Abstract
Background/Aims
Improved data and methods are needed for modeling disease progression in Alzheimer’s disease (AD) for economic evaluation of treatments. The aim is to estimate prediction models for long-term AD progression and subsequently economic outcomes.
Methods
Three-year follow-up data on 435 patients treated with the cholinesterase inhibitor donepezil in clinical practise were analyzed. Regression models were estimated for long-term prediction of decline in cognitive function (ADAS-cog) and activities in daily living (ADL) ability, risk of institutionalization and costs of care.
Results
The cognitive deterioration was estimated at between 1.6 and 4 ADAS-cog points per every 6 months, increasing with disease severity. Cognitive function was an important predictor of ADL-ability, which itself was the most important predictor of the risk of institutionalization and costs of care. Combining all models in a cross-validation process generated accurate predictions of costs of care at each 6 months follow-up.
Conclusion
The proposed methods for representing AD progression and economic outcomes can be used in micro-simulation models for the economic evaluation of new treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia and a major cause of morbidity and mortality in the elderly [1]. The societal costs associated with AD are high and they increase with deteriorating cognitive and functional ability as a consequence of the progression of dementia [2]. Currently available drug treatments provide short-term symptomatic improvement, but different stakeholders disagree on whether the benefits are worth the treatment costs [3]. The evidence from the economic evaluation of these treatments is inconclusive, partly due to methodological constraints [4], and this has limited patients’ access to treatment in many markets. As the next generation of anti-dementia drugs becomes available, improved methods for their economic evaluation (i.e., estimating cost-effectiveness) are needed.
Within trial analysis of data from randomized controlled trials has been used to estimate the cost-effectiveness of AD treatments [5–8], but the external validity of these estimates is limited by factors including the selection of patients and insufficient follow-up [9]. Therefore, economic modeling is needed as a complement to combine efficacy data from clinical trial with epidemiologic and economic data from clinical practise. The economic model is used to predict the long-term consequences of treatment with good external validity of relevance to decision makers [9, 10]. Previous models have been dominated by the use of cognitive function as a single disease indicator to simulate disease progression and link treatment efficacy to cost-effectiveness [11]. With some exceptions including [12, 13] the patient’s functional ability or ability to perform activities in daily living (ADL-ability) has been omitted in most published models, although it has been shown to be the most important determinant of costs of care [14–16]. The British National Institute for Health and Clinical Excellence (NICE) has recognized the need for epidemiologic data to include both cognitive function and ADL-ability for more accurate prediction of disease progression in economic evaluation [17]. Including ADL-ability could potentially provide better evidence of the societal value of available and future treatments [17]. Thus, there is need for improved methods of modeling AD progression, incorporating both cognitive impairment and the gradual loss of ADL-ability.
This study aims to develop new methods for predicting disease progression and economic endpoints for the economic evaluation of AD treatment. We have a unique longitudinal sample of Alzheimer patients in clinical practise. This data were used to develop an economic model with prediction functions capable of simulating the long-term outcomes of a typical AD cohort over time including cognitive function, ADL-ability, care setting (i.e., community dwelling or institution) and costs of care. The model was then tested on its ability to replicate the observed costs of care of the same cohort over time given minimal baseline inputs.
Materials and methods
Patients
Data from the Swedish Alzheimer Treatment Study (SATS): an open-label, prospective, multi-center, longitudinal study on AD patients in a routine clinical setting was analyzed. The sample included 435 patients from 10 memory clinics in Sweden. They were included from 1997 through 2001 and followed up to 3 years. All patients had a clinical diagnosis of dementia as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV) [18] and probable or possible AD, according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and related Disorders Association (NINCDS-ADRDA) [19]. After the baseline assessment, all patients started on ChEI treatment with donepezil (Aricept®, Pfizer). Informed consent was attained from all patients and their closest relative/caregiver. The study was conducted according to the provisions of the Helsinki Declaration and approved by the ethics committee of Lund University, Sweden. For further detail, see Wallin et al. [20].
