Abstract
Noise pollution may impair the cognitive performances of several animal species, producing suboptimal behavioral responses. Involuntary shifts in attention from noise pollution (an irrelevant stimulus) may account for this outcome, specifically, by reducing the available cognitive processing capacity to conduct relevant tasks; the ‘distracted prey hypothesis’. Many reef fish mediate predation risks by pairing with conspecifics and using refuges; two crucially important resources that are often heterogeneously distributed in space. In a T-maze, we tested the cognitive performances of wild-caught tide-pool fish, Sergeant Major (Abudefduf saxatilis), exposed to control (45 dBA) or noise playbacks (100 dBA). After exposure to a model predator, fish could reach an area containing a refuge and conspecifics (target area), located in one arm of the maze. We posited that fish exposed to noise playbacks would require additional time to reach this target, because of impaired cognitive performances (i.e., learning, remembering). While fish learned to reach the target, no statistical difference existed between acoustic treatments. However, fish exposed to additional noise increased their time spent under shelter and reduced their exploratory behavior. As tide-pools are inherently noisy and structurally complex, residents of these habitats may have cognitive abilities that are resilient to acoustic disturbances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much of the noise emitted by human activities is considered a form of pollution, from which escape is increasingly difficult (Goines and Hagler 2007; Hildebrand 2009; Buxton et al. 2017; Bugnot et al. 2019). Over the last decades, a considerable amount of research has been devoted to unraveling the wide-ranging effects that noise pollution impose on humans and non-human animals. Precisely, some of deleterious effects of noise pollution have led to impaired performances associated to hearing, acoustic communication, physiological and mental homeostasis and cognition (reviewed in Stansfeld and Matheson 2003; Rabin et al. 2003; Goines and Hagler 2007; Rabat 2007; Simpson et al. 2011; Cox et al. 2018). Given the importance of cognition on behavior and thereby on fitness, the impacts noise pollution may have on learning and memory were studied in detail. For example, in humans and rodents, increased noise levels were shown to negatively impact several cognitive processes (i.e., perception, learning and memory), which could yield short- and/or long-lasting effects (Lercher et al. 2003; Stansfeld et al. 2005; Rabat 2007; Belanger 2009; Cheng et al. 2011; Tao et al. 2015; Jafari et al. 2019; but see Cui et al. 2009).
Thus far, assessments of potential effects of noise pollution on cognition chiefly focused on terrestrial organisms. However, underwater sound propagation far exceeds that of areal conditions (in distance and speed), making acoustic stimuli exceedingly relevant to fitness of aquatic organisms (Popper and Hawkins 2019; Popper et al. 2019). Concurrently, akin to the terrestrial realm, aquatic habitats are becoming increasingly noisy (Hildebrand 2009), whereby water- and air-borne anthropogenic noises (i.e., within and between realms, respectively; Bugnot et al. 2019) can lead to impacts that are similar (or even more acute) to those found in terrestrial habitats (reviewed in Slabbekoorn et al. 2010; Gill et al. 2015; Cox et al. 2018). Despite this, the potential impacts noise pollution may have on the cognitive performances of aquatic organisms have generally been neglected (but see Ferrari et al. 2018).
Weakening of cognitive performances, following exposures to noise pollution, may be understood in terms of ‘attention’. Attention is the activation of the neuronal representation(s), which can be divided among various tasks/stimuli at a given time (Dukas 2004). Naturally, only a finite amount of information (stimuli) may be processed at a given time by an organism’s neuronal machinery (Washburn and Tagliatatela 2006). For instance, the distracted prey hypothesis (Chan et al. 2010) posits that when exposed to noise pollution, a prey organism may lose some of this ability to detect a predator, fundamentally because of the distraction effect. When an animal divides its attention with a distracting stimulus (e.g., noise), it effectively reduces the total processing capacity that could otherwise be allocated to conduct a relevant task (e.g., detecting predators, feeding; Dukas 2004). For example, the Ambon damselfish (Pomacentrus amboinensis) learned the cues of a novel predator under control conditions, but failed to do so under noisy conditions (Ferrari et al. 2018); this outcome agrees with the notion of attention deficit (i.e., distraction).
For many animals, learning and memory are essential for an array of fitness-enhancing activities. By itself, ‘spatial learning’ (a type of associative learning about an organism’s habitat) is centrally important for fish (Reese 1989; Odling-Smee and Braithwaite 2003a; Sison and Gerlai 2010; Schluessel and Bleckmann 2012; White and Brown 2014). This cognitive ability may have serious fitness consequences as it is involved, for example, in guidance to find food (Noda et al. 1994; Braithwaite et al. 1996; de Kort et al. 2005; Bajer et al. 2010) and adequate habitat (e.g., reaching a refuge to escape predators; Markel 1994; de Kort et al. 2005; Bajer et al. 2010). Thereby, learning to effectively navigate in structurally complex environments may be one strategy providing fish with important fitness benefits.
Rocky/coral reefs and pools may be considered excellent examples of stable (i.e., relative to the lifespan of most animals) yet structurally complex habitats, in which spatial cognition is required for effective navigation (Reese 1989; White and Brown 2014). In these habitats, many reef fish mediate the presence of predator threats by schooling with conspecifics (Morgan and Godin 1985; Magurran 1990) and/or escaping to a refuge (Aronson 1971; Markel 1994; Cooper and Samia 2018); two resources that may be heterogeneously distributed within a fish’s landscape (Nunes et al. 2019). Thus, the interplay between predation and the spatial heterogeneity of these features (i.e., refuges and conspecifics) within a fish’s habitat implies that spatial cognition must play an important fitness role.
