Abstract
Objective
To review and assess the quality of the available evidence on the cost-effectiveness of erlotinib in the first-line treatment of advanced non-small cell lung cancer (NSCLC).
Methods
A systematic review was conducted to identify full-text original economic evaluations of erlotinib in the first-line treatment of advanced NSCLC written in English and published from the year 2000 onwards. Study characteristics and results were recorded and compared. The quality of the studies was assessed by the Quality of Health Economic Studies (QHES) questionnaire.
Results
Eleven out of 130 papers were chosen for this review. Comparative regimens consisted of a best supportive care, reverse strategy, bevacizumab, cisplatin plus pemetrexed, carboplatin plus gemcitabine or gefitinib. The methods most used in these studies were modeling and sensitivity analysis and cost-effectiveness analysis. All of the studies evaluated direct costs and used quality-adjusted life-year (QALY) and life-years gained (LYG) as outcome, with 3% and 3.5% discount rate. The studies assigned ICER that ranged from dominant to I$305,510.31/QALY and from I$31,209.55/LYG to I$66,540.20/LYG. Based on the willingness to pay threshold, seven studies concluded that erlotinib was cost-effective, two studies showed that erlotinib was cost-effective on specific patients with certain conditions, and two studies comparing erlotinib with reverse strategy did not find a difference in cost-effectiveness. The high quality of these studies was confirmed using the QHES tool: the mean score was 75.77 out of 100 (SD 9.38).
Conclusion
Most of these high-quality studies suggested that erlotinib was cost-effective in the first-line treatment of advanced NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The IARC Global Cancer Observatory has estimated that in 2018 the global cancer burden have risen to 18.1 million new cases and 9.6 million deaths in 185 countries [1,2,3]. Several factors as population growth, ageing, social and economic development, lifestyles, diet more typical of industrialized countries are associated with the increasing cancer burden [4,5,6,7,8,9].
Lung cancer is caused mainly by smoking [10,11,12], and represents the leading cause of cancer deaths in the world, with about 1.8 million cases (12.9% of all cancers) and 1.59 million deaths (19.4% of all cancer-related deaths) per year [13,14,15]. Among lung cancers, non-small cell lung cancer (NSCLC) is the most common type (85–90% cases of all lung cancers) [16, 17]. Most patients are diagnosed in the advanced stage (79%) [18] with the presentation of symptoms related to the primary tumor (27%), nonspecific systemic symptoms suggestive of metastases (34%) or symptoms specific to a metastatic site (32%) [19]. Lung cancer is difficult to treat because its pathological mechanisms are still poorly understood. This disease is a significant economic burden not only to patients but also to national healthcare systems.
Recent advances in our understanding of the cell signaling pathways that control cell survival have opened up many new therapies that improve the 5-year survival rate for patients [20]. Among these new methods, targeted therapy with the typical drug, erlotinib (Tarceva), has been found to limit tumor development and progression by inhibiting the epidermal growth factor receptor (EGFR) mutations found in 10–15% of cases in North America and Western Europe and up to 51.4% of cases among Asian patient [21,22,23,24,25].
Erlotinib was approved for second-line therapy by the FDA in 2005, and then for first-line treatment of advanced EGFR mutation-positive NSCLC in 2013, because it was proven superior to chemotherapy for progression-free survival in patients whose tumors harbor sensitizing driver mutations in the EGFR gene [26,27,28]. However, for decision makers, the choice of treatment depends not only on effectiveness but also on treatment costs [29]. The aim of the study was to review the available evidence on the cost-effectiveness of erlotinib in the first-line treatment of advanced NSCLC and evaluate the quality of studies.
Materials and methods
Systematic literature review
A systematic literature search was conducted in PubMed, Cochrane, Scopus and ScienceDirect, all well-known and respected databases, in March 2017 to identify articles published from January 2000 onwards that report a pharmacoeconomic evaluation of erlotinib in first-line treatment of advanced NSCLC. The search process was repeated in December 2017 to ensure up to date results. The main key words, formed using the PICO technique (Table 1), were “advanced non-small cell lung cancer”, “erlotinib”, “first-line”, “cost-effectiveness”, “cost-utility”, “economic evaluation”, “cost-minization”, “cost-benefit” and synonyms identified using the Medical Subject Heading (MeSH) tool. Search terms were combined with boolean searching terms (“AND”, “OR”) and adapted as required for each database, for example, #1 “non-small cell lung cancer” OR “non-small cell bronchial cancer” OR “NSCLC” AND #2 “advanced” OR “stage IV” OR “stage IIIB” OR “metastatic” AND #3 “first-line” AND #4 “erlotinib” AND #5 “economic evaluation” OR “economic analysis” OR “cost minimization” OR “cost effective” OR “cost benefit” OR “cost utility”.
The titles and abstracts of all the publications that met the search criteria were reviewed independently by two researchers. Full-text articles written in English were captured if they provided an original economic evaluation of erlotinib in first-line treatment of NSCLC patients. Clinical trials, review papers, posters, or studies that did not assess the full pharmacoeconomics, or in other words, that did not compare both costs and consequences of interventions or conditions, and studies without a specific effectiveness evaluation were eliminated. Any disagreement between the two reviewers was solved through discussion.