Assessments
Data were collected on cognitive function, ADL-ability and resource utilization at baseline and every 6 months up to 3 years. Cognitive function was assessed with Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) [21]. ADAS-cog ranges from 0 to 70 with increasing cognitive impairment. Basic ADLs were assessed with Physical Self-Maintenance Scale (PSMS) [22]. It consists of 6 domains (toileting, feeding, dressing, grooming, physical ambulation and bathing), which form a total score between 0 and 6 indicating in how many basic ADL domains the patient is independent. Instrumental ADLs were assessed with Instrumental Activities of Daily Living Scale (IADL) [22]. It consists of eight items (using the telephone, shopping, preparing food, housekeeping, laundering, transportation, handling medications and handling finances), which form a total score between 0 and 8 indicating in how many instrumental ADL domains the patient is independent. The resource utilization data included inpatient care, some community care services (formal home help, home-delivered meals and day care) and the date of any move into an institutionalized care setting.
Economic model
An economic model was developed by identifying a pathway from the progression of dementia (represented by cognitive function) through its consequences on care need (ADL-ability) and their impact on the provision of care or economic endpoints (care setting and costs of care), (Fig. 1). That is, we assumed that the cognitive function represents the underlying disease, which may cause loss of ADL-ability but not vice versa. In our model, these two indicators represented the disease trajectory and were the main predictors of the economic endpoints: care setting and costs of care. Separate prediction functions were estimated for each of the disease indicators and the economic endpoints. Additional predictors in all prediction functions were time since disease onset, time since baseline, age and gender. Any non-significant (P > 0.1) predictor was removed for the final prediction functions.
Cognitive function
Wallin et al. [20] reported an initial treatment effect over the first 6-month period followed by a stable deterioration over time in this sample, which is also coherent with the findings of clinical trials of ChEIs [23, 24]. To estimate the disease progression of patients on stable treatment, we, therefore, only included data from 6 months post-baseline and onward. Another 8 observations (0.6%) were excluded due to non-valid scores from subjects not willing to carry out the assessment. We estimated the mean 6 months progression in ADAS-cog (difference between the assessments of two adjacent patient visits) by 10 point ranges of ADAS-cog (at the earlier of each pair of visits) using pooled data from all time points. Small deviations from 182 days between two visits were controlled for by multiplying with 182 divided by the number of days between the visits, thereby assuming a linear decline between every two visits.
ADL-ability
The number of independent domains in PSMS and IADL was used to represent basic and instrumental ADL-ability, respectively. For each of the two, a prediction function for the number of independent domains was estimated using pooled data from all time points. Both present and the 6 months lag of ADAS-cog (i.e., the ADAS-cog score at the assessment 6 months preceding the current assessment) were tested as predictors. Both ADL-ability measures had a curvature relationship to ADAS-cog, which was explored both by using squared outcome variables and adding squared ADAS-cog as predictors. Observations were clustered on each patient to take into account the potential intra-individual correlation (i.e., between multiple assessments of each patient over time). Further, repeated measures models were tested to further explore the sensitivity of the potential intra-individual correlation.
Care setting
All patients resided in the community at baseline. The probability of institutionalization over time was estimated using a survival analysis approach on the number of days from baseline to institutionalization. As the risk of institutionalization was assumed to increase over time at a constant rate, we used a Weibull model capable of accommodating this premise.
Costs of care
Each patient’s use of community care services within a 6-month period was calculated by multiplying the reported hours/visits per week by 26. For inpatient care, data on exact resource use were available for each 6-month period. Estimates of costs of care for each patient and time point were calculated by multiplying the number of resource units used in each 6-months period with a Swedish price vector [25, 26] (inflated for 2005). Institutionalized patients were assumed to have a constant per diem cost of 1,507 SEK (~150 EUR) [25]. For community-dwelling patients, a two-part model was employed [27]. First, a logistic model was applied to estimate the probability of a non-zero cost of community-dwelling patients, since they commonly reported zero resource utilization. Second, the mean costs by ADL-ability scores were examined to predict the cost of community-dwelling patients with a non-zero cost.
