Abstract
In fulfilling their daily activities, animals must expend the least amount of energy possible while feeding in order to optimise their energy balance. Food is removed by congeners as a result of exploitation competition. When a resource becomes limited, an increase in the probability of interference competition (direct competition for the resource) is triggered. While a high social rank may increase foraging time and resource access, this status also has detrimental facets. To explore the benefits of dominance/aggression in a context where true monopolisation of resources could be advantageous, we tested three hypotheses related to the patchiness of resources, agonistic activity (i.e. dominance and aggression) and individual attributes (i.e. morphology and behaviour) in a group of captive mouflon males (Ovis ammon musimon). Feeding performance was analysed using linear mixed models based on predictors about patchiness of the resource, and behavioural and morphological indices. No clear relationship was found between dominance and feeding performance. However, the general pattern showed (i) a decrease in overall feeding performance with the dispersion of the resource; (ii) that the discrepancy in feeding performance among individuals was maximal when confronted with intermediate conditions; and (iii) that alternative tactics allowed subordinate individuals to achieve a similar feeding performance to dominants. The results of this study suggest that, over and above agonistic behaviour and dominance, the motivation of individuals and its variation over time, though difficult to evaluate, could be key to understanding the coexistence of alternative behavioural tactics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals attempt to maximise their energy balance by expending as little energy as possible during feeding (Leblond et al. 2010) while primarily using rich food patches (Shrader et al. 2007). However, this behaviour often generates aggressive interactions that can be especially frequent in social herbivores that graze as a group. In such conditions, depending on food availability, individuals interfere with each other either to acquire a resource or to defend it against conspecifics. Under this framework, and according to the Resource Defense theory based on the economic defendability of resources (Brown 1964), dominant individuals will acquire certain advantages over subordinates when competing for food. Several studies in both cervidae (Appleby 1980; Barrette and Vandal 1986; Thouless 1990) and bovidae (Festa-Bianchet 1991; Fournier and Festa-Bianchet 1995; Masteller and Bailey 1988; Prins 1989; Rutberg 1986) have shown that high-ranking individuals tend to acquire better resources (i.e. in terms of quality and/or quantity) than their lower ranking counterparts. In all of these situations, aggression through the increase of agonistic interactions appears to be the most common mechanism of interference competition (Vahl et al. 2005).
Conversely, this notion of competitive dominance may confuse the concepts of pure dominance, as a dyadic property, and the ability to acquire food (i.e. it is not always the dominant, but the most motivated, that have first access to food, Barrette and Vandal 1986; Drews 1993). Moreover, certain individuals are reported to develop particular tactics of food acquisition to gain preferential access to food resources (e.g. Cervus elaphus, Schmidt and Hoi 1999; Odocoileus vigginianus, Taillon and Côté 2007; Ovis Canadensis, Bonenfant et al. 2009). Other studies (Ceacero et al. 2012; Schmidt et al. 1998) suggest that the most dominated animals do not necessarily eat less, probably because interference for monopolising food resources is frequent only among dominant individuals (Barroso et al. 2000). Feeding interactions and their impact on congeners is well known in invertebrates (Smallegange et al. 2006), fishes (Ryer and Olla 1996; Sundström 2001), amphibians (Gabor and Jaeger 1995) and birds (Enoksson 1988; Gyimesi et al. 2010), but has received much less attention in large mammals (but see Epsmark 1974 in cervids; Rubenstein 1994 in equids; Shi and Dunbar 2006 in caprids). While the functional significance of the relationship relies on the advantages procured by the monopolisation of a resource (Grant and Guha 1993), little is known about the proximate mechanisms underlying resource monopolisation. The probability that an individual will transgress upon a congener’s personal space with aggression is high in food-limited conditions. Thus, the variability of behaviours for food acquisition in such conditions increases, particularly among males depending on their morphological differences and the social system. In intolerant species, like rupicaprids, or in solitary species, dominance is associated with exclusive monopolisation of resources (Vestal and Stoep 1978). This could be far less marked in socially tolerant species such as many wild and feral caprids (Villaret and Bon 1998; Stanley and Dunbar 2013). As an example, aggregation in males has been reported in some species (Capra walie, Dunbar and Dunbar 1981; Capra aegagrus, Schaller 1977; Ovis orientalis, Maisels 1993) with low to median inter-individual distances during feeding. This could reduce the influence of hierarchical position on food intake.
In order to test the links between agonistic activity (dominance and aggression) and a monopolisation of resources during feeding bouts (feeding benefits), we investigated the effect of resource organisation (i.e. patchiness) on relationships among mouflon males constituting a single captive group. Patchiness may be viewed as integrating two slightly different dimensions, namely distribution and accessibility. We developed setups that allowed us to test the two dimensions in our experiments (see “Animals and methods”).
The experiments were conducted on mouflon males (Ovis ammon musimon), which are known to show aggression and dominance behaviour. This behaviour is under the control of the sex hormones (Bouissou 1995), making males the more aggressive sex. We should note that competition among males could be indirectly related to food acquisition as it is known that for some species, males could compete for sites which will attract females during the rut (von Hardenberg et al. 2000; Carranza et al. 1995). We assume that this is not the case in our study, mainly because the experiment was done in later winter, outside the reproductive period.