After reviewing, the following information from these studies was recorded in a table: (1) bibliography as year, authors and location of studies, (2) study design including aim, interventions and alternatives, the study perspective, costs and outcomes, type of model, time horizon and cycle, discount rate and sensitivity analysis, and (3) results of the study.
To compare the results of the studies, all currency values were converted into 2017 international dollars (I$) based on Consumer Price Index (CPI) and Purchasing Power Parity (PPP) [30] by the following formulas:
in which, Costpresent: cost, converted into 2017 I$. Costpast: cost in the year of the study was conducted, CPIpresent: average consumer price index in 2017. CPIpast: consumer price index of the year when the study was conducted.
Quality evaluation
The quality of the studies was assessed by two reviewers who were not given author or affiliation information, using an internationally accepted quality checklist, the Quality of Health Economic Studies (QHES), which was developed by five experts in the field of health economics and three investigators [31]. This questionnaire consists of 16 questions with the dichotomous criteria scores to which the answers yes (full score) or, no, (zero score). In our study, the scale was modified with three answers: no (zero score), yes (full score) and partial (half of full score). The QHES assigns a weight to each question according to its importance (ranging from 1 to 9 points). The total number of points possible is 100 (adding up the score from 1 to 9 assigned to the ‘yes’ answer for each question), thus 0 represents lowest quality and 100 highest quality. In case of a disagreement between the reviewers on any of the questions, a third reviewer acted as a tie-breaker. Since there is no standard interpretation for the ranking of QHES scores, we decided that a score from 75 to 100 indicated good quality, 50–75 medium quality and below 50 low quality. Moreover, the relationship between the quality of the studies (QHES scores) and the independent variables was also evaluated using t test and Pearson’s correlation coefficient. The independent variables consisted of the number of authors, the locations of authors, the years from publication to 2017, funding sources, study design and location of study. A p value of < 0.05 is considered as statistical significance.
Results
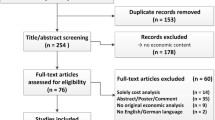
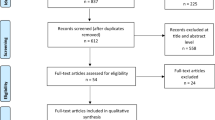
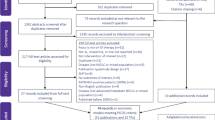
A total of 130 studies met our search criteria: 30 from Pubmeb, 8 from Cochrane, 36 from Scopus and 56 from Sciencedirect. After removing duplicates, 99 studies remained, and once we screened their titles and/or abstracts, 11 studies remained that met the inclusion and exclusion criteria. Thus, 88 studies were eliminated: 4 were not about first-line treatment, 19 did not provide a pharmacoeconomic evaluation, 1 was not a full pharmacoeconomics, 21 did not appraise the cost-effectiveness of erlotinib, 3 did not assess NSCLC disease, 1 was not written in English, 17 were systematic reviews, and 22 were only abstracts or programs from scientific conferences. The 11 articles that met our criteria were read in full and assessed for eligibility, and all were found acceptable (Fig. 1). Their details and content were then summarized in Table 2.
Study characteristics
Studies of economic evaluation of erlotinib were carried out in the interval between 2012 and 2017 in many countries all around the world including Europe (UK, Germany, France, Spain, Italy) [32,33,34,35,36,37,38] Asia (Hongkong, China) [39,40,41] and America (USA) [42], but the largest number of studies were in Europe (64%) and in 2012 and 2015 (54%). Among 11 studies, 5 studies compared erlotinib with the best supportive care, 2 studies dealt with reverse strategy, 1 study compared erlotinib in combination with bevacizumab with bevacizumab alone, the others compared with cisplatin plus pemetrexed, carboplatin plus gemcitabine and gefitinib. The target population in most studies comprised elderly patients with advanced stage EGFR-mutated NSCLC.
The main methods used in most studies were cost-effectiveness analysis (CEA) with modeling method and sensitivity analysis for uncertainty of model, including multi-way probabilistic sensitivity analysis in all studies and one-way deterministic analysis in five studies [34,35,36, 40, 41]. Regarding the modeling method, the health-state transition model was used in the majority of studies (8 of 11 studies), the Markov model being the most chosen (75%). In the health-state transition model, the model cycle was 1 month in the majority of the studies and 3 weeks in the studies conducted in Germany [34] and China [40]. The model horizon was different in each of articles, with a lifetime horizon in 2 studies [39, 42], 10 years in 3 studies [34, 40, 41], 4 years in 1 study [32] and with no specification in 2 studies [35, 36]. In 3 remaining studies, 2 studies used partitioned survival model [37, 38] with a 1 month cycle and a lifetime horizon [38] or 5 a year horizon, while 1 study was based on the case report forms collected monthly [33]. The main clinical data sources of these studies were EURTAC (European Clinical Trials Database No. 2006-003568-73), GFPC 0505 (a prospective randomized phase II trial), SATURN (the randomized multicenter placebo controlled phase 3 patient-level data trial) and OPTIMAL trial (a multicentre open-label randomised phase 3 study).
