Abstract
It is increasingly common to quantify and describe behavioral variation in domestic and wild animals in terms of “personality”. Correlating behavioral traits are referred to as personality “dimensions” or “factors” and different dimensions have been reported in different species. “Boldness” is a well-described personality dimension in several species, although some issues remain unclear. Previous models of boldness include both novelty and risk taking, but recent studies indicate that these types of behaviors may reflect separate personality dimensions. In this study, we developed a behavioral test battery for domestic rabbits, and recorded behaviors of 61 individuals in four different situations (novel object, novel arena, social, and predator interactions). We used domestic rabbits as a model because behavioral variation in rabbits has rarely been quantified in terms of personality dimensions, although rabbit behavior is described. We also wanted to investigate behavioral variation in a Swedish rabbit breed of conservation concern — the Gotland rabbit. Factor analysis of the behavioral test measures suggested three personality dimensions: “exploration”, “boldness”, and “anxiety”. Novel object scores clustered in the exploration and boldness factors, whereas scores associated with predator interactions were explained by “anxiety”, indicating that novel object and anti-predator behavior reflect different personality dimensions in rabbits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral characteristics of an individual that are relatively consistent over time can be described as the individual’s personality, and different dimensions, or factors, of that personality consist of correlating behavioral traits (Koolhaas et al. 1999; Carere and Eens 2005; Groothuis and Carere 2005). Assessment of personality is increasingly used for describing and quantifying behavioral variation in both wild and domestic animal species (Müller and Schrader 2005; Lee et al. 2007; Bell 2011; van Overveld and Matthysen 2013). Personality can be studied in terms of between-individual differences (population level) and within-individual variation (individual level; Uher 2011). In the definition of personality, consistency across situations on the population level has often been assumed (i.e. individuals having a relatively low latency to approach a new object in the home cage also have a relatively low latency to do so in a novel arena; e.g. Sih et al. 2004; Carere and Eens 2005; Réale et al. 2007). In order for cross-situational consistency to be high at the population level, the within-individual variation must be quite low (Stamps and Groothuis 2010; Uher 2011). In reality, consistency across situations at the population level may vary from low to high with, for example, species or the complexity of traits examined (Stevenson-Hinde et al. 1980; Sih et al. 2003; Kralj-Fišer et al. 2007; Uher et al. 2008, 2013a). Therefore, it may not be appropriate to include cross-situational consistency in the general definition of personality (Uher 2011).
Two separate methods are used for identifying animal personality dimensions: (i) behavioral coding, and (ii) subjective rating of traits (Gosling 2001). In behavioral coding individual animals are subject to a fixed set of test situations during which the animal’s behavioral response is recorded (Mater and Anderson 1993; Kurvers et al. 2009). Alternatively, behavioral coding can be used outside of test settings, in behavioral observations (Altmann 1974; Rödel and von Holst 2009; Uher et al. 2013a). The subjective rating method implies that one or two observers who typically know the animal well (e.g. owner, keeper, etc.) rate the animal with respect to a series of personality traits (Stevenson-Hinde and Zunz 1978; Gosling 1998, 2001; Fox and Millam 2010). The two methods are sometimes used interchangeably; however, recent findings suggest that they do not measure the same phenomena (Uher et al. 2013b).
Exactly which behavioral traits constitute personality dimensions in different species is unclear. “Boldness” is a personality dimension that is most commonly described and studied (Carter et al. 2012a), but the definition of boldness is somewhat ambiguous (Carter et al. 2013), and it has been measured using several different tests, including novel object tests (Frost et al. 2007; Kurvers et al. 2012), emergence tests (Lopez et al. 2005), and trapability tests (Réale et al. 2000; Réale and Festa-Bianchet 2003). Thus, models of this personality dimension include both risk taking (Brown and Braithwaite 2004; Lopez et al. 2005) and behavior in novel situations (Bremner-Harrison et al. 2004; Kurvers et al. 2009); correlations between boldness and other personality dimensions have been observed (Barnett et al. 2012; Watanabe et al. 2012). Recently, researchers have found that behavior in novel situations and risk taking may reflect two separate personality dimensions as reported in fish (Wilson and Stevens 2005), birds (Fox et al. 2009), and primates (Carter et al. 2012a).
In this pilot study, we developed a test battery aimed at applying the behavioral coding method to assess potential personality dimensions in the domestic rabbit. The method of using several tests for a more comprehensive exploration of personality dimensions has been frequently employed in personality studies on primates (e.g. Spencer-Booth and Hinde 1969; Uher et al. 2008). The domestic rabbit was chosen as a model for several reasons. First, rabbit behavior has rarely been studied in terms of personality, although several studies have been conducted on aspects of wild and domestic rabbit behavior, such as aggression and sexual behavior (Mykytowycz and Hesterman 1975; Southern 1947), social behavior (Lehmann 1991; Rödel et al. 2006), and anti-predatory responses (Monclús and Rödel 2008; Vitale 1989). Behavioral phenotypes have been documented in wild European rabbits (Rödel and Monclús 2011); however, studies that use batteries of tests for behavioral coding to assess personality dimensions in rabbits are scarce (but see Reyes-Meza et al. 2011; Rödel and Monclús 2011). Second, domestic rabbits are used for a wide set of purposes where knowledge of personality types is of practical interest including as pets, in research, for meat and fur production, for hobby activities, and as therapy animals in medical care (Arrington and Kelley 1976; Reed 1994; Fine 2010).