We tested three predictions related to patchiness of resources, agonistic activity in terms of dominance/aggression and individual characteristics (i.e. morphology and behaviour): (1) we expected an increase in the variance among the individuals’ feeding performances in the most clumped feeding situation (i.e. a single feeding bucket vs three buckets), arising from an increase in feeding competition; (2) the individual that receives the most submissive or avoidance behaviours (i.e. the dominant animal during feeding, not necessarily the most aggressive) is expected to be the most efficient consumer regardless of the patchiness of the resource; and (3) morphological and/or behavioural characteristics may interfere with this rationale, such that a particular behaviour and/or morphological characteristic may allow an individual to overcome the disadvantages (i.e. in terms of feeding) which subordinate status confers.
As we have no quantified information outside of a feeding context, our analysis focused on agonistic interactions during feeding and the feeding consequences for dominants and subordinates. It was not our goal to study whether high dominance rank per se (i.e. pure dominance) is advantageous but rather (i) to question whether dominance in a feeding context confers some advantages and (ii) to describe the social context of this dominance.
Animals and methods
Data collection
Data were collected from a group of five males (see Table 1 for morphological measurements) confined for almost 6 months in a 900-m2 enclosure. The experimental setup was located in the enclosure in which feeding trials of a duration of 6 min were carried out. Considering that morphology under the influence of rearing conditions probably had an effect on the interactions and social organisation among males, their origin may have some significance. The male Bo (4 years old) was separated from its mother after weaning and reared alone. The four 2-year-old males (To, GB, Ca and Ja) were separated early from their mothers and bottle-fed as a group. Although the oldest male Bo had the largest horns, he was also the lightest in weight (Table 1). We chose to include this male in spite of its different history since, with respect to the social dynamics hypothesis (Chase et al. 2002), its social profile contributed to the stability of the group. The animals were exposed to three experimental feeding situations (S) with different degrees of resource patchiness simulated by changing the number and relative position of buckets. The buckets were cylindrical, 13 cm deep × 15 cm in diameter, and mounted at top of a 42-cm-high stake. This height corresponds to the mouflon’s size, making it easy to observe individuals. Situation 1 included one available bucket, so only one male was allowed to feed at any given time (hereafter 1B). Situation 2 included three buckets arranged closely in a triangle with 35 cm between buckets (hereafter 3CB). The same total quantity of alfalfa pellets as in 1B was equally distributed between the three buckets. Three males were able to feed with their horns in contact. Situation 3 was similar to situation 2, but the three buckets were set 2.6 m apart, allowing two individuals to eat at adjacent buckets without physical contact (hereafter 3FB).
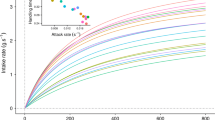
The three feeding situations provided different degrees of patchiness regarding the accessibility and distribution of the food resources. In 1B, the patchiness is introduced through a reduced accessibility to the resource. In this situation, the greatest possible theoretical group feeding rate given the 6-min duration of a trial corresponds to 72 s per individual (i.e. 360 s for five individuals). This corresponds to a shared feeding time among the males. In 3CB and 3FB, accessibility is increased and availability maintained at a similar level, while the distribution is altered (i.e. dispersion is increased in 3FB). The highest possible theoretical group feeding rate here is 216 s (i.e. [360 s × 3] / 5 individuals). These theoretical values in the case of equal feeding duration among males according to food availability are shown in Fig. 1. To take into account the variation in food organisation, we calculated a relative feeding performance (RFP) by dividing the measured feeding duration (FED) for the group feeding rate in each situation tested. Accordingly, an RFP value equal to 1 means that the male reached the theoretical feeding rate according to the level of competition (i.e. food availability and distribution).
Relationship between feeding time distribution and the individual feeding dominance rank during each competitive situation ordered in decreasing order from left to right. a One bucket (1B); b three close buckets (3CB); c three far apart buckets (3FB). Note that the two top-ranked males, Bo and To, switched their rank (added in brackets) after situation 1B. The dotted line denotes the theoretical group feeding rate at equilibrium for each situation (see “Animals and methods”)
Each feeding situation was replicated ten times on ten consecutive days and was separated from other situations by a 10-day interval. For practical aspects, and to avoid excessive modification of mouflon familiarity with the environment, we carried out the feeding scenarios in the following order: one bucket (1B), three close buckets (3CB), and then three far apart buckets (3FB), instead of exposing mouflon to the three scenarios at random. No variations in the rate of interactions were detected over the ten replicates carried out for each situation, so we considered that no habituation to the experimental design occurred. To allow the animals to become familiar with the setup, mouflon were fed with alfalfa pellets in the proximity of the setup (but without the buckets mounted) for 4 weeks before the start of the experiment and were exposed to each new situation (without pellets) over a 10-day period. For each trial (n = 10 per feeding situation), the five males were first stabled together in a sub-enclosure (12 m2) close to the experimental sub-enclosure. Depending on the situation being tested in the experimental enclosure, 1 L of pellets (ca. 650 g) was distributed in a single bucket or equally shared among the three buckets. The five males were then introduced to the experimental section of the enclosure and filmed for 6 min from a 3-m-high blind located to one side of the enclosure. The camera’s field of view covered an area extending 1.5 m beyond the buckets, monitoring in the same sequence all individuals within this field of view. The 6-min trial duration was sufficient for the consumption of almost all of the pellets in a bucket.
Using Observer © software (Noldus, Information Technology, Wageningen, The Netherlands), each recorded trial was viewed five times (i.e. once for each of the five males in each situation), using the focal sampling method (Martin and Bateson 1986) to collate data for each male. This corresponded to 15 h of focal-male footage analysed. Each agonistic act, the initiator, the recipient and its response were systematically recorded.
Between each feeding trial, males were given restricted access to hay but allowed to graze in the enclosure. Occasional observations indicated that all animals were able to feed without harassment by other males, and thus, we assume that these conditions were not responsible for any differences in feeding motivation state. The tests were carried out at the same time each day (9:00 am), such that the males entered the sub-enclosure at a similar stage of their daily nutritional cycle, and hence, their hunger levels were also assumed to be similar.