Regarding the perspectives of these publications, 2 studies by Chouiad were based on a third-party perspective [35, 36] and 7 remaing studies (except the Ting study [42] and the Khan study [33]) were based on the perspective of the health care system [32, 34, 37,38,39,40,41], so they calculated only direct costs consisting of chemotherapy, hospitalization, medications, diagnostic imaging, consumables, treating adverse events, procedures, laboratory tests, and support care. Two studies of Ting [42] and Khan [33] did not mention the perspective, but they did not assess indirect costs, so they were not based on society perspective. Main outcomes obtained in the studies were life-years gain (LYG), quality-adjusted life years (QALY) and progression-free life-years gained (PF-LY). A discount rate of 3% in 5 studies [32, 34, 39, 40, 42] and 3.5% in 5 studies [33, 35,36,37,38] were used for both cost and effectiveness. The Fangbing study did not mention a discount rate [41].
Cost-effectiveness
After converting the currencies used in the studies to I$, using the CPI and PPP, we found that erlotinib in the first-line treatment of advanced NSCLC had incremental cost-effectiveness ratios (ICER) in terms of QALY and LYG (ICER/QALY and ICER/LYG) that ranged from dominant, effectively superior and cost saving, to I$305,510.31/QALY (Fig. 2) and from I$31,209.55/LYG to I$66,540.20/LYG (Fig. 3). In the studies conducted in Europe, the cost-effectiveness acceptability curves resulting from probabilistic sensitivity analyses showed that the probability of erlotinib being cost-effective was over 50%, if the range of willingness-to-pay (WTP) threshold was €50.000–€70.000 [34, 37], even 100% in the research of Walleser et al. [38]. It would be 100% in all studies in Europe if WTP was at least €90.000. About studies in Asia, at the threshold of approximately US$100.000, erlotinib had a 100% probability of being cost-effective in Hong Kong [39], but 50% probability in China [40]. One study showed that erlotinib exhibited about 10% chance of being cost-effective in all patients and about 80% chance in a subset of elderly patients with NSCLC who had poor performance, were deemed unfit for chemotherapy and developed first cycle (28 days) rash in the range of WTP of £20,000–£30,000 [33]. Two other studies in France comparing erlotinib with reverse strategy did not find a difference in cost-effectiveness [36, 43] and 1 study comparing a combination of erlotinib and bevacizumab with bevacizumab alone suggested that the strategy with bevacizumab alone was the best in terms of cost-effectiveness [41].
When comparing the results regarding comparative regimens, a significant discrepancy emerged between the results in comparision erlotinib with best supportive care. Although all these studies were carried out in Europe, the result of Vergnenegre (result in three European countries including Italy (I$42,801.60/LYG), France (I$54,170.50/LYG) and Germany (I$66,540.20/LYG)) [36] was higher than that of Walleser (result in 5 European countries including Italy (I$32,301.04/LYG), France (I$31,209.55/LYG), Germany (I$35,232.67/LYG), UK (I$33,557.67/LYG) and Spain (I$36,688.71/LYG)) [37]. Similarly, studies comparing erlotinib and chemotherapy regimens found significant difference: the results ranged from I$20,434.34/QALY to I$134,696.97/QALY. Regarding the regimen of erlotinib followed by chemotherapy after disease progression compared to the reverse strategy, in the Chouaid study on fit elderly patients, there was no difference in outcomes between the two strategies, but the erlotinib-first strategy was less costly [43]. However, similar research by Chouaid, but on frail elderly patients, found no difference in cost-effectiveness [36].
The quality of the reports
On the results of the quality evaluation through the 16 questions in the QHES quality questionnaire, 7 studies were of high quality, and 4 of medium quality. The mean score of these 11 studies was 75.77 out of 100 (SD 9.38). The more recent studies were among the higher quality ones. The highest score was assigned to the Shcremser article published in 2015 [34] which was assigned a score of 92. The studies by Khan and Ting published in 2015 [33, 42] and Vergnenegre published in 2016 [32] were ranked the second highest with a score of 81–86. Three studies published in 2013, 2014, 2017 were ranked the third highest, with slightly higher scores in 2013 and 2014 (“Appendix 1”).
Regarding our answers to the 16 questions to evaluate the quality of each study, all the studies were assigned a ‘yes’ answer to questions 1,4, 6 and 15, which regard the study objective, pre-specification of the groups at the beginning of the study, performance of incremental analysis between alternatives, and the conclusions/recommendations of the study. In contrast, lowest scores to these studies were given for question 2 (over 50% studies got partial score because of not stating reasons for perspective selection in study), question 7 (over 50% studies got partial score because of lacking of explicit methodological detail on costing/resource use and health/patient measures or above and beyond the source of costs and the name of the scale used to measure health states or other effectiveness outcomes [44]), question 10 and question 12 (about 30% articles did not get full score for these items) and question 14 (all studies did not explicitly discuss direction and magnitude of potential biases) (Fig. 4).
When analysing the relationship between the variables and the quality of studies based on the mean QHES scores, articles that the years since publication were under 3 years had a significantly higher mean score than the studies that the years were from 3 years or more (83.0 versus 69.8; p = 0.01), that is, the more recent studies were the higher quality ones. Regarding the study design, there were not enough articles in each category to conduct meaningful statistical comparison, so we evaluated the relationship between the quality of studies and the types of model used in the studies (Markov model or not). The result showed that there was a significantly higher mean quality score in the studies that used Markov model than the studies that used other models (80.83 compared with 65.75; p = 0.002). For locations of study, locations of authors, number of authors and funding source, there was not significantly related to the mean QHES score (Table 3).
