species. Species Divers 29:91–98
Mochizuki K, Oyama K, Kume G, Motomura H (2023) Review of distributional records of Parascorpaena mcadamsi and P. moultoni (Scorpaenidae) in Japanese waters, and notes on sexual dichromatism and dimorphism of the two species. Ichthy, Nat Hist Fish Jpn 29:39–57 [In Japanese, English abstract]
Mohapatra A, Tudu PC, Ray D (2015) First report of Mcadam's Scorpionfish Parascorpaena mcadamsi (Fowler, 1938) from Indian waters. J Bombay Nat Hist Soc 112:104–106
Motomura H (2009) Sebastapistes taeniophrys (Fowler 1943): a valid scorpionfish (Scorpaenidae) from the Philippines. Ichthyol Res 56:62–68
Motomura H, Aizawa M, Endo H (2014) Sebastapistes perplexa, a new species of scorpionfish (Teleostei: Scorpaenidae) from Japan. Species Divers 19:133–139
Motomura H, Béarez P, Causse R (2011a) Review of Indo-Pacific specimens of the subfamily Scorpaeninae (Scorpaenidae), deposited in the Muséum national d'Histoire naturelle, Paris, with description of a new species of Neomerinthe. Cybium 35:55–73
Motomura H, Fricke R, Eschmeyer WN (2005) Redescription of a poorly known scorpionfish, Scorpaena canariensis (Sauvage), and a first record of Pontinus leda Eschmeyer from the Northern Hemisphere (Scorpaeniformes: Scorpaenidae). Stuttg Beitr Naturk Ser A (Biol) 674:1–15
Motomura H, Habano A, Arita Y, Matsuoka M, Furuta K, Koeda K, Yoshida T, Hibino Y, Jeong B, Tashiro S, Hata H, Fukui Y, Eguchi K, Inaba T, Uejo T, Yoshiura A, Ando Y, Haraguchi Y, Senou H, Kuriiwa K (2015) The ichthyofauna of the Uji Islands, East China Sea: 148 new records of fishes with notes on biogeographical implications. Mem Fac Fish Kagoshima Univ 64:10–34
Motomura H, Ishihara S (2013) Fish collection building and procedures manual, English edn. The Kagoshima University Museum, Kagoshima and the Research Institute for Humanity and Nature, Kyoto
Motomura H, Poss SG, Shao K-T (2007) Scorpaena pepo, a new species of scorpionfish (Scorpaeniformes: Scorpaenidae) from northeastern Taiwan, with a review of S. onaria Jordan and Snyder. Zool Stud 46:35–45
Motomura H, Sakurai Y, Senou H, Ho HC (2009) Morphological comparisons of the Indo-West Pacific scorpionfish, Parascorpaena aurita, with a closely related species, P. picta, with first records of P. aurita from East Asia (Scorpaeniformes: Scorpaenidae). Zootaxa 2191:41–57
Motomura H, Senou H (2008) A new species of the scorpionfish genus Scorpaena (Scorpaenidae) from Izu Peninsula, Pacific coast of Japan. J Fish Biol 72:1761–1772
Motomura H, Struthers CD, McGrouther MA, Stewart AL (2011b) Validity of Scorpaena jacksoniensis and a redescription of S. cardinalis, a senior synonym of S. cookii (Scorpaeniformes: Scorpaenidae). Ichthyol Res 58:315–332
Nakabo T (ed) (2002) Fishes of Japan with pictorial keys to the species, English edn. Tokai Univ Press, Tokyo
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford Univ Press, New York
Nelson JS, Grande TC, Wilson MV (2016) Fishes of the world. John Wiley & Sons, Inc., Hoboken
Palumbi SR, Martin AP, Romano SL, Mcmillan WO, Stice L, Grabowski G (1991) The Simple fool’s guide to PCR. Dept Zool, Univ Hawaii, Honolulu
Peters W (1855) Übersicht der in Mossambique beobachteten seefische. Monatsb Akad Wiss Berlin 1855:428–466
Pietschmann V (1934) Drei neue fische aus den hawaiischen Küstengewässern. Anz Akad Wiss Wien 71:99–100
Poss GS (1999) Scorpaenidae. Scorpionfishes (also, lionfishes, rockfishes, stingfishes, stonefishes, and waspfishes). In: Carpenter KE, Niem VH (eds) FAO species identification guide for fishery purposes. The living marine resources of the western central Pacific. Vol. 4. Bony fishes part 2 (Mugilidae to Carangidae). FAO, Rome, pp 2291–2352
Poss SG, Motomura H (2022) Family Scorpaenidae, Scorpionfishes and lionfishes. In: Heemstra PC, Heemstra E, Ebert DA, Holleman W, Randall JE (eds) Coastal fishes of the western Indian Ocean. Vol. 2. South African Institute for Aquatic Biodiversity, Makhanda, pp 506–549
Randall JE, Eschmeyer WN (2002) Revision of the Indo-Pacific scorpionfish genus Scorpaenopsis, with descriptions of eight new species. Indo-Pacif Fish 34:1–79
Randall JE, Lim KKP (2000) A checklist of the fishes of the South China Sea. Raffles Bull Zool (Suppl) 8:569–667
Richardson J (1842) Contributions to the ichthyology of Australia. Ann Mag Nat Hist (New Ser) 9:207–218