Damselfishes (family Pomacentridae) constitute one of the most conspicuous groups of the fish fauna of coral reefs throughout the world. Many of these species rely on the structural complexity of their habitat for refuge to avoid and/or escape predators (Helfman 1998; Holbrook and Schmitt 2002). Furthermore, many damselfish produce and respond to acoustic cues (Myrberg and Sypre 1972; Leis et al. 2002; Parmentier et al. 2009), despite of the fact that coastal species are increasing subjected to noise pollution (Bittencourt et al. 2014; Sánchez-Sánchez et al. 2015; Nichols et al. 2015; Leduc et al. 2021). Here, we experimentally tested a common fish of rocky reefs and tide-pools of the Western Atlantic, the Sergeant Major (Abudefduf saxatilis). We predicted this fish would have the ability to learn navigating in a novel habitat (i.e., T-maze tank). However, given the detrimental effects of noise pollution on spatial cognition demonstrated in mice (Cheng et al. 2011; Tao et al. 2015; Wang et al. 2017), we predicted that the ability of this reef fish to learn navigating in this novel habitat would be impaired by the extraneous noise.
Materials and methods
All procedures undertaken herein complied with the guidelines of the Ethic Committee for Animal Use of the Federal University of Bahia (Project Number 40/2017). The capture of this fish was authorized by the Institute Chico Mendes for Conservation of Biodiversity (License no 55979-1). We designed this experiment to follow the reduction principle of the 3Rs (HMSO, 1986).
Study animals and holding conditions
We used a common reef fish of the Western tropical Atlantic, the Sergeant Major (A. saxatilis; Pomacentridae). This is an abundant and conspicuous species of coastal shallow reefs and tide-pools (Froese and Pauly 2020), which relies on heterogeneously distributed refuges to avoid predators (AOHCL personal observations). As in many Pomacentridae fish (Myrberg and Spires 1980; Leis et al. 2002; Popper and Schilt 2008; Parmentier et al. 2009), the Sergeant Major is known to make and respond to sounds (Egner and Mann 2005), which allows for testing potential effects of noise pollution on its cognition and behavior.
Using a seine net, we caught wild individuals from tide-pools of Ondina beach (13°0′41.29′′S; 38°30′22.41′′W). These tide-pools are part of shallow rocky reef complex, which harbor up to 70 species of fish (Ferreira et al. 2015; Pinheiro et al. 2018) and are subjected to piscivorous (Ferreira et al. 2015) and avian predators (AOHCL personal obs.). Within tide-pools, juveniles of this species form loose aggregations with similar-size conspecifics (Foster 1989), which allowed us to easily and rapidly capture 32 individuals (mean ± s.d. = 4.31 ± 0.70 cm, standard length, LS). After capture, fish were stored in a 30-L cooler and transported (~ 20 min) to the Institute of Biology of the Federal University of Bahia. There, these fish were kept in four flow-through 70-L seawater (~ 35 ppt)-holding aquaria (70 × 40 × 30 cm) equipped with a terracotta pot as refuge and housed in groups of six, which is a common density for this species (Foster 1989). All aquaria were maintained at the same flow rate (0.5 L min−1), temperature (25 °C), and photoperiod (12 L–12 D).
Before we conducted any experimental manipulations, fish were allowed to acclimate to the laboratory conditions for a period of 2 weeks. Prior manipulations with this species demonstrated the suitability of this period of acclimation to these new conditions, as shown by species-typical behavior (i.e., swimming and feeding ‘normally’) and an absence of apparent fear of experimenters (i.e., fish following experimenters during feedings). During the settling period, we fed these fish ad libitum daily, a combination of brine shrimp (Artemia franciscana) and commercial dry food flakes.
The T-maze apparatus and testing room
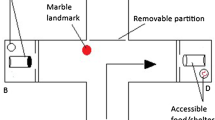
The apparatus and procedures used in this experiment were modified from previous experiments (Odling-Smee and Braithwaite 2003b; Odling-Smee et al. 2008; White and Brown 2015). A T-maze tank (Fig. 1) was constructed from 4 mm glass, lined with a 2-cm layer of triturated coral, and filled with saltwater a depth of 10 cm, with the walls covered with beige/tan paper. The T-maze was placed on two layers of 5-cm polystyrene pads. The walls of the experimental room were covered by black sound-absorbing acoustic foam tiles, and the ceiling was white and equipped with light fixtures. The temperature of the test room was the same as the holding room (25 °C).
Overhead view of experimental T-maze setup. In this schematic, the correct target area (i.e., with terracotta pot as refuge and the sight of conspecifics) is located in the right-side arm, whereas the incorrect target area (devoid of these two rewards) is located in the left-side arm. Schematic representation of visual predatory avian stimulus (kingfisher, top), the loudspeaker system, composed of a left and right speaker (LS and RS, respectively) coupled with a subwoofer (bottom), and the test fish at its initial position for the predator release (top of the maze) are shown
The base of the T-maze consisted of the start ‘box’ (25 × 18 cm), which was divided from the rest of the maze by a vertically sliding opaque guillotine-door (Fig. 1). Once the door was open, the fish had access to the entire maze and could swim to reach the end of both arms (i.e., left and/or right). However, the extremity of each arm of the T-maze was partially blocked by an opaque partition, which had a 2.5-cm-wide vertical opening (slit) that allowed accessing the end of the T-maze’s arm; these were considered ‘target areas’ (detailed below). This opening was directly adjacent to the back wall of the maze, such that a fish that swam to the back wall, typically turned left or right and swam into a target area.
