Abstract
Populations of a species may show variation in mating systems, especially when the species is widely distributed. Aglaoctenus lagotis is a funnel-web wolf spider distributed in South America and with a ‘central Argentina form’ (CA) and a ‘southern Uruguay form’ (SU). Both forms differ in sexual behaviour, population density and copulatory season. This study evaluates the potential level of polyandry of both forms, sequentially exposing females to different males of their form under laboratory conditions. The number of copulations each female accepted and the characteristics of these sexual encounters were registered. CA females accepted more re-copulations than SU females and seemed to maintain more sexual attractiveness after the first copulation. In neither form was female re-copulation influenced by body characteristics, duration of the first copulation, ejaculation frequency or copulatory body shaking of females. Additionally, the PCA showed that both forms could be separated by their copulation behaviours. The higher level of polyandry in the CA form compared to the SU form suggested in our results adds another difference between these forms, currently under study to determine whether they are different species. This study is the first on mating systems in funnel-web wolf spiders, adding knowledge to the discussion about the evolution of sexual strategies in this group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mating systems can vary within a species with distant or wide geographical variations, or between closely related species (Choe and Crespi 1997; Goodwillie et al. 2005). This phenomenon has been more frequently reported in plants (e.g. Brys et al. 2014) than in animals (Shine and Fitzgerald 1995). However, populations of animal species may show variation in mating systems (e.g. the degree of monopolization of mates in short or more extended copulatory seasons) (Emlen and Oring 1977; Elias et al. 2011), as occurs in other traits like morphological and physiological features (Foster and Endler 1999). Mating systems are related to ecological factors in populations, such as duration of mating, female sexual receptivity, length of the mating season, population density and, at the same time, are commonly influenced by climatic factors (Elias et al. 2011; Gowaty 2013; Macías-Ordóñez et al. 2014). That is why, in species with vast geographic distributions, environmental factors usually vary, favouring differences in mating systems (Emlen and Oring 1977; Macías-Ordóñez et al. 2014). In spiders, differences in specific traits associated with geographic or environmental variations have been reported for dispersion (Bonte et al. 2006) and courtship (Miller et al. 1998; Blackburn and Maddison 2014), but never in copulation rates.
Copulating several times could imply high costs for females such as a loss of foraging opportunities, increased risks of predation and transmission of pathogens (Herberstein et al. 2002). However, polyandry is common in the majority of the animals (Arnqvist and Nilsson 2000; Boulton and Shuker 2013; Taylor et al. 2014), and spiders are no exception. Indeed, polyandry seems to be the most frequent mating system in Araneae (Huber 2005), although some cases of monandry and monogyny have been reported (Pérez-Miles et al. 2007), principally associated with the occurrence of mating plugs and sexual cannibalism (Hosken et al. 2009; Kuntner et al. 2009; Nakata 2016). Among the benefits of polyandry for females are ensuring sufficient sperm to fertilize all of their eggs, receiving direct benefits such as nuptial gifts or, more indirectly, avoiding inbreeding and increasing the genetic diversity of the offspring (Eberhard 1996; Maklakov and Lubin 2006; Peretti and Aisenberg 2015). Furthermore, the unique structure of reproductive systems in female spiders offers a unique benefit to mating multiple times, which is the ability to store sperm separately and to exercise cryptic female choice (Eberhard 2004).
In the family Lycosidae (wolf spiders), Schizocosa ocreata and Pardosa astrigera present monandrous females as exceptional cases (Norton and Uetz 2005; Jiao et al. 2011). However, studies usually show low levels of polyandry as the typical scenario for the family (Fernández-Montraveta and Ortega 1990; Fernández-Montraveta and Cuadrado 2003; Aisenberg et al. 2009). These lower levels of polyandry could be related to the increase in female sexual selectivity as well as a decrease in female sexual receptivity after the first copulation (Fernández-Montraveta and Ortega 1990; Norton and Uetz 2005; Uetz and Norton 2007).
Aglaoctenus lagotis is a wolf spider widely distributed within the Neotropics, from Uruguay to Colombia (Piacentini 2011). It is an atypical member of the family Lycosidae because it has a sedentary lifestyle and lives its whole life in webs (Sordi 1996), in contrast to the wandering habit that characterizes the family (Foelix 2011). With an annual life cycle, adults of A. lagotis have a single reproductive period, and females construct only one egg sac in their lives (Santos and Brescovit 2001). There is scarce information available about A. lagotis and the other few members of the group of web lycosids. Recent studies have described courtship and copulation in A. lagotis, and they have led to the distinction of two forms, the central Argentina form (CA form) and the southern Uruguay form (SU form) (Stefani et al. 2011; González et al. 2013), with differences in phenology, microhabitat use (González et al. 2014) and body coloration patterns (González et al. 2015a).