Discussion
This comprehensive review examined studies on the cost-effectiveness of erlotinib in the first-line treatment of advanced NSCLC, and assessed the quality of the studies using the QHES questionnaire, a well-known and trusted tool. Unlike other systematic reviews, this review focused on the first-line treatment of advanced NSCLC instead of later lines of treatment, and on erlotinib regimen rather than other regimens, and drew upon many recent and up-to-date publications, as well as evaluated the quality of studies [35, 45,46,47,48] In doing so, we sought to give healthcare policymakers a sense of the reliability of the research available, to help inform their decisions in the first-line treatment of NSCLC with erlotinib.
An initial keyword search in 4 respected medical databases yielded 130 articles on our topic; after reading the titles and articles to exclude duplicates and publications that did not meet our criteria, 11 studies were identified for this review. The main methods used in most studies were cost-effectiveness analysis (CEA) with modeling method and sensitivity analysis for the uncertainty of model. The use of modeling method in CEA studies regarding treatments for chronic diseases is indispensable because it allows evaluation of costs and clinical outcomes based on a number of types of data from many different sources, and makes it possible to extrapolate efficacy for the general population, especially when there is insufficient data from longer-term or head-to head clinical trials [49]. However, the modeling method is also a key limitation of the erlotinib pharmacoeconomic analyses which leads to a large amount of uncertainty in the analyses. As a result, the authors of most studies built a set of assumptions and sensitivity analysis to solve these problems with multi-way probability sensitive analysis chosen in all studies. In their model, they also set up a series of treatment scenarios that were close to reality and difficult to realize in a clinical trial with sufficient power and an appropriate time horizon.
Through converted results, there was the variability of ICER/QALY (about tenfold). This discrepancy may come from the difference in the comparators and the methods of researches. While other studies used modeling method and chemotherapy or targeted drugs in comparators, Khan et al. [33] chose the real data and the best supportive care in comparison. The choice of the comparator had important implications not only for trial ethics and recruitment but also for results and interpretation and depended mainly on the specific assessment question [50]. Compared to other studies, the study of Khan et at aimed to patients who were unfit for chemotherapy and received only active supportive care due to their poor performance status or presence of comorbidities [33]. This choice was relevant not only for demonstrating the efficacy and cost of erlotinib but also for assessing its place in therapy in comparison with existing therapy [50]. Results of the studies came from the perspective of a health care system and third-party payer were limited by the direct costs, while indirect costs associated with travel, loss of productivity and other factors may accounted for high value. To have comprehensive overview of the economic burden of erlotinib in the treatment of advanced NSCLC, these indirect costs should be assessed as well. Most studies agreed that erlotinib was cost-effective compared with best supportive care and chemotherapy (except the studies by Khan [33] and Fangbing [41]) across the range of possible WTP thresholds, but concluded that it was no more cost-effective than reverse strategy. One of the factors that had significant impacts on the ICER was price of erlotinib, which was shown in the results of the deterministic sensitivity analysis in the studies of Schremser et al. [34] and Wang et al. [40]. The change in the cost of erlotinib caused the second highest change in ICER in the group of selected cost variables. Thus, price improvement was a potential strategy to improve the cost-effectiveness of erlotinib. In China, all eligible Chinese patients (EGFR mutation-positive NSCLC) would be able to use erlotinib free of charge after they have treated with erlotinib continuously for 5 months through the donations of manufacturer [40]. In UK guidance, erlotinib was provided at the discounted price agreed under the patient access scheme [51]. In other countries that lacked this special benefit, erlotinib monotherapy would fail to be cost-effective [40].
Using the QHES instrument, the quality of these 11 studies was assessed. Most of them got a high score (an average of 75.77 out of 100), with the more recent publications earning the higher scores for quality. This could be explained that the authors had reference and followed up-to-date about guidelines for reporting health economic evaluation. This reference is necessary for authors to upgrade the quality of their reports, which make their results valuable and trustworthy for decision-makers. The QHES modification in three answer instead of the dichotomous scores (mid-score was applied if the article only met the half the requirements of the item) made the evaluation closer. For examples in the item 2, most authors stated the research perspective in the studies, which was important to identify the included cost components, but they did not mention to the reasons for its selection that did not deserve full points but did not deserve zero points either. As a result, it was to note the need for improvement in regarding questions 2, 7, 10, 12, 14, on which the studies generally failed to score well.
As usual in pharmacoeconomic evaluations, almost all of the analyses were fully or partially funded by the manufacturer if their medicines were included in the research. The source of funding was made clear in most of the analyses: seven studies specified their source of funding, one study indicated that it received no funding and three studies failed to indicate anything about their support. Among 7 studies funded, 2 received financial support from pharmaceutical companies (28.6%), 2 received funding from government fund (28.6%) and 3 were supported by both pharmaceutical companies and national source (42.8%).
Our study has some limitations. The criterion limiting the articles to those written in English and published from January 2000 onwards meant that we might have excluded many previous and non-English studies. Besides, the choice of only four databases and not including unpublished researches (grey literature) generated some biases. This review evaluated studies’ reporting quality which might not reflect the quality of the study [52]. Moreover, although good inter-rater reliability was noted for scores in QHES scale, other evaluators may score the articles differently [53].
