Abstract
The stink bug Leptocorisa chinensis (Hemiptera: Alydidae), which causes pecky rice grains, emits pungent volatiles when disturbed. To study ecological functions of the volatiles, we investigated the responses of adult L. chinensis in a small observation arena. When an undisturbed individual of the same gender was introduced into the arena, the initially occupying L. chinensis did not show any behavioural responses. However, when a disturbed conspecific of the same gender was introduced, the initial occupant was excited immediately and escaped from the arena through the hole, suggesting that the pungent volatiles from a disturbed conspecific caused excitement/escape behaviour. Chemical analyses using a gas chromatograph–mass spectrometer showed that disturbed adults of both sexes emitted octanal, (E)-2-octenal, octanol, hexyl acetate, (Z)-3-octenyl acetate, octyl acetate and (E)-2-octenyl acetate. (E)-2-Octenal was the major compound. When exposed to (E)-2-octenal and to (E)-2-octenyl acetate, undisturbed females were excited and escaped from the observation arena with a similar proportional response as to disturbed females. Males and females escaped from (E)-2-octenal at ca. 2–10 ppbV in a mesh cage. The possible use of the volatile compounds eliciting excitement/escape behaviour in L. chinensis for control of this species in paddy fields is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When piercing–sucking herbivores feed on rice grains, they leave brownish-black spots called pecks on the grains. Pecky rice grains are shrivelled and of poor quality; therefore, an increase in the proportion of such grains decreases the market value of the crop (Ito 2004). Leptocorisa chinensis Dallas (Hemiptera: Alydidae), which occurs throughout South and Southeast Asia (Grist and Lever 1969), is a major pecky rice-causing stink bug in the area. In Japan, this species occurs in mainland (Takeuchi 2007). It is bivoltine throughout most of its distribution. During spring, wild Gramineae species host L. chinensis, and the first generation of offspring predominantly uses these plants for reproduction. The second generation of adults invades paddy fields and feeds on grains in the milk or soft-dough stages, causing pecky rice (Takeuchi et al. 2004; Takeuchi 2007). Effective and environmentally benign control of this species in paddy fields is needed.
Disturbed stink bugs emit pungent volatile compounds that could have several ecological functions, such as defence against predators or alarm pheromones (Blum 1985; Aldrich 1995). An alarm pheromone function might be used for behavioural control of stink bugs in agroecosystems; For example, in Sri Lanka, farmers collect and squash stink bugs, placing them in bags around the field to reduce damage (Yamashita, personal communication). The volatiles from smashed bugs probably repel stink bugs in the area. We hypothesised that volatiles emitted from disturbed L. chinensis could be used to repel conspecifics in rice fields and reduce the number of pecky rice grains.
Leal et al. (1996) identified the volatiles emitted from L. chinensis that were anaesthetised with CO2 to minimise the release of defensive secretion, and found that a 5:1 mixture of (E)-2-octenyl acetate and octanol, major components of the emissions of undisturbed L. chinensis, was an attractant pheromone. However, the chemistry and ecological functions of volatiles from disturbed L. chinensis individuals have not yet been studied. The objective of this study is to clarify whether the volatiles from disrupted L. chinensis elicit excitement and escape behaviour in conspecifics as a first step to test the above hypothesis. We first investigated whether L. chinensis escaped from disturbed conspecifics in an observation arena under laboratory conditions. We then analysed the volatiles emitted by both disturbed and undisturbed L. chinensis and observed the responses of undisturbed L. chinensis to the components in volatiles from disturbed conspecifics.
Materials and methods
Insects
A colony of L. chinensis was established from adults collected from paddy fields in Kasai, Hyogo Prefecture, Japan, in October 2005, and maintained for two generations prior to the experiments. Males and females were kept in a plastic cage (90 mm diameter, 50 mm height) in a climate-controlled room at 25 ± 0.5 °C under a photoperiod of 16L:8D, with distilled water in a small Petri dish and rice panicles (ca. 50 mm) in the milk-ripe stage as food. After mating occurred in the cage, we used them for the experiments. For chemical analyses and bioassays, an individual bug 3–15 days old was used only once.