In addition to differing in some of the behavioural units of courtship, the CA form has a longer courtship duration (averaging 9 min) and a shorter copulation duration (8 min on average) than the SU form (3 and 61 min on average, respectively). Also, phenological differences exist between the forms, with the CA form showing a spring–summer unified reproductive season and the SU form having its copulatory season during the autumn, the maternal period during spring–summer and the females, mostly mated, overwintering (González et al. 2014). Each form presents its characteristic pattern of body coloration (González et al. 2015a) and different use of microhabitats. The CA form occupies all layers of vegetation, with a higher population density and a broader spatial distribution compared to the SU form. The SU form, which only inhabits the herbaceous layer and in open environments, presents a lower population density and narrower spatial distribution (only reported for Uruguay to date) (González 2015). Therefore, because of the above characteristics, these two forms of A. lagotis would be temporally and spatially unable to meet, but if they did, the repertoires of sexual behavior would prevent matings. This scenario agrees with reproductive isolation between the two forms and with the occurrence of two distinct species (González et al. 2015a).
This study aimed to evaluate the potential level of polyandry, under laboratory conditions, in the two forms of A. lagotis (the CA form and the SU form) (objective 1). We expected significant variations in mating systems between the forms based on the wide geographic distribution of the species and the differences in several characteristics related to copulatory rates already reported between the forms. We also evaluated whether the copulatory traits (copulation duration, number of ejaculations, female body shakings, among others) were correlated with the level of polyandry of each form (objective 2). Several studies show that copulatory traits, such as long copulation durations, a high number of ejaculations and long courtship durations, imply greater energy investments, and decrease the likelihood of re-copulation for both sexes (Singer and Riechert 1995; González and Costa 2008). This is why we expected a higher potential for polyandry in the CA form than in the SU form, given that the shorter duration of copulation reported in the former (González et al. 2013) could be associated with a lower transfer of spermatozoa per copulation. At the same time, the shorter copulatory season (González et al. 2014) and a lower population density of the SU form (González 2015) would diminish re-copulation opportunities compared to the CA form.
Materials and methods
Collecting and housing
We worked with the two forms of A. lagotis from two different localities: individuals from the CA form were collected in the lowlands of the Sierras Chicas mountains, Western Córdoba, Argentina (CA; 30°57′00.10″S, 64°10′00.28″W) and individuals from the SU form were collected in the Piedras de Afilar grasslands, Canelones, Southern Uruguay (SU; 34°36′44.83″S, 54°27′24.22″W). We collected adult males and subadult individuals of the CA form from August to October 2010 and of the SU form from March to April 2010–2011. Males collected in the field were considered virgins because we captured them alone in webs, which would mean that they were still in their own webs, and had not left to look for females (M. González, personal observation; reported for other funnel-web spiders by Singer and Riechert 1995). We captured the spiders during daylight and manually, blocking silk tubes and forcing the spiders to go out to the platform of the web, where we collected them with Falcon tubes. We used 15 females and 75 males of each form for the trials. In this way, we exposed each female (initially virgin) to five males to evaluate the number of copulations that she was capable of accepting. The collecting and trial period was consistent with the copulatory season of each form (reported in González et al. 2014), and trials were developed in the laboratories of each locality (CA form trials in the Laboratorio de Biología Reproductiva y Evolución, UNC, Argentina and SU form trials in the Departamento de Ecología y Biología Evolutiva, IIBCE, Uruguay). In both cases, laboratory temperatures averaged 20–23°C.
Spiders were individually housed in Petri dishes (diameter 9.5 cm, height 1.5 cm) with a piece of cotton embedded in water. We fed all the individuals twice a week with larvae of mealworms Tenebrio sp. (Coleoptera; Tenebrionidae) and Acheta domestica small crickets (Orthoptera; Gryllidae). We monitored individuals daily and recorded the occurrences of moulting in the subadults, aiming to determine the exact date of reaching adulthood. Ten voucher specimens of each form (five females and five males) were deposited in the scientific arachnological collections of the Cátedra de Diversidad Animal I, Facultad de Ciencias Exactas, Físicas y Naturales, UNC, Argentina, and the Facultad de Ciencias, UdelaR, Montevideo, Uruguay.