The behavioural repertoire was based on spatial position and behaviours (Table 2). Following the nomenclature used in Observer ©, we distinguished behavioural states and behavioural events. The behavioural states include all behaviours for which the duration may be used for analyses. The behavioural events were collated as frequencies and corresponded to interactive acts (Table 2).
As proposed by some authors (Bouissou and Gaudioso 1982; Drews 1993), dominance may be viewed as a reduction in timidity rather than an increase in aggressivity. Furthermore, the most dominant individual may not always show the most aggression (Drews 1993; Rowell 1966). Thus, using an approach similar to that of Chase et al. (2002), but following Rowell (1974) in focusing on submissive tendency (i.e. avoidance behaviour), we considered animals receiving the most and performing the least submissive acts (i.e. active and passive withdrawal) during a trial as dominant in a feeding context according to the formula:
where “IDF” is an index of feeding dominance (IDF), “sub r” is the frequency of received passive/active withdrawal and “sub g” is the frequency of performed passive/active withdrawal. Following Guilhem et al. (2000), we considered a lack of response to an agonistic act as an unclear outcome, possibly linked to feeding motivation (Lemel and Wallin 1993; Ceacero et al. 2012). Accordingly, the item was not included in the calculation of the feeding dominance index.
To compare this value with an index of direct aggression (IDA), we calculated for each trial the expression rate of threats (thr), horn blows (hbl) and clashes (cla), whether as an initiator (emiss) or as a respondent (receip) according to the following formula:
From previous observations, it was noticed that some individuals tended to be passive during agonistic interaction, so we used the rate of “no response” in the responsive repertoire of males as an index of passivity (hereafter IP):
where nr is the frequency of no detectable response to an agonistic act and Ta is the total number of agonistic items in which a male is involved.
At the end of the experiment, we observed that the hierarchy resulting from feeding competition was inverted for the first two males of the hierarchy, namely Bo and To, resulting in the following hierarchical order: To > Bo > GB > Ca > Ja for both more-than-one bucket situations. The abscissas in Fig. 1 retain the original order for the three situations. Indices of IDF, IDA and IP were calculated for each trial so as to relate an estimation of immediate agonistic activity with the feeding performance for each male.
To include a general value of the physical attributes (prediction 3) of each male in the analysis, we used a data reduction technique by transforming the morphological data of males to a continuous independent variable (hereafter MoSc) by using the scores of a PCA performed on morphological data (listed in Table 1). Specifically, we used the scores from the first axis of the PCA.
Statistical analysis
Our dataset has some weaknesses as it is limited in terms of sample size (n = 5). In addition, the RFP we calculated as our dependent variable is upper bounded, as the maximal time of feeding is limited by trial duration and, consequently, adjusted values could fall outside this interval. Another issue concerns pseudo-replication due to repeated measures on each individual in each trial; however, mixed models allowed us to control for this. Accordingly, we used linear mixed effects models, including a random term in the model coding the identity of the animal, which allowed us to control for inter-individual variation on parameter estimates (Pinheiro and Bates 2000). The normality of residuals and the condition of homoscedasticity (homogeneity of variance) were verified by visual inspection of normal probability plots.
We used R software (R Development Core Team 2013) and the lmer function of the lme4 package (Bates et al. 2012) to perform a linear mixed effects analysis. We aimed to test the influence of certain variables involved in our predictions on the RFP of individual male mouflon. The fixed effects of the most complex model included the situation variable (S) to test our first prediction about the influence of patchiness of resources, the indexes of dominance during feeding (IDF) and aggressivity (IDA) involved in the second prediction, and finally the IP and the proxy of morphology (MoSc) (see Table 1) involved in the third prediction about individual traits. The reference level for the fixed effect S was 1B as this situation corresponds with the maximal level of competition.
We did not construct a full model (i.e. including all possible interactions of all terms) as our most complex model, because of the risk of over-parameterisation and difficulty of interpretation (i.e. an n/k ratio lower than 40 (i.e. 30), with k being the number of fitted parameters in the most complex model and n the sample size). To test for inter-individual differences per se (differences in intercepts estimating male feeding performance) and in the relationship with covariates that appeared to be the most pertinent during feeding trials (IFD and IP), we introduced a term that estimated the heterogeneity in terms of intercept and slopes of individuals against each of the covariates as a random factor. Note that the identity of males (IdM) appears both as included in the random term and as a covariate (MoSc), as each value of the latter (n = 5) is specific to a given male.
The intercept and slope appeared to be correlated for the two covariates (i.e. IDF and IP) regressed on individual identity. This indicates that the within-subject effect of the covariates depends on the initial values of the dependent variable RFP. Accordingly, we only specified the slope in the random terms of the model.
The dredge function in the MuMIn package (Barton 2012) was used to select the best models on the basis of the Akaike’s information criterion corrected for small sample size (AICc) (Burnham and Anderson 2010). Candidate models were then ranked using this criterion and differences between the AICc value of the best model (i.e. model with the lowest AICc) and other candidate models (ΔAICc) to identify the best competing models. Models with AICc differences <2 were considered as equally supported by the data, but we also considered models with ΔAIC <6 as informative (Bolker et al. 2009; Richards et al. 2011). The Akaike weight (wi), estimating the probability that each candidate model was the best among the selected models, was well below the threshold of 0.9 (i.e. 0.38, Table 3). In such cases, a model-averaging approach is recommended to reduce the risk of relying on a single and weak candidate model (Grueber et al. 2011). Therefore, a final model was obtained by a model averaging analysis (Buckland et al. 1997; Grueber et al. 2011) on the set of models with a ΔAIC <6, using the MuMIn package (Barton 2012) (functions dredge and model.avg). Significance of model-averaged parameter estimates was based on a Wald Z test for maximum likelihood estimates; so in this case, no degree of freedom is reported.