The extremity of each arm of the maze consisted of target area (25 × 18 cm), one of which was the ‘correct’ target (i.e., goal), whereas the other was considered ‘incorrect’. In the correct target, a terracotta pot (~ 8 cm in diameter) was laid on its side as a refuge, and three conspecifics were visible from an adjacent aquarium (these fish took no part in the experimental procedure that took place in the T-maze). We ensured conspecifics and the refuge could only been seen after the test fish had entered the correct target (i.e., passing through the opening). Other experiments have shown that shelters and/or the presence of conspecifics may be considered rewards (Al-Imari and Gerlai 2008). As such, fish that entered the correct target were doubly rewarded. The incorrect target was devoid of shelter and the sight of the adjacent aquarium was blocked (Fig. 1). To control for a potential side bias, for each playback treatment (detailed below), the location of the correct target was either placed at the left- or right-end side of the maze.
Fish behavior was monitored remotely from an Apple tablet (Ipad 3, Apple, Cupertino, CA, USA) remotely connected to a GoPro camera (GoPro®, Hero 3 + Black Edition, San Mateo, CA, USA, 24 fps and 1080 dpi), which was positioned ~1.2 m above the T-maze. To minimize external disturbances, we monitored all trials from an adjacent room.
Playback treatments
As a source of additional noise, we produced a brown noise (i.e., frequency band between 100 and 1000 Hz) wav file made using Audacity (v2.3.0, http://audacity.sourceforge.net/). Previous work has shown that Sergeant Major are most sensitive to frequencies ranging between 100 and 400 Hz (Egner and Mann 2005). For the treatment of additional noise, this sound file was played continuously (i.e., looped) to form a continuous playback track. The sound systems used for the playback consisted of a WAV/MP3 player (Ipod 4, frequency response 10–20k Hz; Apple, Cupertino, CA, USA), connected to a dual-speaker system, which was paired with a subwoofer (Dell 2.1, 33-W Multimedia System, with a 65–17 k Hz response bandwidth, Dell Computer, USA). We measured the amplitude of the playback with a Skill-Tec TM, SKDEC-02 (São Paulo, SP, Brazil) sound pressure level meter (A-weighted, fast response, range 30–130 dB, at 1 s interval) and adjusted playback levels to a volume of 100 dBA at a distance of 1 m from the speaker. This was done daily to ensure using the appropriate acoustic intensities. The underwater acoustic conditions (sound pressure) were measured at mid-water column depth (i.e., 5 cm above tank floor) by placing the hydrophone facing upward, at the entrance of each arm; this allowed us to compare the sound pressure in each arm of the T-maze. The underwater recording system consisted of a hydrophone (SQ26, Cetacean Research Technology, Seattle, USA) with a built-in preamp, connected to a digital audio recorder (PCM-M10, 48 kHz sampling rate, Sony Corporation, Tokyo, Japan). We determined the calibration constants for the recorder by recording pure tones of known amplitudes at different frequencies (40, 400, 800, and 1000 Hz). Using this procedure permitted us to measure sound pressure in absolute units. Using oscilloscope recordings (3425 Differential Picoscope, Pico Technology, Cambridgeshire, UK), the flat response across these frequencies was confirmed and we combined our measurements with the manufacturer-provided hydrophone sensitivity, which allowed us to determine the recording system calibration constant. This let us to convert the underwater audio recordings to µPa for analyses. These recordings were then imported to the statistical software R (Core Team R 2013), allowing extraction of sound pressure levels in third-octave frequency bands using a custom-written R-module.
We acknowledge that the exact sound pressure levels received by fish are not necessarily uniform throughout the tank (Parvulescu 1964). However, here we aimed to test whether cognitive performances of a spatial task differ in relation to increased noise.
Experimental protocol
Before conducting the experiment, we allowed fish to become accustomed (i.e., trained) to the T-maze. For this acclimation phase, fish were placed inside the maze on four different days, each alternated with 24 h in their home tanks. To minimize acute social isolation stress, (sensu Gleason and Weber 1977), acclimation occurred in groups of four, three two and singly, on each of these days, respectively. The acclimation period was conducted under control (i.e., silent condition) and no shelter was present, nor could conspecifics be seen. As fish were not individually identifiable, all fish held in a given holding aquaria were assigned a single combination of treatment, namely either the control or the additional noise treatment, and a specific side for the correct target (i.e., left or right).
To stimulate activity (i.e., food-searching behavior; Bajer et al. 2010), fish were food-deprived for 12 h before testing. Experimental trials were conducted between 10:00 and 16:00 when these fish tend to be most active (sensu Ferreira et al. 1998). At the onset of testing, the sound system was either turned-on, with either the playback of additional noise at 100 dBA, or the same playback was played, but with the sound intensity of the speaker turned to its minimum, which corresponded to the acoustic intensity of the testing room; approximately 45 dBA. A single fish was placed into the start box and given 3 min to settle. After this period we presented an ecologically relevant predatory stimulus, following an approach by Voellmy et al. (2014). This stimulus corresponded to a realistically painted cutout shape of a common predatory bird of the sampled tide-pools (i.e., ringed kingfisher, Megaceryle torquata). This predator model was longitudinally ‘flown’ (via a 2 m rod) above starting position of the test fish, after which the guillotine-door was remotely removed and the fish was allowed access to the maze for a period of 10 min.
At the end of this period, each fish was returned to its designated holding aquarium (detailed above). This procedure was repeated at 24-h intervals, for five repetitions. Between the fourth and fifth repetitions, a number of fish became afflicted with a common bacterial infection. As such, eight and three fish, for each of the control and additional noise treatment (respectively) did not partake in the fifth repetition. Upon completion of the fifth and last repetition, all fish were treated with antibiotics. After having fully healed, they were returned to their native tide-pools.