Experimental design
For the trials, we used virgin females between 10 and 15 days after reaching adulthood, ensuring genital sclerotization and chemical sexual attractiveness (Papke et al. 2001; Baruffaldi and Costa 2010). We used males 7 days after reaching adulthood to ensure cuticle hardness. Adult males from the field were used 4 days after their capture, to ensure captivity acclimatization. We took this decision about the number of days in order to reduce the difference in the ages between both types of males since we do not know, under natural conditions, how many days the males remain in their webs after the final moult. However, they would stay at least 3 days after reaching adulthood under laboratory conditions (M. González, personal observation). Both types of males were randomly used within the experimental groups. We performed the trials during daylight since we have observed copulations in the field during the day (F. G. Costa and M. González, unpublished data). We carried out the experimental trials in glass cages (length 30 x width 16 x height 20 cm), with a layer of 2 cm of sand and 2 cm of wood-chips above as substrate, simulating leaf litter. We also added Y-shaped small plant branches as a refuge and for web support, as well as a water source in small lids. We placed each virgin female in the arena 5 days before the trial, to allow funnel-web construction (González et al. 2015b). After web construction, we sprinkled water over them, simulating dew, to ensure water supply.
We performed an intra-form study, and that is why we carried out the same methodological steps twice, once for the CA form and another time for the SU form. Firstly, we exposed each of 15 females to a randomly selected male which was placed on the margin of the female web. Only the females that copulated continued the experiment (‘first exposure’ = copulation). Secondly, each female was exposed consecutively to four new males (‘subsequent exposures’): immediately after copulation (0 day), 3 days after copulation, 10 days after copulation and 30 days after copulation. In sum, each female was exposed to five males during a month (the estimated time females allocate to copulation before egg sac construction or the death of males — M. González, personal observation). The choice of these time intervals was based on other studies that evaluated the evolution of female sexual receptivity after the first copulation in lycosids (Norton and Uetz 2005; González and Costa 2008). The ‘first exposure’ trials were discarded when males did not court after 30 min, or if the copulation did not occur after 60 min of placing the male on the female web. Individuals were not reused. The ‘subsequent exposure’ trials were discarded only if the male did not court after 30 min of placing him on the female web. In these cases, the female was exposed immediately to another male but, if that male did not court, the female was not re-exposed to another male until the subsequent exposure date. If the males courted but copulation did not occur, or if attacks occurred, the female was not re-exposed to another male until the subsequent exposure date.
We observed whether courtship and copulation occurred at each of the subsequent exposures, as well as their respective characteristics. We recorded the trials with a Sony DCR-SR85 digital video camera and analysed the videos with the JWATCHER software (Blumstein et al. 2000). We registered courtship duration (elapsed time between the first courtship behaviour and male mounting) and copulation duration (elapsed time between male mounting and dismounting). We also observed the occurrence and duration of each sexual behavioural act reported by González et al. (2013). Courtship behaviours of the SU form were web-stretching, striding-forwards, and forelegs-elevating (performed by males), while in the CA form they were web-stretching and leg-tapping (performed by males). We also included the leg-tappings performed by females of both forms (a possible indicator of positive sexual receptivity, González 2015; González et al. 2015a). Courtship intensity was measured as the ratio between the sum of all courtship behaviour and courtship duration. The rate of each courtship behavioural act was measured as the ratio between the sum of occurrences of each act and courtship duration. During copulation we registered insertions (when each male palp was introduced in one of the two female gonopores), ejaculations (estimated by the hematodochal expansions or by the erection of the spines in male hind legs) and female body shakings during copulation (another possible indicator of positive sexual receptivity) (González 2015; González et al. 2015a).
Body dimensions of the copulatory partners (e.g. weight and size) are commonly reported as traits affecting the occurrence of copulations (Andersson 1994). Therefore, we measured carapace width, a common measure of body size in spiders (Eberhard et al. 1998; Foelix 2011), and abdominal width. We considered the ratio between abdominal width and carapace width as an index of the body condition of an individual (Moya-Laraño et al. 2003). Females of both forms did not differ in size (CA: 4.44 ± 0.74 mm; SU: 4.58 ± 0.46 mm; U = 135.5, z = 0, P = 0.99), although males from CA were smaller than SU males (CA: 4.18 ± 0.66 mm; SU: 4.95 ± 0.57 mm; U = 48, z = –2.83, P = 0.003).
Statistical analyses
We analysed the results using the statistical packages PAST, version 1.18 (Hammer et al. 2003) and WINPEPI, version 1.6 (Abramson 2004). We used the chi-square test for independent variables to compare the total number of copulations between the different experimental groups of both forms, with the Bonferroni correction to diminish type I error. Regarding the second objective, we analysed courtships, copulations and re-copulation traits with the Kruskal–Wallis ANOVA test and the Mann–Whitney U test for non-parametric data. We also performed multiple linear regressions to evaluate the relation between the number of copulations each female achieved and body characteristics (size, body condition, and weight), copulation duration, the frequency of ejaculations or frequency of body shakings during copulation. With logistics regressions, we tested the relation between the occurrence of attacks and male body dimensions. We also performed a principal component analysis to visualize whether the two forms can be distinguished by differences in the female tendency to re-copulate.