Bioassays
We constructed the bioassay set-up to evaluate the excitement and escape behaviour of undisturbed L. chinensis to cues from excited conspecifics (Fig. 1. Water (60 cc) was provided in a lidded plastic cup (85 mm diameter, 40 mm height). The lid had a 6-mm-diameter opening, into which a piece of wet cotton towel was inserted. We placed 10 rice seeds at the milk-ripe stage on top of part of the lid as food. We covered the lid with an inverted plastic cup of the same size with one escape hole (18 mm diameter) at the side. We called the inside of the inverted plastic cup the observation arena.
Bioassay set-up to evaluate the responses of Leptocorisa chinensis to alarm odours. An undisturbed adult was introduced into an observation arena made from two plastic cups (85 mm diameter, 40 mm height), and then either (A) an L. chinensis or (B) a piece of filter paper impregnated with a synthetic chemical was introduced through the hole (18 mm diameter)
To introduce an undisturbed individual into the arena, we carefully transferred one L. chinensis from a rearing cage to a test tube (18 mm diameter, 120 mm length), and the opening of the test tube was connected to the opening in the inverted plastic cup to allow the bug (initial occupant) to enter the arena. When the bug stopped moving, it was considered undisturbed. For the control experiment, we introduced an undisturbed bug into the arena in the same way. For experimental treatments, we introduced an individual of the same gender using forceps with gentle nipping to disturb it and cause it to emit the pungent odour. The two individuals were distinguishable because the second, newly introduced one was already excited. After the introduction, the behaviour of the initial occupant was observed for 3 min. When the occupant raised its antennae to scan the air and started walking actively, it was judged to be excited. When it left the arena through the hole, we judged that it had escaped. We measured the duration of time until it showed excitement and escape behaviour. The introduced disturbed individual was actively walking in a circle on the ceiling of the arena. During the observation, the two individuals did not interact in the arena.
To further test the ecological functions of the pungent volatiles from L. chinensis, we conducted the same bioassays using the dominant chemical components. A pure compound (1 or 10 µg) was applied to a piece of filter paper (10 × 10 mm2) and introduced into the observation arena containing an undisturbed L. chinensis through the opening (Fig. 1). The amounts of pure compound [(E)-2-octenal] were determined based on chemical analyses: the amounts released by one disturbed female per minute were ca. 4.5–14 μg. We measured the duration of time until the bug showed excitement and escape behaviour. The bioassays were conducted in a climate-controlled room (25 ± 3 °C, 50–60 % relative humidity; during 10:00–16:00). We repeated each experiment 5–10 times per day on 2–4 experimental days.
We then tested the responses of males and females of L. chinensis to (E)-2-octenal at different concentrations to identify the optimal airborne levels needed to excite the bugs. In this study, we needed (E)-2-octenal to volatilise slowly to measure the air concentrations, so we dissolved (E)-2-octenal in methanol (10 % v/v) and then diluted with distilled water to make 0.1, 0.01, and 0.001 % solutions. For each bioassay, we used 1 mL solution impregnated into a piece of moist cotton wool (2 cm × 2 cm × 0.5 cm) on the same size of aluminium foil. We conducted experiments in a mesh cage (30 × 30 × 50 cm3: 2 mm mesh) with an electric fan (flow rate 50 cm/s; SY124010L, 40 mm × 30 mm × 10 mm thickness; Size Corporation, Tokyo, Japan) in the centre of the cage. We carefully introduced 4–5 undisturbed L. chinensis (either males or females) into the mesh cage as described above. When the bugs stopped moving, they were considered undisturbed. We then placed a piece of impregnated cotton wool on the fan. We observed the flight behaviour for 5 min. In our preliminary behavioural observations of both sexes in the mesh cage, we confirmed that flying individuals did not elicit any behavioural responses in undisturbed individuals. For both males and females, we conducted the experiments 4 times (0.1 % solution and 0.01 % solution) and 3 times (0.001 % solution) on 1–3 experimental days in a climate-controlled room (25 ± 3 °C, 50–60 % relative humidity; during 10:00–16:00).