Conclusion
A searching and screening process led to the identification of 11 studies, which were then analysed and assessed for quality. Depending on the frequently proposed willing to pay threshold of each country, most concluded that erlotinib was cost-effective in the first-line treatment of advanced NSCLC patients, two studies showed that erlotinib was cost-effective on specific types of patients with certain conditions, and two studies found no difference in cost-effectiveness between erlotinib and reverse strategy. The quality of the studies was high, with a mean QHES score of 75.77 (SD 9.38) on a scale from 0 to 100.
References
Siracusa, M., Grappasonni, I., Petrelli, F.: The pharmaceutical care and the rejected constitutional reform: what might have been and what is. Acta Biomed. 88(3), 352–359 (2017). https://doi.org/10.23750/abm.v88i3.6376
Signorelli, C., Odone, A., Gozzini, A., Petrelli, F., Tirani, M., Zangrandi, A., Zoni, R., Florindo, N.: The missed constitutional reform and its possible impact on the sustainability of the Italian National Health Service. Acta Biomed. 88(1), 91–94 (2017). https://doi.org/10.23750/abm.v88i1.6408
International Agency for Research on Cancer, World Health Organization. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Press Release no. 263. https://www.who.int/cancer/PRGlobocanFinal.pdf. Accessed 12 Sept 2018
Petrelli, F., Scuri, S., Tanzi, E., Nguyễn, T.T.C., Grappasonni, I.: Lifestyles and discomfort in a sample of young Romanian students. J Prev Med Hyg. 59(3), E230–E235 (2018). https://doi.org/10.15167/2421-4248/jpmh2018.59.3.985
Grappasonni, I., Scuri, S., Tanzi, E., Kracmarova, L., Petrelli, F.: The economic crisis and lifestyle changes: a survey on frequency of use of medications and of preventive and specialistic medical care, in the Marche Region (Italy). Acta Biomed. 89(1), 87–92 (2018). https://doi.org/10.23750/abm.v89i1.7068
Siracusa, M., Petrelli, F.: Trade of food supplement: food or drug supplement?. Recenti Prog Med. 107(9), 465–471 (2016). https://doi.org/10.1701/2354.25224
Grappasonni, I., Marconi, D., Mazzucchi, F., Petrelli, F., Scuri, S., Amenta, F.: Survey on food hygiene knowledge on board ships. Int Marit Health 64(3), 160–167 (2013)
Grappasonni, I., Petrelli, F., Klusoňová, H., Kračmarová, L.: Level of understanding of medical terms among italian students. Ceska Slov Farm. Winter 65(6), 216–220 (2016)
Spacilova, L., Klusonova, H., Petrelli, F., Signorelli, C., Visnovsky, P., Grappasonni, I.: Substance use and knowledge among Italian high school students. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 153(2), 163–168 (2009)
Kračmarová, L., Klusoňová, H., Petrelli, F., Grappasonni, I.: Tobacco, alcohol and illegal substances: experiences and attitudes among Italian university students. Rev. Assoc. Med. Bras. (1992) 57(5), 523–528 (2011)
Petrelli, F., Grappasonni, I., Peroni, A., Kracmarova, L., Scuri, S.: Survey about the potential effects of economic downturn on alcohol consumption, smoking and quality of life in a sample of Central Italy population. Acta Biomed. 89(1), 93–98 (2018). https://doi.org/10.23750/abm.v89i1.7059
Grappasonni, I., Petrelli, F., Amenta, F.: Deaths on board ships assisted by the Centro Internazionale Radio Medico in the last 25 years. Travel Med Infect Dis. 10(4), 186–191 (2012). https://doi.org/10.1016/j.tmaid.2012.06.006
Ferlay, J.S.-F., Lortet-Tieulent, E., Rosso, J., Coebergh, S., Jan-Willem, W., Comber, H., Forman, D., Bray, F.: Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6), 1374–1403 (2013)
Ferlay, J., Ervik, I.S.,M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D.M., Forman, D., Bray, F.: Cancer Incidence and Mortality Worldwide. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (2012). Accessed 15 Dec 2016
Siegel, R., Naishadham, D., Jemal, A.: Cancer statistics, 2013. CA Cancer J. Clin. 63(1), 11–30 (2013)
Navada, S., Lai, P., Schwartz, A., Kalemkerian, G.: Temporal trends in small cell lung cancer: analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J. Clin. Oncol. 24(18_suppl), 7082–7082 (2006)
Sher, T., Dy, G.K., Adjei, A.A.: Small cell lung cancer. In: Mayo Clinic Proceedings 2008, vol. 3, pp. 355–367. Elsevier
Surveillance, E., and End Results Program: SEER stat fact sheets: lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html (2013). Accessed 15 Oct 2017
Spiro, S.G., Gould, M.K., Colice, G.L.: Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP evidenced-based clinical practice guidelines (2nd edn.). Chest 132(3 Suppl), 149 s–160 s (2007). https://doi.org/10.1378/chest.07-1358