Results
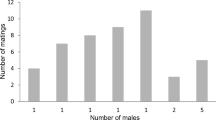
More than half of the females from the SU form (n = 8) never re-copulated, while most females of the CA form (n = 14) re-copulated between one and two times (\(\chi_{4}^{2}\)= 10.8, P = 0.029). CA form females accepted re-copulations earlier than SU form females (\(\chi^{2}\)= 4.03, P = 0.045), and they achieved more copulations compared to the SU ones (U = 75, z = –3.27, P = 0.001) (Fig. 1). Female re-copulation was not influenced by body characteristics, duration of the first copulation, ejaculation frequency or copulatory body shaking, in any of the forms (CA: R = 0.6, F = 0,950, df = 6, P = 0.502; SU: R = 0.290, F = 0,110, df = 6, P = 0.987). Nevertheless, the PCA showed that both forms could be separated by their copulation behaviours (Fig. 2). We did not find differences between durations of copulations and re-copulations. Similarly, we did not observe differences between their respective frequencies of insertions, ejaculations and body shakings (Table 1).
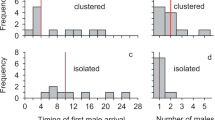
Principal component analysis (PCA) analysing characteristics of both forms (CA and SU): number of re-copulations, duration of (first) copulations, frequencies of ejaculations and body shakings during sexual encounters. It indicates the presence of two groups based on the first two components (PC1 and PC2) corresponding to the two studied forms
Courtship duration of CA form males did not differ between the groups of copulated females (H = 7.213, P = 0.072), although it tended to be shorter in the 3-days group. However, males courted for longer duration to copulated than to virgin females (U = 692.5, z = –3.09, P = 0.002), but without differences in the intensity (U = 748.5, z = –1.58, P = 0.113). Leg-tapping was more commonly performed by virgins (100%) than by copulated females (77%) (\(\chi_{1}^{2}\) = 29.88; P < 0.001), as well as at a higher rate (U = 525.00, z = –4.24, P < 0.001). Only copulated females of the 0-day group attacked males (n = 3). These attacks did not show a relation to lack of male courtship (\(\chi_{1}^{2}\) = 0.051, P = 0.832), courtship intensity (\(\chi_{1}^{2}\) = 1.472, P = 0.225) or ratio between female/male sizes (\(\chi_{1}^{2}\) = 1.152, P = 0.283). However, the attacked males were those in poorest body condition (\(\chi_{1}^{2}\) = 8.789, P = 0.003).
Courtship duration did not differ between the groups of copulated females in the SU form (H = 0.391, P = 0.820). Nevertheless, males courted copulated females for longer (U = 78.00, z = –3.520, P < 0.001) but less intensely than virgin females (U = 106.00, z = –3.065, P = 0.002). Leg-tapping was more commonly performed by virgin females (47%) than by copulated females (11%) (\(\chi_{1}^{2}\) = 12.651; P = 0.010), as well as at a higher rate (U = 4.00, z = –2.562, P = 0.011). Copulated females of the 0-day (n = 9) and 3-days (n = 9) groups attacked males. Attacks were not associated with presence or absence of male courtship (\(\chi_{1}^{2}\) = 0.031, P = 0.873), courtship intensity (\(\chi_{1}^{2}\) = 2.142, P = 0.342), or ratio between female/male sizes (\(\chi_{1}^{2}\) = 0.060, P = 0.806). We found a negative relationship between male body size and female attacks (\(\chi_{1}^{2}\) = 5.424, P = 0.0200), but we did not find a significant relation with male body condition (\(\chi_{1}^{2}\) = 0.636, P = 0.425). No evidence of mating plugs or sperm removal was observed in either form.
Discussion
Our study indicates that the forms of the funnel-web wolf spider A. lagotis are likely to differ in their mating systems. Although females of both forms were exposed to the same number of ‘potential’ mates (i.e. having removed the factor ‘female web access’), a higher level of polyandry was observed in the CA form than in the SU form, in which the majority of females never re-copulated. CA form females were more receptive to copulation (e.g. with more leg-tapping and fewer attacks) than SU females, not only when they were virgins but also after copulating. As is the rule in lycosids (Rypstra et al. 2003; Aisenberg and Costa 2005), after the first copulation females reduce their receptivity (fewer leg-tappings), and also their attractiveness (e.g. males court with less intensity), especially in the SU form.
In the SU form, males courted virgin females more intensely than the copulated ones. Females also performed a higher leg-tapping rate when they were virgins, signalling positive sexual receptivity to males. These observations suggest that both receptivity and attractiveness decrease after the first copulation. Males, in turn, courted copulated females for longer, possibly showing caution about the risk of being attacked, as observed in our study. In the CA form, males did not change their courtship intensity when facing virgin or copulated females. All these differences, together with a low attack rate in the CA form, suggest a significant predisposition to a high level of polyandry in the CA form.