Behavioral analysis
From muted video-recordings, all data were extracted by an observer (RRNS) who was naïve to the treatments and had no prior knowledge of fish cognition or bio-acoustics. We quantified several behavioral endpoints: (i) the latency of the fish to enter the correct target; (ii) the initial (first) arm choice made; (iii) the time spent under shelter and (iv) the combined number of visits made to each arms. The latency to enter the correct target was defined as the time required for the base of the caudal fin to pass the opening leading to the correct target. The initial first choice was measured as a binomial response: whether fish initially entered the correct or the incorrect target. Entering the incorrect target first (instead of the correct one) would correspond to failing to quickly reach a refuge, which under natural settings may have dire consequences for prey when encountering a predator (Brown 2003). These two first behavioral responses aimed to assess whether fish cognitive performances were maintained or impaired by noise pollution. The time spent under shelter was defined as when at least ¾ of the fish’s body was inside the terracotta pot. We measured this behavior because, along with exposures to predatory cues, additional noise tends to increase the propensity for sheltering in fish (McLaughlin and Kunc 2015). Last, we took a basic measure of exploratory activity, which reflects the motivation to search for resources (Burns et al. 2016). This was done counting the total number of visits to the target areas (correct and incorrect), following Sison and Gerlai (2010).
Statistical analysis
To test whether the noise treatments (45 vs. 100 dBA), repetitions (1–5) and sides (left vs. right) had an effect on the latency (s) to arrive at the correct target and on the time spent under shelter (s), we used a Generalized Linear Model (GLM) with a gamma error distribution (with a log link). To test this effect on the total number of visits to any target areas (correct and incorrect), we used a GLM with negative binomial error distribution (with a log link). We used the GLM approach since our data did not follow the prerequisites of normality, and could not be normalized. We considered ‘noise treatments’, ‘repetitions’ and ‘sides’ as fixed factors. We also tested whether additional noise had an overall effect on the number of first correct choices (i.e., directly entering the correct target), using a GLM with a binary logistic error distribution. These statistical analyses were carried out using IBM SPSS v.21 (SPSS Inc., Chicago, IL, USA).
Results
The treatment of extraneous sound resonated in the T-maze. Indeed, low-frequency sounds (30–400 Hz) reached a peak increase of over 30 dB (re µPa/Hz) in sound pressure, compared to baseline control conditions (Fig. 2).
Sound pressure level (SPL; dB re 1 µPa2) in third-octave frequency bands (Hz), measured at mid-water column at the entrance of each arm of the T-maze. The red and green boxplots correspond to measurements under exposure of extraneous noise (100 dBA), taken at the left and right arm of the maze, respectively. The blue and purple boxplots correspond to measurements done under controlled conditions (45 dBA), taken at the left and right arm of the maze, respectively. When compared to the control condition, the extraneous sound treatment led to higher acoustic intensitie across the frequencies of 20 to ~3000 Hz, with a peak increase of 30 dB in 1/3 octave banded sound pressure. No difference between sides was observable
The location of the correct target (i.e., placed either in left- or right-side arm of the maze) yielded no statistically significant difference in any of the comparisons (Table 1). The latency to arrive at the correct target was significantly affected by the variable ‘repetitions’, with progressively lower latencies (Table 1; Fig. 3). Specifically, with repetitions, fish needed significantly less time to reach the correct target, which suggests that learning occurred. However, we found no effect of the additional sound treatment on this measure, nor any interactions between the number of repetitions and treatment (Table 1; Fig. 3). Likewise, we found no overall effect of the acoustic (i.e., noise) treatment on the number of first correct choices (i.e., toward the correct target (Wald Chi-square = 2.3; df = 1; p = 0.125), nor an effect of repetition on this outcome (Wald Chi-square = 5.4; df = 4; p = 0.241). By contrast, we found a statistically significant effect of acoustic treatment on the time sheltering, but the number of repetitions or interaction between these factors yielded no significant effect (Table 1; Fig. 2). The overall number of visits to the T-maze’s arms was statistically reduced with repetitions and the noise treatment, but without significant interaction (Table 1; Fig. 2).
Measurements of the Sergeant Major (Abudefduf saxatilis) exposed to control condition (45 dBA; open bars) or to additional noise (100 dBA; grey bars). a The latency (s) to reach the correct target significantly decreased with the number of repetitions, but the acoustic treatments had no effect on this outcome. b However, the time spent under shelter (s) was significantly higher under the noise treatment, with no effect of repetitions. c By contrast, the number of visits to target areas was lower under the noise treatment, with a significant effect of repetitions on this outcome. The boxplot lines are the median for the measured parameters, the boxes range from the 25th to the 75th percentile, whiskers are 1.5 IQR, circles and asterisks are outliers
Discussion
Considering the steep adaptive gradient imposed by predation, learning and remembering the location of refuges may provide important advantages for prey organisms (Markel 1994; Odling-Smee et al. 2006; Cooper and Samia 2018; Nunes et al. 2019). Thus, behaving adaptively may require of animals to acquire and retain information about their environment, particularly after changes have occurred. Our results suggest that the Sergeant Major may learn navigating into a novel habitat in order to reach a refuge and/or conspecifics following exposure to a predation threat. This conclusion emerges by the shorter time required to reach to correct target with the increasing number of repetitions. However, contrary to our expectations, exposures to the additional noise did not yield any observable impairment of cognitive performances to solve this spatial task; in the noise treatment, no additional time was required to achieve the performances obtained under control conditions. Furthermore, we found no difference in the proportion of initial target choice (i.e., making a correct or incorrect first choice) when comparing treatments. However, akin to others studies (Picciulin et al. 2010; McLaughlin and Kunc 2015), exposures of extraneous noise led to increased propensity to spend time under shelter and to reduced overall activity (Sabet et al. 2016), which was measured in terms of number of visits to the ends of the maze’s arms (sensu Burns et al. 2016). Thus, while it appears that our model detected the extraneous noise, it did not lead to significantly negative effects on the ability to solve a spatial task.