Cuccioloni, M., Bonfili, L., Mozzicafreddo, M., Cecarini, V., Scuri, S., Cocchioni, M., Nabissi, M., Santoni, G., Eleuteri, A.M., Angeletti, M.: Mangiferin blocks proliferation and induces apoptosis of breast cancer cells via suppression of the mevalonate pathway and by proteasome inhibition. Food Funct. 7(10), 4299–4309 (2016)
Shi, Y., Au, J.S., Thongprasert, S., Srinivasan, S., Tsai, C.M., Khoa, M.T., Heeroma, K., Itoh, Y., Cornelio, G., Yang, P.C.: A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 9(2), 154–162 (2014). https://doi.org/10.1097/jto.0000000000000033
Marchetti, A., Martella, C., Felicioni, L., Barassi, F., Salvatore, S., Chella, A., Camplese, P.P., Iarussi, T., Mucilli, F., Mezzetti, A., Cuccurullo, F., Sacco, R., Buttitta, F.: EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 23(4), 857–865 (2005). https://doi.org/10.1200/jco.2005.08.043
Doval, D., Prabhash, K., Patil, S., Chaturvedi, H., Goswami, C., Vaid, A., Desai, S., Dutt, S., Veldore, V., Jambhekar, N., Mehta, A., Hazarika, D., Azam, S., Gawande, S., Gupta, S.: Clinical and epidemiological study of EGFR mutations and EML4-ALK fusion genes among Indian patients with adenocarcinoma of the lung. Oncotargets Ther. 8, 117–123 (2015). https://doi.org/10.2147/ott.s74820
Sharma, S.V., Bell, D.W., Settleman, J., Haber, D.A.: Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7(3), 169–181 (2007). https://doi.org/10.1038/nrc2088
Cioffi, P., Marotta, V., Fanizza, C., Giglioni, A., Natoli, C., Petrelli, F., Grappasonni, I.: Effectiveness and response predictive factors of erlotinib in a non-small cell lung cancer unselected European population previously treated: a retrospective, observational, multicentric study. J. Oncol. Pharm. Pract. 19(3), 246–253 (2013). https://doi.org/10.1177/1078155212465994
De Grève, J., Van Meerbeeck, J., Vansteenkiste, J.F., Decoster, L., Meert, A.P., Vuylsteke, P., Focan, C., Canon, J.L., Humblet, Y., Berchem, G., Colinet, B., Galdermans, D., Bosquée, L., Vermeij, J., Dewaele, A., Geers, C., Schallier, D., Teugels, E.: Prospective evaluation of first-line erlotinib in advanced non-small cell lung cancer (NSCLC) carrying an activating egfr mutation: a multicenter academic phase II study in caucasian patients (FIELT). PLoS One (2016). https://doi.org/10.1371/journal.pone.0147599
Zhou, C., Wu, Y.L., Chen, G., Feng, J., Liu, X.Q., Wang, C., Zhang, S., Wang, J., Zhou, S., Ren, S., Lu, S., Zhang, L., Hu, C., Hu, C., Luo, Y., Chen, L., Ye, M., Huang, J., Zhi, X., Zhang, Y., Xiu, Q., Ma, J., Zhang, L., You, C.: Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12(8), 735–742 (2011). https://doi.org/10.1016/s1470-2045(11)70184-x
Rosell, R., Carcereny, E., Gervais, R., Vergnenegre, A., Massuti, B., Felip, E., Palmero, R., Garcia-Gomez, R., Pallares, C., Sanchez, J.M., Porta, R., Cobo, M., Garrido, P., Longo, F., Moran, T., Insa, A., De Marinis, F., Corre, R., Bover, I., Illiano, A., Dansin, E., de Castro, J., Milella, M., Reguart, N., Altavilla, G., Jimenez, U., Provencio, M., Moreno, M.A., Terrasa, J., Munoz-Langa, J., Valdivia, J., Isla, D., Domine, M., Molinier, O., Mazieres, J., Baize, N., Garcia-Campelo, R., Robinet, G., Rodriguez-Abreu, D., Lopez-Vivanco, G., Gebbia, V., Ferrera-Delgado, L., Bombaron, P., Bernabe, R., Bearz, A., Artal, A., Cortesi, E., Rolfo, C., Sanchez-Ronco, M., Drozdowskyj, A., Queralt, C., de Aguirre, I., Ramirez, J.L., Sanchez, J.J., Molina, M.A., Taron, M., Paz-Ares, L.: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13(3), 239–246 (2012). https://doi.org/10.1016/s1470-2045(11)70393-x
Cioffi, P., Laudadio, L., Nuzzo, A., Belfiglio, M., Petrelli, F., Grappasonni, I.: Gemcitabine-induced posterior reversible encephalopathy syndrome: a case report. J. Oncol. Pharm. Pract. 18(2), 299–302 (2012). https://doi.org/10.1177/1078155211424628
World Bank: World development indicators: consumer price index. http://databank.worldbank.org/data/reports.aspx?source=2&series=FP.CPI.TOTL&country=(2015). Accessed 17 July 2015
Ofman, J.J., Sullivan, S.D., Neumann, P.J., Chiou, C.F., Henning, J.M., Wade, S.W., Hay, J.W.: Examining the value and quality of health economic analyses: implications of utilizing the QHES. J. Manag. Care Pharm. JMCP. 9(1), 53–61 (2003). https://doi.org/10.18553/jmcp.2003.9.1.53
Vergnenegre, A., Massuti, B., de Marinis, F., Carcereny, E., Felip, E., Do, P., Sanchez, J.M., Paz-Arez, L., Chouaid, C., Rosell, R.: Economic analysis of first-line treatment with erlotinib in an EGFR-mutated population with advanced NSCLC. J. Thorac. Oncol. 11(6), 801–807 (2016)
Khan, I., Morris, S., Hackshaw, A., Lee, S.-M.: Cost-effectiveness of first-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy. BMJ Open 5(7), e006733 (2015)
Schremser, K., Rogowski, W.H., Adler-Reichel, S., Tufman, A.L., Huber, R.M., Stollenwerk, B.: Cost-effectiveness of an individualized first-line treatment strategy offering erlotinib based on EGFR mutation testing in advanced lung adenocarcinoma patients in Germany. Pharmacoeconomics 33(11), 1215–1228 (2015)