In contrast, combining our results with those from previous studies of A. lagotis (González et al. 2013, 2014, 2015a), we can suggest that the SU form has a mating system with a low potential level of polyandry. This feature could be linked with a short copulatory season (where males have scarce time to find mates — González et al. 2014), low population density (implying greater energy and time used during mate searching — González 2015) and presence of long copulations (average 61 min — González et al. 2013). Under these constraints, each male could prioritise paternity with at least one female, which could result in investing most of the effort in a single copulation. Meanwhile, females could prioritise having the sperm to fertilize their eggs before winter and male death. Only females survive the winter, storing sperm for fertilization, which occurs next spring (González et al. 2014). This investment priority of the male in a single copulation could even involve the transfer of post-mating substances responsible for the marked decline of female receptivity [receptivity inhibiting substances (RIS)], as has been suggested for the lycosid Schizocosa malitiosa (Aisenberg and Costa 2005; Michalik et al. 2013).
The higher potential level of polyandry in the CA form adds to the previously reported shorter copulations than in the SU form (averaging 8 min) (González et al. 2013), and to the more aggregated distribution of the individuals (González 2015). These factors could favour males that invest in copulations with several females. Similarly, females might have several opportunities to re-copulate over an extended period (4 months; González et al. 2014), and thus counteract adverse effects of inbreeding (Simmons 2005) resulting from high population density, probably involving closeness of relatives (M. González, personal observation).
Differences in the ecological context may explain the level of female receptivity to new re-copulations in many groups of animals (some very distant like birds and wasps — Gowaty 2013; Boulton and Shuker 2016). Since the copulation period of each form occurs in different seasons (autumn and spring, respectively), the copulation frequencies of each form could be explained by ecological factors, for example, temperature. This ecological factor may also influence the duration of copulation, as reported for the lycosid spider Schizocosa malitiosa by Costa and Sotelo (Costa and Sotelo 1994) in which a 1°C) increase in ambient temperature results in a 10% reduction in copulation duration. Micro-environmental preferences of the forms (open areas and herbaceous stratum for the SU form versus open and closed areas and various plant strata for the CA form) (González et al. 2014) cannot be ruled out as another factor linked to the reported differences. Probably the CA form preferences promote a higher population density and, therefore, a greater possibility of a sexual encounter than in the SU form. Current studies show that both forms of the species have an area of overlap and suggest that the sexual and phenological patterns reported for them in allopatry remain in sympatry (González et al. 2013, 2014). If confirmed, environmental factors will also have to be analysed there.
As effects of individual weight and size on the occurrence of copulation have been reported in other spiders (Andersson 1994; Riechert and Johns 2003; Schafer and Uhl 2004; Costa-Schmidt and Machado 2012), we expected that bigger females or those in better body condition would achieve a higher number of copulations due to their higher fecundity. However, in both forms of A. lagotis, acceptance of new copulations was not determined by the size and body condition of the female. Likewise, Norton and Uetz (2005) found no relationship between male and female size and the probability of copulation in the wolf spider Schizocosa ocreata. In our study, females from both forms were more likely to attack males when they had already copulated at least once, males were smaller (in the SU form) or had poorer body condition (in the CA form). Similar results have been found in studies of other lycosids (Persons and Uetz 2005; Fernández-Montraveta et al. 2014), and an increased risk of cannibalism has also been observed in smaller individuals of other invertebrates, such as amphipods (Dick 1995) and cephalopods (Hanlon and Forsythe 2008). As the attacked males were not those with different courtship intensity, we do not know how the females perceived the differences and decided to attack them. Probably the signals differed during courtship and were detected by the females, but we were unable to detect and evaluate them.
Regardless of how the differences arose, each form of A. lagotis seems to have a unique copulatory strategy (low rate of potential polyandry in the SU form and high rate in the CA form), suggesting another essential feature of differentiation between them. As we mentioned before, these forms also differ in phenological patterns (González et al. 2014), body pigmentation patterns of individuals (González et al. 2015a) and sexual repertoires (González et al. 2013), and the two do not intercross except rarely under laboratory conditions (González et al. 2015a). A process of reproductive divergence could explain these inter-form differences and, consequently, speciation, as has been suggested for other arachnids (Blackburn and Maddison 2014; Olivero et al. 2017), amphibians (Barboza 2014), Gasterosteus aculeatus fish (Ishikawa et al. 2006) and other marine species (Palumbi 1994).