The relative importance (RI) of each explanatory variable in the final model was calculated as the sum of all the model weights in which that variable appeared; accordingly, a variable appearing in all models would have a relative importance of 1. To complete the information criterion, which only gives a relative value of models, we evaluated the variance explained by the models in terms of marginal (R 2 m) and conditional (R 2 c) coefficients of determination (or a goodness-of-fit statistic) by using the function “r.squaredGLMM” of package MuMin (Barton 2012). These two coefficients furnish an indication of how well the fixed effects fit the statistical model and how much variation is explained by the variables (fixed and random effects) included in the model, respectively. Note that the coefficients tend to favour the most complex models. To calculate accurate P values for significance of the slope of random effects (i.e. test whether the variance of a random effect was 0), we compared models with a single random effect (IDF then IP) to models with no random term by using parametric bootstrapping (package RLRsim and exactLRT function) (Scheipl 2010).
For statistical accuracy, model comparisons with different random effect structures were fitted using restricted maximum likelihood (REML) and those comparing models with different fixed effect structures were fitted using maximum likelihood (ML) (see Bates 2005 and Baayen et al. 2008 on this issue). The estimates for fixed effects were extracted after removing non-significant random terms (i.e. slope of IDF on male identity).
Spearman’s rank correlation (r s) was used to test the relationship between rank and feeding performance for each situation tested. Statistical significance was assumed for P < 0.05.
Results
The distribution of FED in relation to male identity and the situation tested are given in Fig. 1. Males are ranked in descending order according to the values of the IDF during the first competitive situation (i.e. 1B). Situation 1B was characterised by a lower FED for all males when compared to the three-bucket situations. Additionally, male GB (i.e. third position in the hierarchical order) can be singled out due to a reduced variance in its performance across trials (Fig. 1) and male Ca due to a low value of FED. On a broader level, feeding performance varied markedly among situations (mixed model, S(3CB): Wald Z = 5.73, P < 0.001; S(3FB): Wald Z = 7.72, P < 0.001, Table 4). Note that this variation corresponds to a mean decrease in RFP of 0.18 and 0.25 for 3CB and 3FB, respectively, relative to the reference level for the factor, which is 1B.
Figure 1 highlights the lack of a relationship, irrespective of the feeding situation, between overall dominance rank (abscissa ordering) and feeding duration measured as the time spent with the muzzle inside a bucket, for 1B and 3FB (r s = 0.06, n = 50, NS, and r s = 0.22, n = 50, NS, respectively). However, a positive correlation was present between dominance and feeding duration for 3CB (r s = 0.34, n = 49, P < 0.05) (Fig. 1b).
When the dispersion, but not the quantity, of food increased (3CB and 3FB; Fig. 1b, c, respectively), FED attained higher values. The possibility for a male to feed in a bucket was higher. However, the deviation between the observed values of FED and the theoretical feeding time at equilibrium between males (dotted line in Fig. 1) increased. This is particularly true for the subordinate male Ca for 3FB (Fig. 1c). The variation in feeding duration among males was clearest in 3CB (Fig. 1b). Counter-intuitively, the gap between the theoretical group feeding rate and the observed feeding duration was at its minimum for the most competitive situation (Fig. 1a). Three of five males had a feeding duration near the theoretical value. This was not the case for the three-bucket situations, particularly 3FB where the variation in feeding duration among males was lower (Fig. 1c).
Quite unexpectedly, the weak relationship between dominance and feeding duration appears contradictory to the main results generated by the mixed model approach. Indeed, the predictor IDF appears in each of the models of interest based on selection by the AICc criterion (Tab. 3) and with a significant effect on RFP (Wald Z = 3.15, P < 0.005, Table 4). In fact, this is due to different evaluations of the dominance index, one being estimated at the scale of the situation (used for ordering abscissas in Fig. 1), while IDF is an index measuring transitory dominance during each trial (n = 149). However, the significance of IDF and other fixed factors in the models must be weighed against the low values of the marginal coefficient of determination (R 2 m) (Tab.3), which express the small contribution of fixed factors alone in the models, as well as the high contribution of individual variation (i.e., deduced from the high values of conditional R 2: R 2 c) (Table 3). Figure 2, based on input data, shows the consistent dispersion of the response variable RFP for both factors IDF and IP as well as the higher increase in rate of RFP relative to IP.
In summary, the situation 1B, the most competitive, corresponds to the shortest feeding duration, but if we consider this relative to the possible at equilibrium (72 s of feeding per male), it is in this situation that the overall consumption is closest to this value. This is not the case for the situations with three buckets.
There is no clear relationship between dominance and feeding duration, but when considering the dominance scores during trials, the linear model indicates a significant relationship with the RFP. When a male subordinate succeeds temporarily in increasing his index of dominance during a trial, this tends to be associated with an increase in his feeding performance. This relationship was also true for the passivity index.
At a global level, it should be noted that the model-based estimate (slope) of the dependent variable RFP indicates that for an increase of one unit in the IDF and the IP indices, the RFP increases by 0.36 (confidence interval (CI) 0.14–0.58) and by 0.42 (CI 0.06–0.77) respectively, with a larger value and CI for the latter (Table 4). With regard to the influence of direct aggression (IDA) and morphological features (MoSc), there was no significant direct effect of these variables on RFP.