Roy S, Ray D, Mishra A, Mohanty SR, Mohapatra A (2022) Range extension of a poorly known fish species Hoplosebastes armatus Schmidt, 1929 (Scorpaeniformes: Scorpaenidae) from the Northwest Pacific to the Indian Ocean. Thalassas 38:13–20
Rüppell WPES (1835–38) Neue wirbelthiere zu der fauna von Abyssinien gehörig. Fische des Rothen Meeres. Siegmund Schmerber, Frankfurt-am-Main
Sabaj MH (2020) Codes for natural history collections in ichthyology and herpetology. Copeia 108:593–669
Sauvage HE (1873) Notice sur quelques poissons d'espèces nouvelles ou peu connues provenant des mers de l'Inde et de la Chine. Nouv Arch Mus Hist Nat Paris 8:49–62
Sauvage HE (1878) Description de poissons nouveaux ou imparfaitement connus de la collection du Muséum d’Histoire Naturelle. Famille des Scorpénidées, des Platycéphalidées et des Triglidées. Nouv Arch Mus Hist Nat Paris (Sér 2) 1:109–158
Schmidt P (1929) On Hoplosebastes armatus, a new genus and new species of the family Scorpaenidae from Japan. C R Acad Sci URSS, Leningrad 8:194–196
Smith JLB (1957) The fishes of the family Scorpaenidae in the western Indian Ocean. Part I. The sub-family Scorpaeninae. Ichthyol Bull J L B Smith Inst Ichthyol 4:49–72
Steindachner F (1866) Über die Fische von Port Jackson in Australien. Anz K Akad Wiss Math-Naturwiss Cl 3:50–54
Streets TH (1877) Contributions to the natural history of the Hawaiian and Fanning Islands and Lower California, made in connection with the United States North Pacific Surveying Expedition, 1873–75. Bull U S Natl Mus 7:1–172
Temminck CJ, Schlegel H (1843) Pisces. Parts 2–4. In: von Siebold PF (ed) Fauna Japonica, sive descriptio animalium quae in itinere per Japoniam suscepto annis 1823–30 collegit, notis observationibus et adumbrationibus illustravit. J Müller & Co, Amsterdam, pp 21–72
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vaillant LL, Sauvage HE (1875) Note sur quelques espèces nouvelles de poissons des îles Sandwich. Rev Mag Zool (Sér 3) 3:278–287
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia's fish species. Philos Trans R Soc Lond B 360:1847–1857
Weber M (1913) Die Fische der Siboga-Expedition. E. J. Brill, Leiden
Whitley GP (1961) A new scorpion fish from Queensland. N Qld Nat 29:9–10
Wibowo K, Motomura H (2021) Review of Indo-Pacific species of the scorpionfish genus Scorpaena (Teleostei: Scorpaenidae), with descriptions of two new species from the west coast of Australia. Ichthyol Res. https://doi.org/10.1007/s10228-021-00827-0
Acknowledgments
We extend our sincere gratitude to staff at various institutions for their invaluable contributions to this study. We thank M. McGrouther, A. Hay and S. Reader (AMS), M. Sabaj and M. Arce (ANSP), H. Senou and H. Wada (KPM), G. Shinohara, M. Nakae, K. Fujiwara, and M. Sato (NSMT), R. Fricke (SMNS), J. Williams, L. Palmer, S. Raredon, K. Murphy, D. Pitassy, K. Bemis, A. Nonaka, and A. Reft (USNM), and R. Winterbottom, D. Stacey, and N. Lujan (ROM) for opportunities to examine material. Special thanks to K. Barnuevo for assisting in molecular analysis. We also thank all volunteers, graduates, and students of KAUM for their assistance with specimen collection and curatorial support. We are also grateful to G. Hardy (Ngunguru, New Zealand) for reading the manuscript and assisting with English. We also appreciate the invaluable feedback from the two anonymous reviewers and editors that significantly improved the manuscript, with special gratitude to M. Matsunuma for his exceptional guidance as section editor. This study was supported in part by JSPS KAKENHI Grant Numbers 20H03311, 21H03651, and 23K20304; the JSPS Core-to-core CREPSUM JPJSCCB20200009; and the ″Establishment of Glocal Research and Education Network in the Amami Islands″ project of Kagoshima University, adopted by the Ministry of Education, Culture, Sports, Science and Technology, Japan. This research is a part of dissertation of the first author. All authors agree with the article publication and dissertation submission.
Funding
Open access funding provided by Kagoshima University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as A211D720-F67F-4BB9-8BE2-D0E08B04D7C2.
This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Cabebe-Barnuevo, R., Mochizuki, K. & Motomura, H. Monophyly and re-definition of the Indo-Pacific scorpionfish genus Parascorpaena Bleeker 1876 (Scorpaenidae). Ichthyol Res (2024). https://doi.org/10.1007/s10228-024-00991-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10228-024-00991-z