Volatile collection and chemical analysis
Either a male or a female L. chinensis was used for volatile sampling. For collection from a disturbed individual, it was nipped with forceps. Immediately afterwards, it was put in a 200-mL glass bottle with an air inlet and outlet (55 mm diameter, 110 mm height). For the collection of volatiles from an undisturbed individual, it was allowed to walk into the volatile collection vial from the rearing cage. An undisturbed individual was motionless, walking, or flying in the bottle. Volatile collections were done on four individuals per gender and treatment during daytime (25 ± 3 °C, 50–60 % relative humidity; during 10:00–15:00).
The headspace volatiles in the glass bottles were collected for 1 min (disturbed adult) or 10 min (undisturbed) at flow rate of 100 mL/min using Tenax adsorbent in a glass tube (Tenax TA 20/35 100 mg; 3-mm inner diameter (ID), 160 mm long; GL Science, Tokyo, Japan). The volatile collection time was determined based on preliminary chemical analyses. The trapped compounds from disturbed adults were eluted with 2 mL n-hexane (Wako Pure Chemical Industries Ltd.) containing n-eicosane (0.5 μg; Wako Pure Chemical Industries Ltd.) as internal standard for the recovery rate. The eluate was concentrated by nitrogen gas flow to 10 μl. One microlitre of concentrated eluate was injected into the injection port (250 °C) of a gas chromatograph–mass spectrometer (GC–MS; GC: Agilent 6890 with HP-5MS capillary column: 30 m long, 0.25 mm i.d. and 0.25 µm film thickness; MS: Agilent 5973 mass selective detector, 70 eV with He as carrier gas; Agilent, Santa Clara, CA, USA). The GC oven temperature was programmed to rise from 40 °C (5 min hold) to 280 °C at 15 °C/min. The compounds were identified by comparing their mass spectra and retention times with those of authentic compounds and quantified using a calibration curve of the respective compound.
Air in the flight cage with volatilised (E)-2-octenal was collected for 3 min at flow rate of 100 mL/min using Tenax adsorbent in a glass tube [Tenax TA 20/35 100 mg; 3-mm inner diameter (ID), 160 mm long]. The collections were repeated four times for each of the four concentrations. The volatile collection time was determined based on preliminary chemical analyses. The collected volatile compounds were analysed by GC–MS as described above, except for the injection method. The GC–MS was equipped with a thermal desorption cold trap injector (TCT; CP4010, Chrompack, The Netherlands). Headspace volatiles collected on Tenax-TA were released in the TCT thermal desorption unit at 220 °C for 8 min in He flow. The desorbed compounds were collected in the TCT cold trap unit (SIL5CB-coated fused silica capillary) at −130 °C. Flash heating of the cold trap unit provided sharp injection of the compounds into the capillary column of the GC.
Chemicals
Hexyl acetate, octyl acetate, octanal, (E)-2-octenal and octanol were purchased from Wako Pure Chemical Industries Ltd., Osaka, Japan. (E)-2-Octenyl acetate and (Z)-2-octenyl acetate were synthesised by Sumika Technoservice Corporation (Hyogo, Japan).
Statistical analyses
The amounts of each volatile compound emitted from males and females were compared by t test. The time durations needed for excitation and escape were tested by the Kaplan–Meier time-to-event model using a log-rank test statistic. All statistical tests, except multiple-comparison tests, had α significance of 0.05. For multiple comparisons of duration, we adjusted the significance according to Holm’s sequentially rejective Bonferroni test to reduce type I errors. The multiple comparisons involved three log-rank tests, therefore the lowest of the three P values was compared with α = 0.0167 (0.05/3), the second lowest with α = 0.025 (0.05/2), and the third lowest with α = 0.05 (0.05/1). The proportions of flight responses of L. chinensis to various concentrations of (E)-2-octenal were analysed using two-way analysis of variance (ANOVA) with factors concentration and sex, and their interaction. The data of the flight proportion were arcsine square root transformed before two-way ANOVA, and were weighted in the analysis by the number of individuals released into the mesh cage. All statistical analyses were conducted using the JMP software package (version 9.0.2; SAS Institute, Cary, NC, USA).