Discussion
We consider the main result of our experiment to be the unexpected similarities in feeding performances for four of five males tested, regardless of both the level of competition (i.e. feeding situation) and the dominance hierarchy established during feeding trials. Concerning the similarity in feeding performance, the dominance index we used yielded contrasting results depending on the scale at which it was measured. While the index of dominance per trial appeared to be one of the best predictors of the RFP, this was not the case when dominance was estimated at the larger scale of situations where the correlation with feeding duration was, at best, weak. This reflects the fact that a subordinate may not only stay and feed despite pressure from a dominant (low correlation) but may also increase its feeding performance through transitory dominance during some trials (high correlation). Indeed, this is possible in species where close proximity is tolerated (i.e. social tolerance), which is not common in social ungulates. Furthermore, high-ranking individuals may spend more time defending limited food at the expense of feeding to the point that correlation may be reduced (Craig 1986) or even reversed (Sherwin 1990; Brouns and Edwards 1994). These considerations could explain the lower values of FED in situation 1B, which could be in line with the first prediction (i.e. reduced feeding time). The two top-ranking males spent much time in agonistic interactions, which decreased their feeding duration and, to some extent, that of other males. This is in line with the fact that interference for monopolising food resources is frequent only among dominant individuals (Barroso et al. 2000) and that it correlatively incurs a loss at the expense of feeding (Csermely and Wood‐Gush 1990). However, when considering the theoretical group feeding threshold, the gap with the observed feeding duration is less marked for this one-bucket situation. As a result, our prediction is not fully confirmed. Contrary to trends described in highly hierarchically organised species (e.g. Vestal and Stoep 1978), the dominant individual did not monopolise the only feeding source. If this was the case, the feeding duration of the top-ranking males would have reached higher values than the theoretical value, the reverse being true for the other individuals. Even if the opportunity to feed at a bucket was increased, moving between food patches is time consuming, and at the expenses of feeding, particularly because moving between buckets affected not only high-ranking males, but all males, contrary to observations by Wierenga (1990) in dairy cows. Inter-individual variability in feeding in goats is also reported to decrease when the space provided is increased (Jørgensen et al. 2007). This result also contradicts our second prediction; the top-ranking males were not able to monopolise food, even though the median value of their feeding duration was among the highest. The tendency to be passive during agonistic bouts (i.e. to display no response to an agonistic actor) falls clearly within the field of the third prediction concerning morphometric and behavioural characteristics. The two males showing this tendency obtained the highest feeding durations for the study. The male Ja was also the most subordinate; that is, this male never provoked an active withdrawal or submissive behaviour from another male. As the most clumped situation is expected to be highly competitive and therefore to provoke many agonistic interactions and direct aggression, the individuals exhibiting the most “no response” behaviours were particularly able to gain time for feeding when exposed to this challenging situation. This pattern (i.e. similar feeding score for top- and bottom-ranked individuals) is inconceivable for species bearing butcher’s hook-like horns, known to perform a higher rate of aggressive interactions than other gregarious ungulates (i.e. rupicaprids, Fournier and Festa-Bianchet 1995; Vestal and Stoep 1978). For these species, a highly competitive situation will result in a strict feeding hierarchy in which the dominant animal will never be disturbed by subordinates during feeding (chamois: Vestal and Stoep 1978; but see Côté 2000 for salt block access in Rocky Mountain goat).
So, as dominance does not fully explain feeding success (food gain) in a situation of acute competition, consideration should be given to establish how dominance interacts with other characteristics allowing subordinates to acquire desired food. For most caprids, age, horn length and body weight are auto-correlated among males (Granados et al. 1997; Santiago-Moreno et al. 2005 but see Rowell and Rowell 1993), but due to rearing conditions, this was not the case in the studied group. As a consequence, the male Bo was disadvantaged by his lighter weight and we assume that this contributed significantly to rank loss after the intense competition during the test with only one bucket. Interestingly, this male was also the most aggressive, which suggests some independence from dominance. Shifts in dominance order have been reported in groups of familiar males in bovids (Hass and Jenni 1991; Roden et al. 2005) and also in cervids (De Young et al. 2006), making dominance difficult to predict. Differences in weight could be linked to aspects affecting consequences of dominance, even if the effects are not direct (i.e. withstand assaults by congeners as shown by males GB and Ja). We suggest that the behaviour most used by the males (i.e. horn push) favours passivity (i.e. no response) by the recipient and does not elicit an aggressive escalation by the actor. Note that this feature is not a general pattern, as for example in the bird genus Carduelis, a lack of response leads to an agonistic escalation (Senar 1990). This particular way to seek access to food is also reported as common for merino sheep (Sherwin 1990). Other studies on sheep have reported that either horn push or horn butt are used to gain access to food (Sherwin 1990; Erhard et al. 2004). However, when horn push is preferred, this could be indicative that motivation rather than dominance is determining the priority to feed (Arnold and Maller 1974). Over and above body weight, individual features (e.g. cognitive abilities, Krueger and Flauger 2008; personality, Michelena et al. 2009; past experience, Rowell and Rowell 1993, Hansen et al. 2009; Searle et al. 2009) are considered to be involved in competitive feeding performance. Similarly, aspects related to motivation in diverse contexts (e.g. food access and reproduction) deserve more attention. In goats, diet selection is influenced by rank (Barroso et al. 2000) but the nutritional value of patches (i.e. amount of food) may modulate this relationship, as an increase in the availability of food motivates subordinates to accept confrontation with dominant animals (Stears et al. 2014). In deer, only the feeding time of highly ranked hinds is related to their hierarchical position (Veiberg et al. 2004). Although there was little empirical support, this pattern could exist in our study since in the three situations, the feeding duration of the top-ranked male was greater than that of the second-ranked.