Chouaid, C., Crequit, P., Borget, I., Vergnenegre, A.: Economic evaluation of first-line and maintenance treatments for advanced non-small cell lung cancer: a systematic review. Clinicoecon. Outcomes Res. CEOR. 7, 9–15 (2015). https://doi.org/10.2147/ceor.s43328
Chouaid, C., Le Caer, H., Corre, R., Crequit, J., Locher, C., Falchero, L., Dujon, C., Berard, H., Monnet, I., Vergnenegre, A.: Cost analysis of erlotinib versus chemotherapy for first-line treatment of non-small-cell lung cancer in frail elderly patients participating in a prospective phase 2 study (GFPC 0505). Clin. Lung Cancer 14(2), 103–107 (2013)
Vergnenègre, A., Ray, J.A., Chouaid, C., Grossi, F., Bischoff, H.G., Heigener, D.F., Walzer, S.: Cross-market cost-effectiveness analysis of erlotinib as first-line maintenance treatment for patients with stable non-small cell lung cancer. Clinicoecon. Outcomes Res. CEOR 4, 31 (2012)
Walleser, S., Ray, J., Bischoff, H., Vergnenègre, A., Rosery, H., Chouaid, C., Heigener, D., de Castro Carpeño, J., Tiseo, M., Walzer, S.: Maintenance erlotinib in advanced nonsmall cell lung cancer: cost-effectiveness in EGFR wild-type across Europe. Clinicoecon. Outcomes Res. CEOR 4, 269 (2012)
Lee, V.W., Schwander, B., Lee, V.H.: Effectiveness and cost-effectiveness of erlotinib versus gefitinib in first-line treatment of epidermal growth factor receptor-activating mutation-positive non-small-cell lung cancer patients in Hong Kong. Hong Kong Med. J. 20(3), 178–186 (2014)
Wang, S., Peng, L., Li, J., Zeng, X., Ouyang, L., Tan, C., Lu, Q.: A trial-based cost-effectiveness analysis of erlotinib alone versus platinum-based doublet chemotherapy as first-line therapy for Eastern Asian nonsquamous non-small-cell lung cancer. PLoS One 8(3), e55917 (2013)
Lv, F., Yu, K., Gao, W., Yu, S.: Cost-utility of erlotinib combined with bevacizumab versus bevacizumab alone after completion of chemotherapy with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J. Chin. Pharm. Sci. 26(6), 447–454 (2017)
Ting, J., Ho, P.T., Xiang, P., Sugay, A., Abdel-Sattar, M., Wilson, L.: Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mutation-positive non-small-cell lung cancer in the United States. Value Health 18(6), 774–782 (2015)
Christos, C., Chrystelle, L., Cecile, D., Pascal, T., Bernard, A.J., Isabelle, M., Alain, V.: Cost effectivenes of erlotinib versus chemotherapy for first-line treatment of non small cell lung cancer (NSCLC) in fit elderly patients participating in a prospective phase 2 study (GFPC 0504). BMC Cancer 12(1), 301 (2012)
Marshall, D.A., Donald, F., Lacny, S., Reid, K., Bryant-Lukosius, D., Carter, N., Charbonneau-Smith, R., Harbman, P., Kaasalainen, S., Kilpatrick, K.: Assessing the quality of economic evaluations of clinical nurse specialists and nurse practitioners: a systematic review of cost-effectiveness. NursingPlus Open 1, 11–17 (2015)
Jäkel, A., Plested, M., Dharamshi, K., Modha, R., Bridge, S., Johns, A.: A systematic review of economic evaluations in second and later lines of therapy for the treatment of non-small cell lung cancer. Appl. Health Econ. Health Policy 11(1), 27–43 (2013)
Lange, A., Prenzler, A., Frank, M., Golpon, H., Welte, T., von der Schulenburg, J.-M.: A systematic review of the cost-effectiveness of targeted therapies for metastatic non-small cell lung cancer (NSCLC). BMC Pulm. Med. 14(1), 192 (2014)
Lyseng-Williamson, K.A.: Erlotinib: a pharmacoeconomic review of its use in advanced non-small cell lung cancer. Pharmacoeconomics. 28(1), 75–92 (2010). https://doi.org/10.2165/10482880-000000000-00000
Bongers, M.L., Coupe, V.M., Jansma, E.P., Smit, E.F., Uyl-de Groot, C.A.: Cost effectiveness of treatment with new agents in advanced non-small-cell lung cancer: a systematic review. Pharmacoeconomics. 30(1), 17–34 (2012). https://doi.org/10.2165/11595000-000000000-00000
Siebert, U.: When Should Decision-Analytic Modeling be Used in the Economic Evaluation of Health Care? Springer, Berlin (2003)
Luijn, J.C.v., Loenen, A.C.v., Gribnau, F.W., Leufkens, H.G.: Choice of comparator in active control trials of new drugs. Ann. Pharmacother. 42(11), 1605–1615 (2008). https://doi.org/10.1345/aph.1L115
National Institute for Health and Clinical Excellence: Erlotinib and gefitinib for treating non-small-cell lung cancer that has progressed after prior chemotherapy. https://www.nice.org.uk/guidance/ta374/chapter/1-Guidance (2015). Accessed 20 Nov 2018
Littlewood, C., Ashton, J., Chance-Larsen, K., May, S., Sturrock, B.: The quality of reporting might not reflect the quality of the study: implications for undertaking and appraising a systematic review. J. Man. Manip. Ther. 20(3), 130–134 (2012). https://doi.org/10.1179/2042618611y.0000000013