Results
Response of L. chinensis to volatiles emitted from conspecifics
Following the introduction of a disturbed same-sex conspecific, all undisturbed females (n = 19) and males (n = 16) exhibited excitement within 40 s (Fig. 2a) and escaped the arena within 3 min (Fig. 2b), with no differences between the sexes (P = 0.14 and P = 0.72 log-rank test, respectively). When an excited individual touched the hole (18 mm diameter) with antennae, the individual immediately walked out of the arena through the hole and flew to the fluorescent lights on the ceiling. In contrast, addition of an undisturbed same-sex conspecific elicited no excitement or escape behaviours in the first adult (Fig. 2a, b).
Proportions of excitement and escape of undisturbed Leptocorisa chinensis females (n = 19) and males (n = 16) when exposed to undisturbed or disturbed conspecifics of the same gender. Lines with the same lower-case letter are not significantly different (Holm’s sequentially rejective Bonferroni test after Kaplan–Meier time-to-event model using a log-rank test statistic, P < 0.05). Females (n = 19) and males (n = 16) exposed to an undisturbed individual showed no excitement/escape behaviour
Chemical analyses of volatiles emitted from L. chinensis
Volatiles from undisturbed males and females were below detectable levels (data not shown). When disturbed, both male and female L. chinensis adults (n = 4) emitted hexyl acetate, octyl acetate, (Z)-3-octenyl acetate, (E)-2-octenyl acetate, octanal, (E)-2-octenal and octanol (Table 1). Females emitted significantly higher amounts of hexyl acetate, octyl acetate, (E)-2-octenyl acetate, (E)-2-octenal and octanol than males (t test) (Table 1).
Response of L. chinensis to synthetic compounds of volatiles emitted from a disturbed individual
We studied the responses of L. chinensis females to three volatile compounds, i.e. octyl acetate, (E)-2-octenyl acetate and (E)-2-octenal, which were predominant in volatiles emitted from a disturbed male and/or female (Table 1). The numbers of individuals tested for each compound is shown in Fig. 3. Octyl acetate was the least active in eliciting excitement/escape behaviour at two doses (Holm’s sequentially rejective Bonferroni test after log-rank test) (Fig. 3a, b). At 1-µg dose, no individuals escaped from the arena when offered octyl acetate, while 60–90 % of individuals escaped when offered (E)-2-octenyl acetate or (E)-2-octenal with no significant differences between the compounds (P = 0.29, log-rank test) (Fig. 3c). At the 10-µg dose, the proportion of escape for octyl acetate was significantly lower than for the other two compounds, while there were no significant differences between (E)-2-octenyl acetate and (E)-2-octenal (Holm’s sequentially rejective Bonferroni test after log-rank test) (Fig. 3d).
Proportions of excitement (a, b) and escape (c, d) of undisturbed Leptocorisa chinensis females when exposed to synthetic chemicals at different concentrations of (a, c) 1 μg and (b, d) 10 μg. Lines with the same lower-case letter are not significantly different (Holm’s sequentially rejective Bonferroni test after Kaplan–Meier time-to-event model using a log-rank test statistic, P < 0.05). n = 19 for (E)-2-octenal, 17 for (E)-2-octenyl acetate, 7 for octyl acetate
Concentration of (E)-2-octenal in air needed to induce escape behaviour in L. chinensis
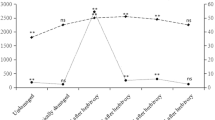
Males started showing flight behaviour when exposed to 0.001 % solution of (E)-2-octenal, while females started showing flight behaviour when exposed to 0.01 % solution (Fig. 4). The solution concentration significantly affected the flight behaviour (F 2,16 = 10.19, P = 0.001). However, effects of sex and the interaction (concentration × sex) were not significant (sex: F 1,16 = 10.13, P = 0.72; interaction: F 2,16 = 2.44, P = 0.30). The headspace analyses of air in the cage showed that 0.01 and 0.1 % solutions of (E)-2-octenal resulted in 1.5 ppbV and 9.3 ppbV, respectively. The concentration of (E)-2-octenal with the 0.001 % solution was below the detectable level.