The most common mating system in spiders is polygamy (Elgar 1998), and lycosids are no exception (Fernández-Montraveta and Ortega 1990; Rypstra et al. 2003; Aisenberg and Costa 2005). While the benefits of polyandry are not specifically known for the family, the two independent spermathecae present in female spiders imply their ability to store sperm from different mates, separately and for long periods, turning into a unique opportunity to exercise cryptic female choice (Eberhard 2004). This benefit seems to be particularly crucial in Aglaoctenus females which are likely to oviposit a single egg sac in their lifetime (Santos and Brescovit 2001), so an increase in the number of eggs is not expected as a benefit. However, the number of fertilized eggs or quality of the offspring may still vary within the egg sac, which could be another benefit of polyandry. Other plausible benefits include the passage of nutrients by the male with the ejaculate (for the female or offspring) and increased genetic variability of offspring, thus reducing the risk of inbreeding (Reynolds 1996; Elgar 1998).
What happens with the male mating strategies, which was not addressed in this study, is currently being explored for both forms. In-depth knowledge of the level of polygyny as well as field studies documenting male visits to female webs will be constructive in improving the understanding of the mating systems of these forms. It would also be enlightening to track the number of sperm transferred by males per copulation, whether the amount differs between copulations and re-copulations, and to determine whether a female’s decision to re-copulate is influenced by the sperm stored (taking into account that females build a single egg sac in their lifetime). Progress on these issues will be useful in uncovering the benefits and costs of polygamy in this atypical group of wolf spiders and animals in general, and in evaluating the relationships between mating systems and lifestyle habits (a sedentary group of lycosids in a family of wandering members).
References
Abramson JH (2004) WINPEPI (PEPI-for-Windows): computer programs for epidemiologists. Epidemiol Perspect Innov 1: 1–6
Aisenberg A, Costa FG (2005) Females mated without sperm transfer maintain high sexual receptivity in the wolf spider Schizocosa malitiosa. Ethology 111:545–558
Aisenberg A, Estramil N, Toscano-Gadea C, González M (2009) Timing of female sexual unreceptivity and male adjustment of copulatory behaviour under competition risk in the wolf spider Schizocosa malitiosa. J Ethol 27:43–50
Andersson M (1994) Sexual selection. Princeton University Press, New Jersey
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Barboza FR (2014) Retomando el concepto de plasticidad fenotípica en el estudio de los modos reproductivos de anfibios anuros. Bol Soc Zool Uruguay 1(2):16–29
Baruffaldi L, Costa FG (2010) Changes in male sexual responses from silk cues of females at different reproductive states in the wolf spider Schizocosa malitiosa. J Ethol 28:75–85
Blackburn GS, Maddison WP (2014) Stark sexual display divergence among jumping spider populations in the face of gene flow. Mol Ecol 23:5208–5223
Blumstein DT, Evans CS, Daniel JC (2000) JWatcher. http://galliform.psy.mq.edu.au/jwatcher/Accessed 13 Mar 2009
Bonte D, Vanden Borre J, Lens L, Maelfait JP (2006) Geographical variation in wolf spider dispersal behavior is related to landscape structure. Anim Behav 72:655–662
Boulton RA, Shuker DM (2013) Polyandry. Curr Biol 23(24):1080–1081
Boulton RA, Shuker DM (2016) Polyandry is context dependent but not convenient in a mostly monandrous wasp. Anim Behav 112:119–125
Brys R, Broeck AV, Mergeay J, Jacquemynl H (2014) The contribution of mating system variation to reproductive isolation in two closely related Centaurium species (Gentianaceae) with a generalized flower morphology. Evolution 68(5):1281–1293
Choe JC, Crespi BJ (1997) The evolution of mating systems in insects and arachnids. Cambridge University Press, Cambridge
Costa FG, Sotelo JR (1994) Stereotypy and versatility of the copulatory pattern in Lycosa malitiosa (Araneae, Lycosidae) at cool versus warm temperatures. J Arachnol 22:200–204
Costa-Schmidt LE, Machado G (2012) Reproductive interference between two sibling species of gift-giving spiders. Anim Behav 84(5):1201–1211
Dick JTA (1995) The cannibalistic behaviour of two Gammarus species (Crustacea: Amphipoda). J Zool 236:697–706
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Eberhard WG (2004) Why study spider sex: special traits of spiders facilitate studies of sperm competition and cryptic female choice. J Arachnol 32(3):545–556