Economic model validation
All prediction functions were combined into an economic model with the ability to predict the long-term outcomes of an Alzheimer cohort with minimal baseline inputs. That is, the prediction functions were used to predict the disease severity measures and economic outcomes over time, each time using the available baseline and already predicted data as inputs into the prediction functions. The economic model was tested by comparing the fit of the predictions on the observed data using a cross-validation approach [28]. An iterative process was programmed in which half of the SATS data sample was randomly drawn without replacement for re-estimation of all prediction functions. These prediction functions were then used to replicate the other half of the data, i.e., predict the disease trajectory and economic endpoints over time of the other half using their baseline inputs only. A patient was assumed to move into institution, the first time their predicted risk (between 0 and 1) was higher than a random number (between 0 and 1), which was drawn for each patient. Similarly, a community-dwelling patient was assumed to have a non-zero cost if their predicted risk was higher than another random number drawn for each patient. The mean predicted and observed costs of care across all patients in the prediction sample were then calculated for each 6-month period following baseline and saved as one observation in a separate data set. This process was performed with 1,000 iterations, each with a new random draw of estimation/prediction samples.
Technical specifications
All calculations were performed in STATA 9.0 for Windows.
Results
Descriptive statistics
Descriptive statistics on all data from this cohort of SATS patients except for costs of care have been presented in previous publications [20, 29] and are, therefore, only briefly described here. The mean ± standard deviation (SD) age at baseline was 74.6 ± 6.5 years; number of years since disease onset, 3.1 ± 2.3; baseline ADAS-cog score, 20.7 ± 10.0; and 65% were female. Of the 435 patients at baseline, 166 remained in the study throughout the 3 years of follow-up. Twenty-five (5.7%) of the patients died, and the most common causes of dropping out were institutionalization (n = 69, 15.9%) and suspected side effects (n = 35, 8.0%). Twenty-four (5.5%) of the patients withdrew because of unsatisfactory effect of treatment or deterioration of the disease.
Cognitive function
The mean 6 months deterioration including 95% confidence intervals over ADAS-cog levels (10 point ranges) is shown in Fig. 2. The 6-months rate of decline increased with level of cognitive function as measured by ADAS-cog, ranging from 1.6 points in the lowest level (0 < ADAS-cog score < 10) and 4.0 points in the second highest (50 < ADAS-cog score < 60). The confidence intervals were wider in the higher levels due to fewer observations, and the number of observations in the highest level (60 < ADAS-cog score < 70) was too low for a reliable estimate of mean deterioration (n = 3). The standard errors were too large for identifying any significant linear relationship between the 6-months rate of decline and ADAS-cog level. However, t tests showed significant differences comparing ADAS-cog levels higher/lower than 30, 40 and 50 points (P values; 0.02, 0.01 and 0.03), respectively. No other significant predictors of the 6-months rate of decline could be identified in the regression analysis.
ADL-ability
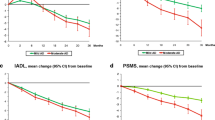
The ADL-ability models with the best fit are presented in Table 1. Modeling squared PSMS gave a better fit than modeling untransformed PSMS while the opposite was found for IADL. The significant predictors were similar for both ADL-ability measures. Both basic and instrumental ADL-ability were lower for older and more cognitively impaired patients. Squared ADAS-cog had a negative impact on ADL-ability indicating that the relationship between ADL-ability and ADAS-cog was stronger in the upper ranges of ADAS-cog where patients have a more severe cognitive impairment. Further, the 6-months lagged ADAS-cog also had a negative impact on ADL-ability indicating that patients who had been cognitively impaired for long had a relatively lower ADL-ability. The IADL model also predicted lower abilities for men than for women. IADL had more missing values than the other instruments and more so for men where only 52% (n = 79) had a complete assessment at baseline, compared to 72% (n = 203) for women. The random effects model gave similar results for both PSMS and IADL but with reduced fit of the model predictions to the observed data in cross-validation.
Care setting
Of the 435 patients, 130 moved into an institutionalized setting during the study. Significant determinants of the probability of institutionalization were ADL-ability and gender (Table 2). The probability of institutionalization increased over time, which was represented by using a Weibull distribution with a shape parameter larger than unity (P = 0.02). The model indicated that the probability of a patient moving into an institution is higher for patients with lower ADL-ability and for female patients. The survival model estimated the mean ± SD time to institutionalization at 4.7 ± 1.8 years from baseline.