Discussion
Undisturbed males and females of L. chinensis became excited and escaped from the arena when exposed to previously excited individuals of the same gender. Their behaviours suggested that they responded to volatiles emitted from disturbed conspecifics. Alternatively, visual/physical cues, such as walking and sounds associated with movement, might have affected the excitation and escape behaviour. To clarify the effects of the volatiles, we conducted chemical analyses and tested the effects of the volatile components on the behaviour of L. chinensis.
Most of the volatiles found from the disrupted individuals were C8 aldehydes, alcohol and acetates. The major compounds found in the headspace of disturbed males and females were (E)-2-octenal and octyl acetate. (E)-2-Octenyl acetate was also one of the major compounds in the headspace of disrupted females. Gunawardena and Bandumathie (1993) reported that the chemical compositions of defensive secretions produced by disturbed Leptocorisa oratorius males and females were similar; the two major components in both were (E)-2-octenal (76 % v/v) and octyl acetate (16 % w/w). In this study, in total, females emitted ~3 times more volatiles than males. A similar trend was reported in disturbed and undisturbed Lygus lineolaris (Wardle et al. 2003).
The relative amounts of (E)-2-octenal and (E)-2-octenyl acetate were 87–92 % and 0.8–3.6 %, respectively. Thus, we concluded that (E)-2-octenal was one of the major factors eliciting the excitement and escape behaviour in L. chinensis females in the arena when a disturbed individual of the same gender was introduced. No significant differences in escape proportions were observed between males and females (Fig. 2b), so we hypothesised that (E)-2-octenal was also a major factor eliciting excitement and escape behaviour in L. chinensis males. This conclusion was supported by the bioassays using emulsified (E)-2-octenal in the mesh cage.
The proportions of escape in response to a disturbed female and to synthetic volatile compounds [(E)-2-octenal and (E)-2-octenyl acetate (1-µg dose)] were not significantly different (P = 0.23, log-rank test). The low activity of hexyl acetate suggested that the location of the double bond at the (E)-2 position was more important than the presence of the aldehyde group. Further studies are needed to evaluate this idea. Further, we did not test the effects of minor compounds (less than ca. 1 % in the blends from both genders: hexyl acetate, (Z)-3-octenyl acetate, octanal and octanol) on excitement and escape behaviours. Studies on the ecological functions of such volatiles are needed as well.
Leptocorisa chinensis males are strongly attracted to a 5:1 mixture of (E)-2-octenyl acetate and octanol (Leal et al. 1996; Watanabe et al. 2009; Fukatsu et al. 2012). These compounds were also detected from males and females at ratios of approximately 2:1 and 20:1, respectively, in our study. Leal et al. (1996) also reported that a whole blend did not attract males and the attractiveness of a binary mixture decreased with addition of (Z)-3-octenyl acetate. In this study, we also detected (Z)-3-octenyl acetate as one of the minor compounds. The attractiveness of (E)-2-octenyl acetate and octanol emitted from a disrupted adult, if any, would have been hampered by the different ratios and/or by the presence of other compounds, such as (Z)-3-octenyl acetate.
Leal et al. (1996) identified compounds found in this study as well as nonanal and (E)-2-octenol in the headspace and hexane extracts of both male and female L. chinensis that were anaesthetised with CO2 to minimize the release of defensive secretion. CO2-induced anaesthesia might have resulted in production of small amounts of volatile compounds. Our inability to detect volatiles from undisturbed males and females was probably due to differences in collection methods.