Indeed, situations of limited food accessibility may contribute through variation in motivation to the appearance of novel feeding tactics (Ceacero et al. 2012; Hollis et al. 2004; Schmidt and Hoi 1999; Thompson et al. 2008). For example, the tendency to evade conflicts (i.e. low reactivity in response to aggression) or the ability to resist some agonistic contacts (i.e. horn push) could be interpreted as mechanisms that increase the time devoted to feeding by some subordinates, compared to high- and mid-ranked congeners. This tactic is observed in pronghorns (Dennehy 2001), domestic goats (Barroso et al. 2000; Miranda-de la Lama et al. 2011) and red deer hinds (Ceacero et al. 2012). All of these studies showed that this tactic resulted in an increase in feeding duration. On the other hand, we might expect that in a competitive context, such changes in status/responses to confrontation by subordinate individuals will not persist in the face of pressure from dominants.
Thus, similar to the importance given to physical attributes in determining the rank order of individuals in hierarchies, the effects of individual characteristics on rank inconsistency over time deserves further consideration. One way to broaden the range of information is to establish individual behavioural profiles as proposed by Kiley-Worthington (1978) to investigate the dominance hierarchy and the organisation of herds in zoos. More recently, the Elo-rating method (Neumann et al. 2011) have been proposed to update dominance ratings by looking at interactions sequentially. As a result, dominance ranks can be monitored on the desired timescale. In a recent publication on repeatability in behavioural studies, Biro and Stamps (2015) warn against ignoring time-related changes in repeatability, and by doing so, increasing the risk of obtaining biased results and erroneous conclusions.
The growing interest in personality and its possible relationship to both feeding behaviour (Bergvall et al. 2010; Hirata et al. 2010) and food intake (Kurvers et al. 2011) may allow a better understanding of how motivational aspects interplay with personality to produce apparently risky behaviours. Similarly, the cases in which dominance appears as a poor predictor of food intake (including carnivores, Gill and Helfield 2012) could benefit from studies on cognitive aspects of motivation and propensity to take risk, particularly in subordinate individuals (Hollis et al. 2004). We argue that further research on this question could improve our understanding of the proximal causes of alternative behaviours.
References
Appleby MC (1980) Social rank and food access in red deer stags. Behaviour 74:294–309
Arnold GW, Maller RA (1974) Some aspects of competition between sheep for supplementary feed. Anim Prod 19:309–319
Baayen RH, Davidson DJ, Bates DM (2008) Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang 59:390–412
Barrette C, Vandal D (1986) Social rank, dominance, antler size, and access to food in snow-bound wild woodland caribou. Behaviour 97:118–146
Barroso FG, Alados CL, Boza J (2000) Social hierarchy in the domestic goat, effect on food habits and production. Appl Anim Behav Sci 69:35–53
Barton K (2012) Package ‘MuMIn’: model selection and model average based on information criteria (AICc and alike). CRAN R Project. Available from http://cran.r-project.org/web/packages/ MuMIn/MuMIn.pdf (accessed April 2015)
Bates D (2005) Fitting linear mixed models in R. R news 5:27–30
Bates D, Maechler M, Bolker B (2012). lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-0. http://CRAN.R-project.org/package=lme4
Bergvall UA, Schäpers A, Kjellander P, Weiss A (2010) Personality and foraging decisions in fallow deer, Dama dama. Anim Behav 81:101–112
Biro PA, Stamps JA (2015) Using repeatability to study physiological and behavioural traits: ignore time-related change at your peril. Anim Behav 105:223–230
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bonenfant C, Pelletier F, Garel M, Bergeron P (2009) Age-dependent relationship between horn growth and survival in wild sheep. J Anim Ecol 78:161–171
Bouissou MF (1995) Social relationships, aggressive behaviour and emotional reactivity in ungulates, influences of sex steroids. Prod Anim 8:71–82
Bouissou MF, Gaudioso (1982) Effect of early androgen treatment on subsequent social behaviour in heifers. Horm Behav 16:132–146
Brouns F, Edwards SA (1994) Social rank and feeding behaviour of group-housed sows fed competitively or ad libitum. Appl Anim Behav Sci 39:225–235
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull 76:160–169
Buckland ST, Burnham KP, Augustin NH (1997) Model selection: an integral part of inference. Biometrics 53:603–618
Burnham KP, Anderson DR (2010) Model selection and multi-model inference: a practical information-theoretic approach. Springer, New York
Carranza J, Garcia-Muñoz AJ, deDios Vargas J (1995) Experimental shifting from harem defence to territoriality in rutting red deer. Anim Behav 49:551–554
Ceacero F, García AJ, Landete-Castillejos T, Bartosova J, Bartos L, Gallego L (2012) Benefits for dominant red deer hinds under a competitive feeding system, food access behaviour, diet and nutrient selection. PLoS One 7, e32780. doi:10.1371/journal.pone.0032780
Chase ID, Tovey C, Spangler-Martin D, Manfredonia M (2002) Individual differences versus social dynamics in the formation of animal dominance hierarchies. PNAS 99:744–749