Eberhard WG, Huber BA, Rodríguez SRL, Briceño RD, Salas L, Rodríguez V (1998) One size fits all? Relationships between the size and degree of variation in genitalia and other body parts in twenty species of insects and spiders. Evolution 52(2):415–431
Elgar MA (1998) Sperm competition and sexual selection in spiders and other arachnids. In: Birkhead TR, Moller AP (eds) Sperm competition and sexual selection. Academic Press, California, pp 307–332
Elias DO, Andrade MCB, Kasumovic MM (2011) Dynamic population structure and the evolution of spider mating systems. In: Casas J (ed) Advances in insect physiology. Academic Press, Burlington, pp 65–114
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Fernández-Montraveta C, Cuadrado M (2003) Timing and patterns of mating in a free-ranging population of Lycosa tarantula (Araneae, Lycosidae) from central Spain. Can J Zool 81:552–555
Fernández-Montraveta C, Ortega J (1990) Some aspects of the reproductive behavior of Lycosa tarantula fasciiventris (Araneae, Lycosidae). J Arachnol 18:257–262
Fernández-Montraveta C, González JM, Cuadrado M (2014) Male vulnerability explains the occurrence of sexual cannibalism in a moderately sexually dimorphic wolf spider. Behav Process 105:53–59
Foelix RF (2011) Biology of spiders, 3rd edn. Oxford University Press, New York, p 419
Foster SA, Endler JA (1999) Introductions and aims. In: Foster SA, Endler JA (eds) Geographic variation in behavior. Perspectives on evolutionary mechanisms. Oxford University Press, New York
González M (2015) Aspectos reproductivos de Aglaoctenus lagotis: estudio interpoblacional de una araña lobo sedentaria de gran variabilidad fenotípica. PhD thesis, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Argentina, p 254
González M, Costa FG (2008) Persistence of sexual reluctance in mated females and the importance of regular copulation in a wolf spider. Ethol Ecol Evol 20:115–124
González M, Peretti AV, Viera C, Costa FG (2013) Differences in sexual behavior of two distant populations of the funnel-web wolf spider Aglaoctenus lagotis. J Ethol 31:175–184
González M, Costa FG, Peretti AV (2014) Strong phenological differences between two populations of a Neotropical funnel-web wolf spider. J Nat Hist 48:2183–2197
González M, Costa FG, Peretti AV (2015a) Funnel-web construction and estimated immune costs in Aglaoctenus lagotis (Araneae: Lycosidae). J Arachnol 43:158–167
González M, Peretti AV, Costa FG (2015b) Reproductive isolation between two populations of Aglaoctenus lagotis, a funnel-web wolf spider. Biol J Linn Soc 114:646–658
Goodwillie C, Kalisz S, Eckert CG (2005) The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annu Rev Ecol Evol Syst 36:47–79
Gowaty PA (2013) Adaptively flexible polyandry. Anim Behav 86:877–884
Hammer O, Harper DAT, Ryan PD (2003) Past palaeontological, version 1.18. Copyright Hammer and Harper. http://folk.uio.no/ohammer/past. Accessed 15 Mar 2009
Hanlon RT, Forsythe JW (2008) Sexual cannibalism by Octopus cyanea on a Pacific coral reef. Mar Freshw Behav Phy 41(1):19–28
Herberstein ME, Schneider JM, Elgar MA (2002) Costs of courtship and mating in a sexually cannibalistic orb-web spider: female mating strategies and their consequences for males. Behav Ecol Sociob 51:440–446
Hosken DJ, Stockley P, Tregenza T, Wedell N (2009) Monogamy and the battle of the sexes. Annu Rev Entomol 54:361–378
Huber BA (2005) Sexual selection research on spiders: progress and biases. Biol Rev 80:363–385
Ishikawa M, Mori S, Nagata Y (2006) Intraspecific differences in patterns of courtship behaviours between the Pacific Ocean and Japan Sea forms of the three-spined stickleback Gasterosteus aculeatus. J Fish Biol 69:938–944
Jiao X, Guo L, Chen Z, Wu J, Chen J, Liu F, Li D (2011) Experimental evidence for female-driven monandry in the wolf spider, Pardosa astrigera. Behav Ecol Sociobiol 65:2117–2123
Kuntner M, Kralj-Fiser S, Schneider JM, Li D (2009) Mate plugging via genital mutilation in nephilid spiders: an evolutionary hypothesis. J Zool 277:257–266
Macías-Ordóñez R, Machado G, Macedo RH (2014) Macroecology of sexual selection: large-scale influence of climate on sexually selected traits. In: Macedo RH, Machado G (eds) Sexual selection: perspectives and models from the Neotropics. Academic Press, USA, pp 1–32
Maklakov AA, Lubin Y (2006) Indirect genetic benefits of polyandry in a spider with direct costs of mating. Behav Ecol Sociobiol 61:31–38
Michalik P, Aisenberg A, Postiglioni R, Lipke E (2013) Spermatozoa and spermiogenesis of the wolf spider Schizocosa malitiosa (Lycosidae, Araneae) and its functional and phylogenetic implications. Zoomorphology 132:11–21
Miller GL, Stratton GE, Miller PR, Hebets E (1998) Geographical variation in male courtship behaviour and sexual isolation in wolf spiders of the genus Schizocosa. Anim Behav 56:937–951