As mentioned in the “Introduction,” in Sri Lanka, farmers protect rice plants by putting smashed adult stink bugs, including Leptocorisa species, around their fields (Yamashita, personal communication). Our data suggest that the volatiles from these smashed bugs would have repelled stink bugs from the agricultural fields. In this study, we clarified that (E)-2-octenal and (E)-2-octenyl acetate are involved in the excitement/escape behaviour of L. chinensis. Continual release of synthetic (E)-2-octenal in paddy fields at concentration of ca. 2 ppbV or higher is expected to protect rice grains from L. chinensis damage during critical stages of the growing season. Our forthcoming paper will report on field experiments.
References
Aldrich JR (1995) Chemical communication in the true bugs and parasitoid exploitation. In: Cardé RT, Bell WJ (eds) Chemical ecology of insects II. Chapman and Hall, New York, pp 318–363
Blum MS (1985) Alarm pheromones. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 9. Pergamon, Oxford, pp 193–224
Fukatsu T, Watanabe T, Hu H, Yoichi H, Hirafuji M (2012) Field monitoring support system for the occurrence of Leptocorisa chinensis Dallas (Hemiptera: Alydidae) using synthetic attractants, field servers, and image analysis. Comput Electron Agric 80:8–16
Grist DH, Lever RJAW (1969) Pests of rice, tropical science series. Longmans Green, London
Gunawardena NE, Bandumathie MK (1993) Defensive secretion of rice bug Leptocorisa oratorius Fabricius (Hymenoptera, Coreidae)—a unique chemical combination and its toxic, repellent and alarm properties. J Chem Ecol 19:851–856
Ito K (2004) The role of the feeding habits of Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) on the production of pecky rice grains with special reference to the occurrence of split-hull paddy. Jpn J Appl Entomol Zool 48:23–32
Leal SW, Ueda Y, Ono M (1996) Attractant pheromone for male rice bug, Leptocorisa chinensis: semiochemicals produced by both male and female. J Chem Ecol 22:1429–1437
Takeuchi H (2007) Population dynamics of Leptocorisa chinensis (Hemiptera: Alydidae) and forecasting of damage occurrence in rice fields. Bull Natl Agric Res Center 9:17–74
Takeuchi H, Watanabe T, Suzuki Y (2004) Ripening stages of rice spikelets selectively damaged by four species of rice bugs, Leptocorisa chinensis Dallas (Hemiptera: Alydidae), Lagynotomus elongatus (Dallas) (Hemiptera: Pentatomidae), Cletus punctiger (Dallas) (Hemiptera: Coreidae) and Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae). Jpn J Appl Entomol Zool 48:281–287
Wardle AR, Borden JH, Poerce HD Jr, Gries (2003) Volatile compounds released by disturbed and calm adults of the tarnished plant bug, Lygus lineoraris. J Chem Ecol 29:931–944
Watanabe T, Takeuchi H, Ishizaki M, Yasuda T, Tachibana S, Sasaki R, Nagano K, Okutani-Akamatsu Y, Matsuki N (2009) Seasonal attraction of the rice bug, Leptocorisa chinensis Dallas (Heteroptera: Alydidae), to synthetic attractant. J Appl Entomol Zool 44:155–164
Acknowledgments
We thank Yoshito Suzuki, Toshikazu Adachi, Hiroaki Fujimoto, Atsuo Akayama, Yoshiki Takashima and two anonymous reviewers for their useful comments. This study was supported by the Research Project for Utilizing Advanced Technologies in Agriculture, Forestry, and Fisheries (Ministry of Agriculture, Forestry, and Fisheries, Japan).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamashita, Ki., Isayama, S., Ozawa, R. et al. A pecky rice-causing stink bug Leptocorisa chinensis escapes from volatiles emitted by excited conspecifics. J Ethol 34, 1–7 (2016). https://doi.org/10.1007/s10164-015-0437-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-015-0437-5