The observed lack of difference between the control and additional noise treatments is unlikely to be due to our model's inability to detect the additional sound. In fact, out-of-water speakers generate a uniform sound pressure field within the tank (Parvulescu 1964), which our model species should detect; the Sergeant Major is most sensitive to frequencies ranging 100 and 400 Hz (Egner and Mann 2005), which were ~40 dB (re µPa/Hz) louder inside the T-maze during the noise playbacks (Fig. 2). Rather, the lack of effect of the extraneous noise on cognitive performances may be rooted in our model species. The test fish used herein were all wild-caught from tide-pools and these habitats (and rocky reefs) are some of the noisiest of the aquatic realm (Lugli 2010). For instance, tide-pools and rocky reefs may have natural acoustic intensities that nearly compare to the maximum acoustic intensity presented in our experiment, i.e., 120 vs. 130 dB re µPa/Hz, respectively (Lugli 2010). The Sergeant Major appeared to have kept the ability to focus its attention on acquiring and retaining the location of the correct target, which may be understood as mapping its surroundings.
Our results may be at odds with those of Ferrari et al. (2018), which demonstrated that the Ambon damselfish (P. amboinensis) could learn novels cues (i.e., from a predator) under control conditions, but failed to do so under noisy conditions. This divergence in outcome may be rooted in the evolutionary life-history differences in the model organisms used in between these studies. Indeed, the natural habitat of the Ambon damselfish may be characterized as quieter than that of juvenile Sergeant Majors; the former is from lagoons and from deeper habitats (up to 40 m), whereas the latter typically occurs in tide pools. Furthermore, Ferrari et al. (2018) relied on fish collected from the pelagic larval stage, whereas we relied on juveniles collected from their benthic habitat (i.e., tide pools), which may be, by comparison, far nosier (Lugli 2010). Additionally, the cognitive tasks diverged between these experiments; specifically one involved ‘predator recognition’ vs. whereas in the other, ‘route finding’ was the task involved.
Not only are tide-pool and rocky shore habitats noisy, these are also typically structurally complex, in which an individual’s abilities to map its surroundings is likely to be paramount for its survival (Reese 1989). It follows that resilience against potential distraction effects resulting from noisy conditions (which should reduce shifts in attention) ought to be adaptive. A clear follow-up question involves comparing cognitive performances to noise pollution in fish species resident of habitats characterized by naturally different noise levels (e.g., quiet/lagoons vs. loud/tide-pools).
Spatial cognition may rely on at least two non-mutually exclusive paradigms. In one, features of the landscape are detected and used for guidance (i.e., allocentric strategy), while the second concentrates on an individual’s body movements (e.g., turning left or right) relative to a pathway (i.e., egocentric strategy; Braithwaite and de Perera 2006). These two strategies have been used separately and together in learned guidance (Rodrigues et al. 1994). Here, test fish needed to rely on egocentric cues, given no landmark was provided inside the T-maze. However, it may be possible that landmarks above or outside of the T-maze (e.g., overhead camera, light fixtures) provided features upon which allocentric guidance was possible. In fact, visual cues may provide fish with a salient type of information (Rodrigues et al. 1994; Holbrook and de Perera 2011). Nonetheless, compared to several other experimental investigations (e.g., Odling-Smee and Braithwaite 2003b; Odling-Smee et al. 2008; White and Brown 2015), the spatial task presented herein may have been easier to solve. In these aforementioned experiments, reaching the target required passing through a circular opening, located on the central part of a side panel (which separated a target area from the main maze). Our comparatively larger vertical opening, allowing passage at any depths, was located against the back wall of the T-maze (Fig. 1). Thus, fish only required turning left or right to find a target area after reaching the back wall. An interesting possibility involves solving comparatively more difficult spatial tasks to discern whether the additional noise may impair this species’ cognitive performances. Indeed, as spatial tasks increase in complexity, animals need increased cognitive ability to process spatial information (Braithwaite and de Perera 2006).
Another possibility for the present result involves the intensity of the noise disturbance used herein. Despite that the intensity of the noise treatment was ~40 dB (re 1 µPa2) louder than the control condition (Fig. 2), it was only ~10 dB (re 1 µPa2) louder than the natural sound intensity of these fish’s tide-pools (Lugli 2010; Leduc et al. 2021). Future examinations of potential impairments of cognitive performances by noise pollution should aim to expose fish to sound intensity that far exceeds the natural range of their natural habitat. In fact, being a hearing generalist, the Sergeant Major has a high auditory threshold (Egner and Mann 2005). Thus, while it may be assumed that less sensitive species would show reduced response to sounds (or fail to respond), this may not be the case. Indeed, studies compared the responses of Danio rerio and Lake Victoria cichlids (e.g., Haplochromis piceatus) to extraneous sound exposures. Although the former species is characterized by a higher sensitivity (lower auditory thresholds) and a far wider frequency range than the latter, both species demonstrated a significant reduction in swimming velocity, not readily associated to differences in hearing abilities (Sabet et al. 2016). Likewise, in their experiment, Hawkins et al. (2014) showed that changes in the behavior of schools of wild sprat Sprattus sprattus and Scomber scombrus to extraneous sound occurred at similar sound levels, despite the strikingly different hearing abilities of these species. Thus, it appears that the relatively low hearing sensitivity of the Sergeant Major (Egner and Mann 2005) should not have impeded ‘normal’ threat response associated to exposures to extraneous sound (which is confirmed by the general reduction in activity level), but may have rendered this species resilient against a distraction effect from exposures to extraneous noise.