Costs of care
Care setting was an important determinant of costs of care. The 6 months costs of a patient residing in an institutionalized care setting (274,274 SEK; ~27,000 EUR) were about 12 times higher than that of an average patient residing in the community (22,210 SEK; ~2,200 EUR). For community-dwelling patients, 1,082 (59%) of all cost observations were equal to zero. The probability of non-zero costs was higher for female patients with a lower basic and instrumental ADL-ability and of higher age (Table 2). The level of costs for patients with a non-zero cost correlated similarly with both PSMS and IADL (Fig. 3), but IADL was used as a single predictor for the final model as PSMS did not add precision to the estimates. Similarly, cognitive function also correlated with costs of care but not when controlling for PSMS and IADL (not shown).
Economic model validation
The combination of all prediction functions resulted in an economic model capable of predicting the long-term outcomes of an Alzheimer cohort with the following baseline inputs: age, sex, cognitive function and ADL-ability. First, the cognitive function (ADAS-cog) at each 6 months cycle was predicted according to the estimated rate of decline over 6 months (Fig. 2). Second, the ADL-ability (PSMS and IADL) at each 6 months cycle was predicted according to the equations in Table 1. Third, the probabilities of institutionalization and non-zero costs were predicted according to the equations in Table 2. Given these probabilities, a stochastic process then determined whether the patient was predicted to be institutionalized or live in the community with a non-zero cost. Finally, this cost was predicted according to the estimates of costs by IADL scores (Fig. 3).
The results from the cross-validation process are shown in Fig. 4. The economic model predictions correspond well with the observed costs of care with overlapping confidence intervals at all visits. Similarly, the predicted proportions of institutionalized patients and community-dwelling patients with non-zero costs were comparable with the observed proportions, with overlapping confidence intervals at all visits (not shown).
Discussion
In this study, we developed new methods for predicting disease progression and economic endpoints for the economic evaluation of AD treatment. We estimated the rate of disease progression in patients treated with ChEI donepezil in clinical practice, incorporating both cognitive and functional ability, and developed models for assessing the risk of institutionalization and the costs of care in relation to disease severity in these two domains. Cross-validation of the combined prediction models showed good correspondence of predicted and observed costs of care with overlapping confidence intervals at all visits. This is an important finding, since it shows that prediction models for each of the individual endpoints can be combined to represent the complex disease progression of AD patients and accurately estimate economic endpoints over time.
No significant predictors of the 6-months rate of decline in ADAS-cog after 6 months of treatment could be identified, but there was a consistent and monotonic trend of cognitive decline across ADAS-cog levels, which also increased the accuracy of the model predictions. Stern et al. [30] demonstrated similar results in untreated patients with the outcome of ADAS-cog over time dependent on initial ADAS-cog score. Wattmo et al. [29] reported that the long-term cognitive outcome measured by MMSE and ADAS-cog in ChEI treated patients was dependent on the baseline scores and time since start of treatment. Our findings suggest a steady although non-significant increase in the rate of cognitive decline with increasing cognitive function, whereas other studies based on untreated patients have found an inverse u-shaped relationship regarding cognition between the rate of decline and severity with the fastest deterioration in moderate dementia [30–32].
We assumed that cognitive decline would cause deterioration in ADL-ability but not vice versa. The rationale for this was purely practical, because the more complex covariance structure between several measures of disease severity would be difficult to assess. Although useful for the purpose of this study, our assumption may be an oversimplification of the reality and we do not claim to have provided any evidence thereof.
ADL-ability decreased with time and deteriorating cognitive function, whereas ADL-ability itself was a predictor of both the risk of institutionalization and the costs of care of community-dwelling patients. Although both resource utilization endpoints correlated with cognitive function as well, this variance was almost totally removed when controlling for ADL-ability. This suggests that the effect of cognitive function on resource utilization is mediated through functional ability and incorporating ADL-ability in economic modeling is, therefore, important for more accurate predictions of costs of care, as proposed in the latest NICE report of ChEIs and memantine treatment in the UK [17].