Côté SD (2000) Determining social rank in ungulates, a comparison of aggressive interactions recorded at a bait site and under natural conditions. Ethology 106:945–955
Craig JV (1986) Measuring social behavior. J Anim Sci 62:1120–1129
Csermely D, Wood‐Gush DG (1990) Agonistic behaviour in grouped sows II How social rank affects feeding and drinking behaviour. Italian J Zool 57:55–58
De Young RW, Demarais S, Honeycutt RL, Gee KL, Gonzales RA (2006) Social dominance and male breeding success in captive white-tailed deer. Wildl Soc Bull 34:31–136
Dennehy JJ (2001) Influence of social dominance on habitat selection by free-ranging ungulates. Behav Ecol 12:177–181
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www R-project org
Drews C (1993) The concept and definition of dominance in animal behaviour. Behaviour 125:283–313
Dunbar RIM, Dunbar E (1981) The grouping behaviour of male Walia ibex with special reference to the rut. Afr J Ecol 19:251–263
Enoksson B (1988) Age- and sex-related differences in dominance and foraging behaviour of nuthatches Sitta europae. Anim Behav 36:231–238
Epsmark Y (1974) Social behaviour of roe deer at winter feeding stations. Appl Anim Ethol 1:35–47
Erhard HW, Fàbrega E, Stanworth G, Elston DA (2004) Assessing dominance in sheep in a competitive situation: level of motivation and test duration. Appl Anim Behav Sci 85:277–292
Festa-Bianchet M (1991) The social system of bighorn sheep: grouping patterns, kinship, and female dominance rank. Anim Behav 42:71–82
Fournier F, Festa-Bianchet M (1995) Social dominance and conflict reduction in rutting male Alpine ibex, Capra ibex. Anim Behav 49:1449–1459
Gabor CR, Jaeger R (1995) Resource quality affects the agonistic behaviour of territorial salamanders. Anim Behav 49:71–79
Gill ID, Helfield JM (2012) Alternative foraging strategies among bears fishing for salmon: a test of the dominance hypothesis. Can J Zool 90:766–775
Granados JE, Perez JM, Soriguer RC, Fandos P, Ruiz-Martinez I (1997) On the biometry of the Spanish ibex, Capra pyrenaica, from Sierra Nevada (Southern Spain). Folia Zool 46:9–14
Grant JWA, Guha R (1993) Spatial clumping of food and social dominance affect interference competition among ruddy turnstones. Behav Ecol 4:293–296
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711
Guilhem C, Bideau E, Gerard JF, Maublanc ML (2000) Agonistic and proximity patterns in enclosed mouflon (Ovis gmelini) ewes in relation to age, reproductive status and kinship. Behav Process 50:101–112
Gyimesi A, Stillman RA, Nolet BA (2010) Cryptic interference competition in swans foraging on cryptic prey. Anim Behav 80:791–797
Hansen BB, Aanes R, Herfindal I, Sæther BE, Henrikse (2009) Winter habitat-space use in a large arctic herbivore facing contrasting forage abundance. Polar Biol 32:971–984
Hass CC, Jenni DA (1991) Structure and ontogeny of dominance relationships among bighorn rams. Can J Zool 69:471–476
Hirata M, Nakayama Y, Tobisa M (2010) Interindividual variability in feeding station behavior in cattle: a preliminary study. Grassl Sci 56:108–115
Hollis KL, Langworthy-Lam KS, Blouin LA, Romano MC (2004) Novel strategies of subordinate fish competing for food, learning when to fold. Anim Behav 68:1155–1164
Jørgensen GHM, Andersen IL, Bøe KE (2007) Feed intake and social interactions in dairy goats—the effects of feeding space and type of roughage. Appl Anim Behav Sci 107:239–251
Kiley-Worthington M (1978) The social organization of a small captive group of eland, oryx and roan antelope with an analysis of personality profiles. Behaviour 66:32–55
Krueger K, Flauger B (2008) Social feeding decisions in horses (Equus caballus). Behav Process 78:76–83
Kurvers RHJM, van Sarten de Hoog SI, van Wieren SE, Ydenberg RC, Prins HH (2011) No evidence for negative frequency-dependent feeding performance in relation to personality. Behav Ecol 23:51–57
Leblond M, Dussault S, Ouellet J-P (2010) What drives fine-scale movements of large herbivores? A case study using moose. Ecography 33:1102–1112
Lemel J, Wallin K (1993) Status signalling motivational condition and dominance, an experimental study in the great tit, Parus major. Anim Behav 34:549–558
Maisels FG (1993) Seasonal variation in grouping patterns of the forest-dwelling Cyprus mouflon Ovis orientalis. J Zool 229:527–532
Martin P, Bateson P (1986) Measuring behaviour, an introductory guide. Cambridge university press, Cambridge
Masteller MA, Bailey J (1988) Agonistic behavior among mountain goats foraging in winter. Can J Zool 66:2585–2588
Michelena P, Sibbald AM, Erhard HW, McLeod JE (2009) Effects of artificial food on the winter activity of White-tailed Deer Odocoileus virginianus living in the north of the area of their distribution. Behav Ecol 20:145–152
Miranda-de la Lama GC, Sepúlveda WS, Montaldo HH, María GA, Galindo F (2011) Social strategies associated with identity profiles in dairy goats. Appl Anim Behav Sci 134:48–55
Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A (2011) Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim Behav 82:911–921
Pinheiro JC, Bates D (2000) Mixed-effects Models in S and S-plus. Springer-Verlag, New York, NY
Prins HHT (1989) Buffalo herd structure and its repercussions for condition of individual African buffalo cows. Ethology 81:47–71
Richards SA, Whittingham MJ, Stephens PA (2011) Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behav Ecol Sociobiol 65:77–89