Moya-Laraño J, Pascual J, Wise DH (2003) Mating patterns in late-maturing female Mediterranean tarantulas may reflect the costs and benefits of sexual cannibalism. Anim Behav 66:469–476
Nakata K (2016) Female genital mutilation and monandry in an orb-web spider. Biol Lett 12:20150912. https://doi.org/10.1098/rsbl.2015.0912
Norton S, Uetz GW (2005) Mating frequency in Schizocosa ocreata (Hentz) wolf spiders: evidence for a mating system with female monandry and male polygyny. J Arachnol 33:16–24
Olivero PA, Mattoni CI, Peretti AV (2017) Differences in mating behavior between two allopatric populations of a Neotropical scorpion. Zoology 123:71–78
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Evol Syst 25:547–572
Papke MD, Riechert SE, Schulz S (2001) An airborne female pheromone associated with male attraction and courtship in a desert spider. Anim Behav 61:877–886
Peretti AV, Aisenberg A (2015) Cryptic female choice in arthropods: patterns, mechanisms and prospects. Springer International Publishing, Switzerland
Pérez-Miles F, Postiglioni R, Montes-de-Oca L, Baruffaldi L, Costa FG (2007) Mating system in the tarantula spider Eupalaestrus weijenberghi (Thorell, 1894): evidences of monandry and polygyny. Zoology 110:253–260
Persons MH, Uetz GW (2005) Sexual cannibalism and mate choice decisions in wolf spiders: influence of male size and secondary sexual characters. Anim Behav 69:83–94
Piacentini L (2011) Three new species and new records in the wolf spider subfamily Sosippinae from Argentina (Araneae: Lycosidae). Zootaxa 3018:27–49
Reynolds JD (1996) Animal breeding systems. Tree 11(2):68–72
Riechert SE, Johns PM (2003) Do female spiders select heavier males for the genes for behavioral aggressiveness they offer their offspring? Evolution 57(6):1367–1373
Rypstra AL, Wieg C, Walker SE, Persons MH (2003) Mutual mate assessment in wolf spiders: differences in the cues used by males and females. Ethology 109:315–325
Santos AJ, Brescovit AD (2001) A revision of the South American spider genus Aglaoctenus Tullgren, 1905 (Araneae, Lycosidae, Sosippinae). Andrias 15:75–90
Schafer MA, Uhl G (2004) Sequential mate encounters: female but not male body size influences female remating behavior. Behav Ecol 12(2):461–466
Shine R, Fitzgerald M (1995) Variation in mating systems and sexual size dimorphism between populations of the Australian python Morelia spilota (Serpentes: Pythonidae). Oecologia 103:490–498
Simmons LW (2005) The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu Rev Ecol Evol Syst 36:125–146
Singer F, Riechert SE (1995) Mating system and mating success of the desert spider Agelenopsis aperta. Behav Ecol Sociobiol 36:313–322
Sordi S (1996) Ecologia de populaçoes da aranha Porrimosa lagotis (Lycosidae) nas reservas Mata de Santa Genebra, Campinas (SP) e Serra do Japi, Jundai (SP) PhD thesis, Universidade Estadual de Campinas, Sao Paulo, Brasil
Stefani V, Del-Claro K, Silva LA, Guimaraes B, Tizo-Pedroso E (2011) Mating behavior and maternal care in the tropical savanna funnel-web spider Aglaoctenus lagotis Holmberg (Araneae: Lycosidae). J Nat Hist 45:1119–1129
Taylor ML, Price TAR, Wedell N (2014) Polyandry in nature: a global analysis. Home. Trends Ecol Evol 29(7):376–383
Uetz GW, Norton S (2007) Preference for male traits in female wolf spiders varies with the choice of available males, female age and reproductive state. Behav Ecol Sociobiol 61:631–641
Acknowledgements
We are very grateful to Anita Aisenberg, María José Albo, Fabiana Baldenegro, Luciana Baruffaldi, Silvana Burela, Soledad Ghione, Gabriel de Simone and María Elena Pérez for their help during the fieldwork. Laura Montes de Oca helped us in housing and breeding individuals in the laboratory, and John Henderson revised the language. We also thank Santiago Benitez-Vieyra, Margarita Chiaraviglio and Martín Ramirez (members of Macarena González’s Ph.D. monitoring committee) for their useful contributions. Financial support was provided by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Fondo para la Investigación Científica y Tecnológica (FONCYT) and Secretaría de Ciencia y Tecnología (SECYT), Universidad Nacional de Córdoba. Finally, we thank the anonymous reviewers who have contributed to the improvement of the manuscript in a substantial way.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
González, M., Costa, F.G. & Peretti, A.V. Different levels of polyandry in two populations of the funnel-web wolf spider Aglaoctenus lagotis from South America. J Ethol 37, 325–333 (2019). https://doi.org/10.1007/s10164-019-00606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-019-00606-5