For costs of care, we differentiated between institutionalized patients (for which a constant per diem cost was assumed) and community-dwelling patients (for which a two-part model was utilized to estimate costs of care). This enables representation of the skewed distribution of costs of care characteristic for AD patients; from zero, through the bulk of low and up to the few high cost estimates. Thus, our model can generate predictions of the distribution of costs of care of individual patients, which makes it suitable for micro-simulation modeling.
This study shares the limitation of most longitudinal studies in the problem of patients lost to follow-up. Of the 435 patients entering the study, only 166 remained after 3 years. Wallin et al. [20] presented a dropout analysis of the same sample where they found that patients who dropped out were older and more severely cognitively impaired at baseline. As they were not assessed after their withdrawal, it is not known whether those who dropped out were worse off over time than those completing the study. However, since deterioration and institutionalization were common causes of withdrawal and are both symptoms of worsening, there is a risk for underestimation of both disease progression and costs of care. This risk was reduced by using a survival model for modeling institutionalization, which handles censored data efficiently. Further, since the cost of institutionalized patients was assumed constant, the dropouts should not affect the costs of these patients. The risk of underestimation rather lies in the deterioration of patients residing in the community and its implications on costs of care. The extent of this problem is not known and should be explored further in future studies.
The IADL measure is not complete for patients who have never carried out certain tasks included in the assessment [22]. This produces some missing values and reduces the number of observations included in models where IADL is either the outcome variable or a predictor. In addition to limiting the sample size, this may imply that the model is invalid for patients who have never carried out the IADL tasks included in the instrument.
The methods for taking into account intra-individual correlation as well as covariance between cognitive function and ADL-ability needs to be further explored in future analysis. At present, we have modeled ADL-ability as a function of cognition, although this probably does not explain their interdependence in total. Further, we tried to account for intra-individual correlation with a random effects model of PSMS and IADL but it did not substantially change the results, although it reduced the fit of the model predictions to the observed data in cross-validation.
The resource utilization data collected in the SATS study are limited as it does not include important health care resources such as informal and outpatient care. Neither was behavioral symptoms included which, in addition to cognitive function and ADL-ability, constitute an important disease indicator with high correlation to costs of care [33]. It may, therefore, be of interest to re-estimate the cost model in future cohorts with additional data available. Further to this point, the focus of this study was not to assess the detailed resource utilization or costs of Alzheimer patients by disease severity or over time and we have, therefore, not presented detailed data on the resource utilization or unit costs used for analysis. This has been done more elegantly in recent publications [34, 35], and our cost estimates primarily serve the purpose of the primary objectives of this study.
In conclusion, the SATS study provides unique patient level data for estimating disease progression in both cognitive function and ADL-ability and how this relates to risks of institutionalization and costs of care over time. The methods developed in this study for representing AD progression and economic outcomes can be used in micro-simulation models for the economic evaluation of new treatments. As the SATS study continues to follow AD patients treated with ChEIs in clinical practise, the models may be refined in future analysis of a larger sample.