Attention is associated with the selection of which stimulus to process and/or which response to execute (Washburn and Tagliatatela 2006). A wide array of all behaviors that may be performed are, at least partly, regulated by attention (Konstantinou et al. 2014). Although some stimuli (e.g., noise) are typically difficult to block, even if it is not advantageous to focus on them, the Sergeant Major appears to have been resilient to the additional sound stimulus when learning its way in a novel habitat. Despite the ecological relevance of a task, it was expected that some attention should be lost. Although attention provides protection from distractions, it should not completely prevent animals obtaining information unrelated to the current task, for example when the necessity to react adequately to surprising (and conceivably dangerous) events occurs (Berti and Schroger 2003). Under this paradigm, we expected the Sergeant Major’s attention to dissipate under the noise treatment, and thereby to show impaired cognitive performances. Contrary to these expectations, however, when mapping its new habitat, this tide-pool fish appeared resilient against a distraction effect generated by noise pollution. Whether this species shows resilience to a distraction effect to noise levels far exceeding the natural range of its habitat remains to be tested.
References
Al-Imari L, Gerlai R (2008) Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio). Behav Brain Res 189:216–219. https://doi.org/10.1016/j.bbr.2007.12.007
Aronson LR (1971) Further studies on orientation and jumping behavior in the gobiid fish Bathygobius soporator. Ann N Y Acad Sci 188:378–392
Bajer PG, Lim H, Travaline MJ, Miller BD, Sorensen PW (2010) Cognitive aspects of food searching behavior in free-ranging wild Common Carp. Env Biol Fish 88:295–300. https://doi.org/10.1163/156853907781347781
Berti S, Schröger E (2003) Working memory controls involuntary attention switching: evidence from an auditory distraction paradigm. Euro J Neurosci 17:1119–1122
Belanger HG (2009) Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J Int Neuropsychol Soc 15:1–8
Bittencourt L, Carvalho RR, Lailson-Brito J, Azevedo AF (2014) Underwater noise pollution in a coastal tropical environment. Mar Poll Bull 83:331–336. https://doi.org/10.1016/j.marpolbul.2014.04.026
Braithwaite VA, de Perera TB (2006) Short-range orientation in fish: how fish map space. Mar Fresh Behav Physiol 39:37–47. https://doi.org/10.1080/10236240600562844
Braithwaite VA, Armstrong JD, McAdam HM, Huntingford FA (1996) Can juvenile Atlantic salmon use multiple cue systems in spatial learning? Anim Behav 51:1409–1415
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4:227–234
Bugnot AB, Hose GC, Walsh CJ, Floerl O, French K, Dafforn KA et al (2019) Urban impacts across realms: making the case for inter-realm monitoring and management. Sci Total Environ 648:711–719. https://doi.org/10.1016/j.scitotenv.2018.08.134
Burns JG, Price AC, Thomson JD, Thomson JD, Hughes KA, Rodd H (2016) Environmental and genetic effects on exploratory behavior of high- and low-predation guppies (Poecilia reticulata). Behav Ecol Sociobiol 70:1–10. https://doi.org/10.1007/s00265-016-2127-x
Buxton RT, McKenna MF, Mennitt D, Fristrup K, Crooks K, Angeloni L et al (2017) Noise pollution is pervasive in U.S. protected areas. Science 356:531–533. https://doi.org/10.1126/science.aah4783
Chan AAYH, Giraldo-Perez P, Smith S, Blumstein DT (2010) Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol Let 6:458–461. https://doi.org/10.1037/h0022681
Cheng L, Wang S-H, Chen Q-C, Liao X-M (2011) Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol Behav 104:981–988. https://doi.org/10.1016/j.physbeh.2011.06.018
Cooper WE Jr, Samia DSM (2018) Choosing among alternative refuges: distances and directions. Ethology 124:209–217. https://doi.org/10.1016/S0065-3454(08)60192-8
Core Team R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from https://www.r-project.org
Cox K, Brennan LP, Gerwing TG, Brennan LP, Gerwing TG, Duda SE, Juanes F (2018) Sound the alarm: a meta-analysis on the effect of aquatic noise on fish behavior and physiology. Global Change Biol 24:3105–3116. https://doi.org/10.1007/s10162-004-4043-4
Cui B, Wu M, She X (2009) Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alteration in the hippocampus. J Occup Health 51:152–158
de Kort SR, Dickinson A, Clayton NS (2005) Retrospective cognition by food-caching western scrub-jays. Lear Motiv 36:159–176. https://doi.org/10.1016/j.lmot.2005.02.008
Dukas R (2004) Causes and consequences of limited attention. Brain Behav Evol 63:197–210. https://doi.org/10.1159/000076781
Egner SA, Mann DA (2005) Auditory sensitivity of sergeant major damselfish Abudefduf saxatilis from post-settlement juvenile to adult. Mar Ecol Prog Ser 285:213–222
Ferrari MCO, Mccormick MI, Meekan MG, Simpson SD, Nedelec SL, Chivers DP (2018) School is out on noisy reefs: the effect of boat noise on predator learning and survival of juvenile coral reef fishes. Proc R Soc London Ser B 285:20180033. https://doi.org/10.1111/gcb.12191
Ferreira CM, Gonçalves J, Coutinho R (1998) Herbivory by the dusky damselfish Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: effects on the benthic community. J Exp Mar Biol Ecol 229:241–264
Ferreira CM, Cavalcanti Coni EO, Medeiros DV, Sampaio CLS, Reis-Filho JA, Barros F et al (2015) Community structure of shallow rocky shore fish in a tropical bay of the southwestern Atlantic. Braz J Oceanogr 63:83–99. https://doi.org/10.1590/S1679-87592015074706304