Desai, P.R., Chandwani, H.S., Rascati, K.L.: Assessing the quality of pharmacoeconomic studies in India. Pharmacoeconomics 30(9), 749–762 (2012)
Acknowledgements
No funding has been received for the conduct of this study and/or preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
CTTN: study rationale and design, literature search, literature selection, quality assessment of studies, interpretation and reflection, writing of the manuscript. FP: literature search, literature selection, quality assessment of studies, revising and reviewing of the manuscript. SS: literature selection, interpretation and reflection, revising and reviewing of the manuscript. BTN: study rationale and design, revising and reviewing the manuscript. IG: quality assessment of studies, interpretation and reflection, providing critical comments, revising and reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest exist in this work. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Location—published year—author | Europe—2016—Vergnenegre [32] | USA—2015—Ting [42] | UK—2015—Khan [33] | Germany—2015—Schremser [34] | Hong Kong—2014—Lee [39] | China—2013—Wang [40] | Europe—2012—Walleser [38] | France—2013—Chouaid [36] | France—2012—Chouaid [43] | Europe—2012—Vergnenègre [37] | China—2017—Fangbing [41] |
|---|---|---|---|---|---|---|---|---|---|---|---|
QHES question (y = full score, p = partial score, n = zero score) | |||||||||||
1. Was the study objective presented in a clear, specific, and measurable manner? | y | y | y | y | y | y | y | y | y | y | y |
2. Were the perspective of the analysis (societal, third-party payer, etc.) and reasons for its selection stated? | p | n | n | p | p | y | y | p | p | p | n |
3. Were variable estimates used in the analysis from the best available source (i.e., randomized control trial - best, expert opinion - worst)? | y | y | y | y | y | p | p | y | y | y | y |
4. If estimates came from a subgroup analysis, were the groups prespecified at the beginning of the study? | y | y | y | y | y | y | y | y | y | y | y |
5. Was uncertainty handled by (1) statistical analysis to address random events, (2) sensitivity analysis to cover a range of assumptions? | y | y | y | y | y | y | y | y | y | n | y |
6. Was incremental analysis performed between alternatives for resources and costs? | y | y | y | y | y | y | y | y | y | y | y |
7. Was the methodology for data abstraction (including the value of health states and other benefits) stated? | p | p | y | y | p | y | p | p | p | n | y |
8. Did the analytic horizon allow time for all relevant and important outcomes? Were benefits and costs that went beyond 1 year discounted (3–5%) and justification given for the discount rate? | y | y | y | y | y | y | y | n | n | y | n |
9. Was the measurement of costs appropriate and the methodology for the estimation of quantities and unit costs clearly described? | y | y | y | y | p | y | n | y | y | y | y |
10. Were the primary outcome measure(s) for the economic evaluation clearly stated and did they include the major short-term? was justification given for the measures/scales used? | y | p | y | y | p | n | y | p | p | n | y |
11. Were the health outcomes measures/scales valid and reliable? If previously tested valid and reliable measures were not available, was justification given for the measures/scales used? | p | p | y | y | y | n | y | y | y | y | n |
12. Were the economic model (including structure), study methods and analysis, and the components of the numerator and denominator displayed in a clear, transparent manner? | y | y | p | y | p | y | p | n | n | y | y |
13. Were the choice of economic model, main assumptions, and limitations of the study stated and justified? | y | y | y | y | y | y | p | n | n | n | y |
14. Did the author(s) explicitly discuss direction and magnitude of potential biases? | n | n | n | n | n | n | n | n | n | n | n |
15. Were the conclusions/recommendations of the study justified and based on the study results? | y | y | y | y | y | y | y | y | y | y | y |
16. Was there a statement disclosing the source of funding for the study? | n | y | y | y | y | y | n | y | y | y | n |
Total score | 83 | 81 | 86 | 92 | 79 | 77 | 69 | 64.5 | 64.5 | 65 | 73 |
Rights and permissions
About this article
Cite this article
Nguyen, C.T.T., Petrelli, F., Scuri, S. et al. A systematic review of pharmacoeconomic evaluations of erlotinib in the first-line treatment of advanced non-small cell lung cancer. Eur J Health Econ 20, 763–777 (2019). https://doi.org/10.1007/s10198-019-01040-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01040-7