References
Henderson, A.S., Jorm, A.F.: Definition and epidemiology of dementia: a review. In: Maj, M., Sartorius, N. (eds.) Dementia. Wiley, Chichester (2000)
Jönsson, L., Berr, C.: Cost of dementia in Europe. Eur. J. Neurol. 12(1), 50–53 (2005)
Lemstra, A.W., Richard, E., van Gool, W.A.: : Cholinesterase inhibitors in dementia: yes, no, or maybe? Age Ageing 36, 625–627 (2007)
Clegg, A., Bryant, J., Nicholson, T., McIntyre, L., De Broe, S., Gerard, K., Waugh, N.: Clinical and cost-effectiveness of donepezil, rivastigmine, and galantamine for Alzheimer’s disease. A systematic review. Int. J. Technol. Assess. Health Care 18, 497–507 (2002)
Feldman, H., Gauthier, S., Hecker, J., Vellas, B., Subbiah, P., Whalen, E.: A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology 57, 613–620 (2001)
Wimo, A., Winblad, B., Engedal, K., Soininen, H., Verhey, F., Waldemar, G., Wetterholm, A.L., Mastey, V., Haglund, A., Zhang, R., Miceli, R., Chin, W., Subbiah, P.: An economic evaluation of donepezil in mild to moderate Alzheimer’s disease: results of a 1-year, double-blind, randomized trial. Dement. Geriatr. Cogn. Disord. 15, 44–54 (2003)
Wimo, A., Winblad, B., Stoffler, A., Wirth, Y., Mobius, H.J.: Resource utilisation and cost analysis of memantine in patients with moderate to severe Alzheimer’s disease. Pharmacoeconomics 21, 327–340 (2003)
Black, S.E., Doody, R., Li, H., McRae, T., Jambor, K.M., Xu, Y., Sun, Y., Perdomo, C.A., Richardson, S.: Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 69, 459–469 (2007)
Jonsson, L.: Assessing health economic outcome in Alzheimer’s disease clinical trials. J. Nutr. Health Aging 11, 353–355 (2007)
Jonsson, L., Jonsson, B., Wimo, A., Whitehouse, P., Winblad, B.: Second International Pharmacoeconomic Conference on Alzheimer’ s Disease. Alzheimer Dis. Assoc. Disord. 14, 137–140 (2000)
Cohen, J.T., Neumann, P.J.: Decision analytic models for Alzheimer’s disease: state of the art and future directions. Alzheimers Dement 4, 212–222 (2008)
Jones, R.W., McCrone, P., Guilhaume, C.: Cost effectiveness of memantine in Alzheimer’s disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Aging 21, 607–620 (2004)
Fagnani, F., Lafuma, A., Pechevis, M., Rigaud, A.S., Traykov, L., Seux, M.L., Forette, F.: Donepezil for the treatment of mild to moderate Alzheimer’s disease in France: the economic implications. Dement. Geriatr. Cogn. Disord. 17, 5–13 (2004)
Zhu, C.W., Scarmeas, N., Torgan, R., Albert, M., Brandt, J., Blacker, D., Sano, M., Stern, Y.: Clinical characteristics and longitudinal changes of informal cost of Alzheimer’s disease in the community. J. Am. Geriatr. Soc. 54, 1596–1602 (2006)
Zhu, C.W., Scarmeas, N., Torgan, R., Albert, M., Brandt, J., Blacker, D., Sano, M., Stern, Y.: Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology 67, 998–1005 (2006)
Murman, D.L., Von Eye, A., Sherwood, P.R., Liang, J., Colenda, C.C.: Evaluated need, costs of care, and payer perspective in degenerative dementia patients cared for in the United States. Alzheimer Dis. Assoc. Disord. 21, 39–48 (2007)
Loveman, E., Green, C., Kirby, J., Takeda, A., Picot, J., Payne, E., Clegg, A.: The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease. Health Technol. Assess. 10:iii–iv, ix–xi, 1–160 (2006)
Frances, A.: American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders: DSM-IV: Prepared by the Task Force on DSM-IV, 4th edn. American Psychiatric Association, Washington, DC (1994)
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., Stadlan, E.M.: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 (1984)
Wallin, A.K., Andreasen, N., Eriksson, S., Batsman, S., Nasman, B., Ekdahl, A., Kilander, L., Grut, M., Ryden, M., Wallin, A., Jonsson, M., Olofsson, H., Londos, E., Wattmo, C., Eriksdotter Jonhagen, M., Minthon, L.: Donepezil in Alzheimer’s disease: what to expect after 3 years of treatment in a routine clinical setting. Dement. Geriatr. Cogn. Disord. 23, 150–160 (2007)
Rosen, W.G., Mohs, R.C., Davis, K.L.: A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 141, 1356–1364 (1984)