Foster SA (1989) The implications of divergence in spatial nesting patterns in the geminate Caribbean and Pacific sergeant major damselfishes. Anim Behav 37:465–476
Froese R, Pauly D (2020) Editor Fishbase. World Wide Web electronic publication www.fishbase.org
Gill SA, Job JR, Myers K, Naghshineh K, Vonhofa MJ (2015) Toward a broader characterization of anthropogenic noise and its effects on wildlife. Behav Ecol 26:328–333. https://doi.org/10.1093/beheco/aru219
Gleason PE, Weber PG (1977) Effect of group size on avoidance learning in zebra fish, Brachydanio rerio (Pisces: Cyprinidae). Anim Lear Behav 5:213–216
Goines L, Hagler L (2007) Noise pollution: a modern plague. South Med J 100:287–294
Hawkins AD, Roberts L, Cheesman S (2014) Responses of free-living coastal pelagic fish to impulsive sounds. J Acoust Soc Am 135:3101–3116
Helfman GS (1998) Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav Ecol Sociobiol 24:47–58
Hildebrand JA (2009) Anthropogenic and natural sources of ambient noise in the ocean. Mar Ecol Prog Ser 395:5–20. https://doi.org/10.3354/meps08353
HMSO (1986) Animals (Scientific Procedures) Act. Amendment Regulations
Holbrook RI, de Perera TB (2011) Three-dimensional spatial cognition: information in the vertical dimension overrides information from the horizontal. Anim Cogn 14:613–619
Holbrook SJ, Schmitt R (2002) Competition for shelter space causes density-dependent predation mortality in damselfish. Ecology 83:2855–2868
Jafari Z, Kolb BE, Mohajerani MH (2019) Noise exposure accelerates the risk of cognitive impairment and Alzheimer’s disease: adulthood, gestational, and prenatal mechanistic evidence from animal studies. Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2019.04.001
Konstantinou N, Beal E, King J-R, Lavie N (2014) Working memory load and distraction: dissociable effects of visual maintenance and cognitive control. Atten Percept Psycol 76:1985–1997. https://doi.org/10.3758/s13414-014-0742-z
Leduc AOHC, Nunes JACC, de Araújo CB et al (2021) Land-based noise pollution impairs reef fish behavior: a case study with a Brazilian carnival. Biol Conserv 253:108910. https://doi.org/10.1016/j.biocon.2020.108910
Leis JM, Carson-Ewart BM, Cato DH (2002) Sound detection in situ by the larvae of a coral-reef damselfish (Pomacentridae). Mar Ecol Prog Ser 232:259–268
Lercher P, Evans PW, Meis M (2003) Ambient noise and cognitive processes among primary school children. Environ Behav 35:725–735. https://doi.org/10.1177/0013916503256260
Lugli M (2010) Sounds of shallow water fishes pitch within the quiet window of the habitat ambient noise. J Comp Physiol A 196:439–451. https://doi.org/10.1121/1.2713661
Magurran AE (1990) The adaptive significance of schooling as an anti-predator defense. Ann Zool Fenn 17:51–66
Markel RW (1994) An adaptive value of spatial learning and memory in the blackeye goby, Coriphopterus nicholsi. Anim Behav 47:1462–1464
McLaughlin KE, Kunc HP (2015) Changes in the acoustic environment alter the foraging and sheltering behaviour of the cichlid Amititlania nigrofasciata. Behav Proc 116:75–79. https://doi.org/10.1016/j.beproc.2015.04.012
Morgan MJ, Godin J-GJ (1985) Antipredator benefits of schooling behaviour in a Cyprinidontid fish, the Banded Killifish (Fundulus diaphanus). J Compar Ethol 70:236–246. https://doi.org/10.1111/j.1439-0310.1985.tb00515.x
Myrberg AA, Spires JY (1980) Hearing in damselfishes: an analysis of signal detection among closely related species. J Compar Physiol 140:135–144
Myrberg AA, Sypre JY (1972) Sound discrimination by the Bicolor damselfish, Eupomacentrus patitus. J Exp Biol 57:727–735
Nichols TA, Anderson TW, Širović A (2015) Intermittent noise induces physiological stress in a coastal marine fish. PLoS One 10:e0139157. https://doi.org/10.1371/journal.pone.0139157.s007
Noda M, Gushima K, Kakuda S (1994) Local prey search based on spatial memory and expectation in the planktivorous reef fish, Chromis chrysurus (Pomacentridae). Anim Behav 47:1413–1422
Nunes JACC, Leduc AOHC, Miranda RJ, Cipresso PH, Alves JP, Mariano-Neto E et al (2019) Refuge choice specificity increases with predation risk in a rocky reef fish. J Exp Mar Biol Ecol 520:151207. https://doi.org/10.1016/j.jembe.2019.151207
Odling-Smee L, Braithwaite V (2003a) The role of learning in fish orientation. Fish Fish 4:235–246
Odling-Smee L, Braithwaite VA (2003b) The influence of habitat stability on landmark use during spatial learning in the three-spined stickleback. Anim Behav 65:701–707. https://doi.org/10.1006/anbe.2003.2082
Odling-Smee L, Simpson SD, Braithwaite VA (2006) Orientation and spatial behaviour in fish. In: Brown C, Laland K (eds) Fish cognition and behaviour. Blackwell Publishing, London, pp 119–138
Odling-Smee LC, Boughman JW, Braithwaite VA (2008) Sympatric species of three spine stickleback differ in their performance in a spatial learning task. Behav Ecol Sociobiol 62:1935–1945. https://doi.org/10.1007/s00265-008-0625-1
Parmentier E, Lecchini D, Frederich B, Brie C, Mann D (2009) Sound production in four damselfish (Dascyllus) species: phyletic relationships? Biol J Linn Soc 97:928–940
Parvulescu A (1964) The acoustics of small tanks. In: Tavolga WN (ed) Marine bioacoustics II. Pergamon, Oxford, pp 7–13