Roden C, Vervaecke H, Van Elsacker L (2005) Dominance, age and weight in American bison males (Bison bison) during non-rut in semi-natural conditions. Appl Anim Behav Sci 92:169–177
Rowell TE (1966) Hierarchy in the organization of a captive baboon group. Anim Behav 14:430–433
Rowell TE (1974) The concept of social dominance. Behav Biol 11:131–154
Rowell TE, Rowell CA (1993) The social organization of feral Ovis aries ram groups in the pre-rut period. Ethology 95:213–232
Rubenstein DI (1994) The ecology of female social behavior in horses, zebras, and asses. In: Jarman P, Rossiter A (eds) Animal Societies: Individuals, Interactions, and Organization. Kyoto University Press, Kyoto, pp 13–28
Rutberg AT (1986) Dominance and its fitness consequences in American bison cows. Behaviour 96:62–91
Ryer CH, Olla B (1996) Social behavior of juvenile chum salmon, Oncorhynchus keta, under risk of predation, the influence of food distribution. Environ Biol Fishes 45:75–83
Santiago-Moreno J, Gómez-Brunet A, Toledano-Díaz A, González-Bulnes A, Picazo RA, Lopez-Sebastian A (2005) Influence of age on the relationship between annual changes in horn growth rate and prolactin secretion in the European mouflon (Ovis gmelini musimon). Anim Reprod Sci 85:251–261
Schaller GB (1977) Mountain Monarchs, Wild sheep and goats of the Himalaya. The Univ, Chicago Press, Chicago
Scheipl F (2010) Rlrsim: Exact (Restricted) Likelihood Ratio Tests for Mixed and Additive Models. R package, version 2.0e5. published online 9 January 2007
Schmidt KT, Hoi H (1999) Feeding tactics of low-ranking red deer stags at supplementary feeding sites. Ethology 105:349–360
Schmidt KT, Seivwright LJ, Hoi H, Staines BW (1998) The effect of depletion and predictability of distinct food patches on the timing of aggression in red deer stags. Ecography 21:415–422
Searle KR, Hunt LP, Gordon IJ (2009) Individualistic herds, individual variation in herbivore foraging behavior and application to rangeland management. Appl Anim Behav Sci 122:1–12
Senar JC (1990) Agonistic communication in social species: What is communicated? Behaviour 112:270–283
Sherwin CM (1990) Priority of access to limited feed, butting hierarchy and movement order in a large group of sheep. Appl Anim Behav Sci 25:9–24
Shi J, Dunbar RIM (2006) Feeding competition within a feral goat population on the Isle of Rum, NW Scotland. J Ethol 24:117–124
Shrader AM, Kerley GI, Kotler BP, Brown JS (2007) Social information, social feeding, and competition in group-living goats (Capra hircus). Behav Ecol 18:103–107
Smallegange IM, van der Meer J, Kurvers RHJM (2006) Disentangling interference competition from exploitative competition in a crabbivalve system using a novel experimental approach. Oikos 113:157–167
Stanley CR, Dunbar RIM (2013) Consistent social structure and optimal clique size revealed by social network analysis of feral goats, Capra hircus. Anim Behav 85:771–779
Stears K, Kerley GI, Shrader AM (2014) Group-living herbivores weigh up food availability and dominance status when making patch-joining decisions. PLoS One 9(10):e109011
Sundström LF (2001) Experience and social environment influence the ability of young brown trout to forage on live novel prey. Anim Behav 61:249–255
Taillon J, Côté SD (2007) Social rank and winter forage quality affect aggressiveness in white-tailed deer fawns. Anim Behav 74:265–275
Thompson AK, Samuel MD, DEELEN TR (2008) Alternative feeding strategies and potential disease transmission in Wisconsin white‐tailed deer. J Wildl Manag 72:416–421
Thouless CR (1990) Feeding competition between grazing red deer hinds. Anim Behav 40:105–111
Vahl WK, Lok T, Meer J, van der Piersma T, Weissing F (2005) Spatial clumping of food increases its monopolisation and defence by convict cichlids, Cichlasoma nigrofasciatum. Behav Ecol 16:834–844
Veiberg V, Loe LE, Mysterud A, Langvatn R, Stenseth N (2004) Social rank, feeding and winter weight loss in red deer, any evidence of interference competition? Oecologia 138:135–142
Vestal BM, Stoep A (1978) Effect of distance between feeders on aggression in captive chamois (Rupicapra rupicapra). Appl Anim Ethol 4:253–260
Villaret J-C, Bon R (1998) Sociality and relationships in Alpine ibex (Capra ibex). Rev Ecol (Terre Vie) 53:153–170
von Hardenberg A, Bassano P, Peracino A, Lovari S (2000) Male alpine chamois occupy territories at hotspots before the mating season. Ethology 106:617–630
Wierenga HK (1990) Social dominance in dairy cattle and the influences of housing and management. Appl Anim Behav Sci 27:201–229
Acknowledgments
The authors would like to thank the technical staff of the CEFS laboratory, especially J.-M. Angibault, N. Cèbe and L. Desneux, for their support in the field. Some anonymous referees made helpful comments on a previous version of the manuscript. E. Serrano was supported by the postdoctoral program (SFRH/BPD/96637/2013) of the Fundação para a Ciência ea Tecnologia, Portugal. We are grateful to Mark Hewison for his valuable revision and suggestions and to Peter Winterton and Sarah Young for polishing up the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kidjo, N., Serrano, E., Bideau, E. et al. Is dominance the only factor determining access to food in an agonistic context? An experiment with captive male mouflon. acta ethol 19, 69–79 (2016). https://doi.org/10.1007/s10211-015-0226-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-015-0226-8