Lawton, M.P., Brody, E.M.: Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186 (1969)
Winblad, B., Engedal, K., Soininen, H., Verhey, F., Waldemar, G., Wimo, A., Wetterholm, A.L., Zhang, R., Haglund, A., Subbiah, P.: A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57, 489–495 (2001)
Winblad, B., Wimo, A., Engedal, K., Soininen, H., Verhey, F., Waldemar, G., Wetterholm, A.L., Haglund, A., Zhang, R., Schindler, R.: 3-year study of donepezil therapy in Alzheimer’s disease: effects of early and continuous therapy. Dement. Geriatr. Cogn. Disord. 21, 353–363 (2006)
Jönsson, L., Eriksdotter Jonhagen, M., Kilander, L., Soininen, H., Hallikainen, M., Waldemar, G., Nygaard, H., Andreasen, N., Winblad, B., Wimo, A.: Determinants of costs of care for patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 21, 449–459 (2006)
Socialstyrelsen.: Kostnad per brukare i kommunal vård och omsorg. http://www.skl.se/MediaBinaryLoader.axd?MediaArchive_FileID=7cd49b6c-30eb-4a00-8635-c9919a5656a5 (2005). Accessed 5 July 2011
Dunn, G., Mirandola, M., Amaddeo, F., Tansella, M.: Describing, explaining or predicting mental health care costs: a guide to regression models. Methodological review. Br. J. Psychiatry 183, 398–404 (2003)
Faber, N.M., Rajko, R.: How to avoid over-fitting in multivariate calibration–the conventional validation approach and an alternative. Anal. Chim. Acta 595, 98–106 (2007)
Wattmo, C., Hansson, O., Wallin, A.K., Londos, E., Minthon, L.: Predicting long-term cognitive outcome with new regression models in donepezil-treated Alzheimer patients in a naturalistic setting. Dement. Geriatr. Cogn. Disord. 26, 203–211 (2008)
Stern, R.G., Mohs, R.C., Davidson, M., Schmeidler, J., Silverman, J., Kramer-Ginsberg, E., Searcey, T., Bierer, L., Davis, K.L.: A longitudinal study of Alzheimer’s disease: measurement, rate, and predictors of cognitive deterioration. Am. J. Psychiatry 151, 390–396 (1994)
Galasko, D.R., Gould, R.L., Abramson, I.S., Salmon, D.P.: Measuring cognitive change in a cohort of patients with Alzheimer’s disease. Stat. Med. 19, 1421–1432 (2000)
Liu, X., Teresi, J.A., Waternaux, C.: Modelling the decline pattern in functional measures from a prevalent cohort study. Stat. Med. 19, 1593–1606 (2000)
Murman, D.L., Chen, Q., Powell, M.C., Kuo, S.B., Bradley, C.J., Colenda, C.C.: The incremental direct costs associated with behavioral symptoms in AD. Neurology 59, 1721–1729 (2002)
Mesterton, J., Wimo, A., By, A., Langworth, S., Winblad, B., Jonsson, L.: Cross sectional observational study on the societal costs of Alzheimer’s disease. Curr. Alzheimer Res. 7, 358–367 (2010)
Gustavsson, A., Brinck, P., Bergvall, N., Kolasa, K., Wimo, A., Winblad, B., Jonsson, L.: Predictors of costs of care in Alzheimer’s disease: a multinational sample of 1222 patients. Alzheimers Dement 7, 318–327 (2011)
Acknowledgments
The authors would like to thank Swedish Brain Power for their support to this study.
Conflict of interest
Anders Gustavsson has acted as consultant to and received research grants from pharmaceutical companies including Wyeth, Pfizer, AstraZeneca, Sanofi-Aventis, Lilly and GSK. Linus Jönsson has acted as consultant to and received research grants from pharmaceutical companies including Wyeth, AstraZeneca, Sanofi-Aventis, Lilly, Pfizer, GSK and Roche. Åsa K. Wallin received funding for a trip from Jansen Cilag. Johan Parmler, Niels Andreasen, Carina Wattmo and Lennart Minthon have no conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gustavsson, A., Jönsson, L., Parmler, J. et al. Disease progression and costs of care in Alzheimer’s disease patients treated with donepezil: a longitudinal naturalistic cohort. Eur J Health Econ 13, 561–568 (2012). https://doi.org/10.1007/s10198-011-0334-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-011-0334-y
Keywords
- Alzheimer’s disease
- Cost of care
- Economic modeling
- Institutionalization
- Cholinesterase inhibitors
- Long-term
- ADL
- Cognition