Picciulin M, Sebastianutto L, Codarin A, Farina A, Ferrero EA (2010) In situ behavioural responses to boat noise exposure of Gobius cruentatus (Gmelin, 1789; fam. Gobiidae) and Chromis chromis (Linnaeus, 1758; fam. Pomacentridae) living in a marine protected area. J Exp Mar Biol Ecol 386:125–132. https://doi.org/10.1016/j.jembe.2010.02.012
Pinheiro HT, Rocha LA, Macieira RM, Carvalho-Filho A, Anderson AB, Bender MG et al (2018) South-Western Atlantic reef fishes: zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Diver Distrib 514:518–543. https://doi.org/10.1007/978-0-387-87458-6
Popper AN, Hawkins AD (2019) An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J Fish Biol 94:692–713. https://doi.org/10.1016/j.biocon.2005.10.020
Popper AN, Schilt CR (2008) Hearing and acoustic behavior: basic and applied considerations. In: Webb JF, Fay RR, Popper AN (eds) Fish bioacoustics. Springer, New York, pp 17–48
Popper AN, Hawkins AD, Sand O, Sisneros JA (2019) Examining the hearing abilities of fishes. J Acoust Soc Am 146:1–9. https://doi.org/10.1121/1.5120185
Prior H (2006) Effects of the acoustic environment on learning in rats. Physiol Behav 87:162–165. https://doi.org/10.1016/j.physbeh.2005.09.012
Rabat A (2007) Extra-auditory effects of noise in laboratory animals: the relationship between noise and sleep. J Am Assoc Lab Anim Sci 46:35–41
Rabin LA, McCowan B, Hooper SL, Owings DH (2003) Anthropogenic noise and its effect on animal communication: an interface between comparative psychology and conservation biology. I J Comp Psychol 16:172–192
Reese ES (1989) Orientation behavior of butterflyfishes (family Chaetodontidae) on coral reefs: spatial learning of route specific landmarks and cognitive maps. Environ Biol Fish 25:79–86
Rodrigues F, Duran E, Vargas JP, Torres B, Salas C (1994) Performance of goldfish trained in allocentric and egocentric maze procedures suggests the presence of a cognitive mapping system in fishes. Anim Lear Behav 22:409–420
Sabet SS, Wesdorp K, Campbell J, Snelderwaard P, Slabbekoorn H (2016) Behavioural responses to sound exposure in captivity by two fish species with different hearing ability. Anim Behav 116:1–11. https://doi.org/10.1016/j.anbehav.2016.03.027
Sánchez-Sánchez R, Fortes-Garrido JC, Bolívar JP (2015) Characterization and evaluation of noise pollution in a tourist coastal town with an adjacent nature reserve. Appl Acoust 95:70–76. https://doi.org/10.1016/j.apacoust.2015.02.004
Schluessel V, Bleckmann H (2012) Spatial learning and memory retention in the grey bamboo shark (Chiloscyllium griseum). Zoology 115:346–353. https://doi.org/10.1016/j.zool.2012.05.001
Simpson SD, Purser J, Radford AN (2011) How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol Let 14:1052–1061. https://doi.org/10.1016/j.aquaculture.2007.07.225
Sison M, Gerlai R (2010) Associative learning in zebrafish (Danio rerio) in the plus maze. Behav Brain Res 207:99–104. https://doi.org/10.1016/j.bbr.2009.09.043
Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN (2010) A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol Evol 11:305–313. https://doi.org/10.1016/j.tree.2010.04.005
Stansfeld SA, Matheson MP (2003) Noise pollution: non-auditory effects on health. Brit Med Bull 68:243–257. https://doi.org/10.1093/bmb/ldg033
Stansfeld SA, Berglund B, Clark C, Lopez-Barrio I, Fischer P, Öhrström E et al (2005) Aircraft and road traffic noise and children’s cognition and health: a cross-national study. Lancet 365:1942–1949
Tao S, Liu L, Shi L, Li X, Shen P, Xun Q, Guo X et al (2015) Spatial learning and memory deficits in young adult mice exposed to a brief intense noise at postnatal age. J Otol 10:21–28. https://doi.org/10.1016/j.joto.2015.07.001
Voellmy IK, Purser J, Simpson SD, Radford AN (2014) Increased noise levels have different impacts on the anti-predator behaviour of two sympatric fish species. PLoS One 9:e102946. https://doi.org/10.1371/journal.pone.0102946.s001
Wang ST, Yu Y, Feng Y, Zou F, Zhang X (2017) Protective effect of the orientin on noise-induced cognitive impairments in mice. Behav Brain Res 296:290–300
Washburn DA, Tagliatatela LA (2006) Attention as it is manifested across species. In: Wasserman A, Zentall TR (eds) Comparative cognition: experimental explorations of animals intelligence. Oxford University Press, New York, NY, pp 127–142
White GE, Brown C (2014) A comparison of spatial learning and memory capabilities in intertidal gobies. Behav Ecol Sociobiol 68:1393–1401. https://doi.org/10.1007/s00265-014-1747-2
White GE, Brown C (2015) Cue choice and spatial learning ability are affected by habitat complexity in intertidal gobies. Behav Ecol 26:178–184. https://doi.org/10.1093/beheco/aru178
Acknowledgments
We are grateful to the Institute of Biology of the Federal University of Bahia for allowing us to use the infrastructures to conduct this experiment, to Hans Slabbekoorn for providing the acoustic stimulus, and to James Campbell for insightful comments on the hydro-acoustics of small tanks. AOHCL is grateful for a CAPES PNPD Fellowship (88882.305953/2018-01). The capture of fish was authorized by the Institute Chico Mendes for Conservation of Biodiversity (License no 55979-1) and all procedures were approved by UFBA Ethic Committee (no 40-2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Leduc, A.O.H.C., Costa, J.S.O., do Nascimento Silva, R.R. et al. Spatial cognitive abilities of a tide-pool fish show resilience to noise pollution. J Ethol 39, 225–234 (2021). https://doi.org/10.1007/s10164-021-00697-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-021-00697-z