Further, for domestic breeds of conservation concern there is an interest in mapping various types of biological diversity including behavior. Many rabbit breeds currently are bred under strong selection for production of meat and fur or for morphology, but there are a few old traditional breeds left. Old breeds are frequently referred to as “landraces” implying local varieties of domestic animals that have developed largely by adaptation to the natural and cultural environment in which they live (Sponenberg and Bixby 2007). Such breeds have been identified as potential genetic resources important to conserve (Baumung et al. 2004; EEA 2009; Laikre 2010; Bruford et al. 2003; Cardellino and Boyazoglu 2009) as outlined in several international agreements (e.g. United Nations Convention on Biological Diversity 1992; FAO 2007a). In Sweden, the Gotland rabbit has been identified as a breed of national conservation importance by the Swedish Board of Agriculture who calls for describing this and other landrace breeds with respect to biological variability traits including behavior (Swedish Board of Agriculture 2009).
We were particularly interested in investigating whether behaviors associated with novel situations and risk taking are linked and represent the same personality dimension, and we designed a battery of five behavioral tests including novel objects, social, and predator threats. We also developed a questionnaire for the subjective rating method and applied it to a limited set of rabbits of which the majority were also assessed with the behavioral test using the behavioral coding method. This provided an opportunity to compare the two techniques for assessing personality dimensions in domestic rabbits.
Materials and methods
Experiments and animals
Sixty-one rabbits, 32 males and 29 females, of seven different breeds (French lop, n = 4; Gotland rabbit, n = 40; Mellerud rabbit, n = 2; mini lop, n = 2; lionhead lop, n = 9; Havana, n = 2; and mixed breed, n = 2) were used in the tests for behavioral coding (Table S1, electronic supplementary materials). The Gotland rabbit and the Mellerud rabbit are traditional landrace breeds whereas the others are bred under selection for primarily physical appearance. Twenty-nine of the 61 rabbits (16 males and 13 females, representing lionhead, French, and mini lops, Gotland, Havana, and mix breeds) were also rated in the subjective rating method, using a questionnaire that we constructed (Appendix, electronic supplementary materials). Additionally, six rabbits (Gotland breed, two males and four females), not used in the behavioral tests, were rated in the questionnaire, resulting in a total of 35 rated rabbits (Table S1, electronic supplementary materials). Of these 35 rabbits, six (Gotland breed, two males and four females) were rated by two observers and the remaining 29 by one. The six rabbits rated by two observers were also used in the behavioral test.
The age of the animals ranged from 3.5 months to 7 years. We classified rabbits under 1 year old as juveniles (n = 14) and 1 year or older as adults (n = 32). Exact age was missing for some animals (n = 15), which were estimated to be between 1 and 7 years old (i.e. adults; Table S1, electronic supplementary materials), resulting in a total of 47 adults. Of the 61 rabbits used for behavioral coding, 40 were not neutered (landrace breeds n = 38; selected breeds n = 2), seven were neutered (selected breeds; three females and four males), and data was missing for 14 animals (landrace breeds; three males and one female, and selected breeds; five of each sex). The six Gotland rabbits used for subjective rating only were classified as adults and neutering data was missing for all six animals. The rabbits were housed indoors in cages or pens with hay or sawdust bedding at 4H club farms, open-air museums, or private breeders in the Swedish Counties of Stockholm, Västermanland, Skåne, Gotland, Östergötland, and Hälsingland.
The tests for behavioral coding
The test battery for behavioral coding was designed to assess personality traits associated with explorative, social, and anti-predatory behaviors, and consisted of seven tests; two different novel object tests in the home cage, a novel arena test, two different novel object tests in the novel arena, a predator test, and a social test. All tests, except novel object in home cage, were carried out in a new environment (novel arena) using an experimental enclosure (180 cm × 180 cm × 70 cm) made of metallic fences (70 × 90 cm) with green plastic covering. The home cages varied in size and were most often rectangular or square.
Novel object in home cage was repeated twice (indicated I and II) as was novel object in novel arena (III and IV), each with a different object (for description of objects see Table S2, electronic supplementary materials). The behavioral measures from the novel object tests and the novel arena test were recorded using paper check sheets and a pen. The social and predator tests were recorded on film with a Fujifilm Finepix S2000HD digital camera and analyzed as described below. The tests in the home cage were carried out before the tests in the new environment.
Novel object in home cage
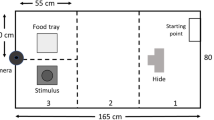
The novel object tests were used to examine the rabbit’s willingness to approach novel objects. The home cage was divided into 4 zones, and at the start of a test the rabbit was placed in the middle corner in zone 1, while the novel object was placed in the opposite corner, in zone 4 (Fig. 1a). We used instantaneous sampling (Altmann 1974) and recorded the rabbit’s zone position with 10 s intervals for 5 min. We also recorded the number of object contacts, time to the first contact, and handling intensity e.g. tasting, biting or eating (see Table S3, electronic supplementary materials for description of objects and scoring). The test was terminated for safety reasons if the rabbit started to eat the object. The novel object in home cage test was repeated twice.
Novel arena
We used novel arena to test the rabbit’s willingness to explore a new environment. The experimental enclosure was divided in 4 zones (Fig. 1b) and the rabbit’s position was recorded using instantaneous sampling (Altmann 1974) with 10 s intervals for 5 min.
Novel object in novel arena
In this test a new object (Table S2, electronic supplementary materials) was added to the novel arena. The test was performed in the experimental enclosure where the middle corner in zone 1 was covered with brown paper (140 × 70 cm) to create a sheltered place for the rabbit (Fig. 1a). The test was repeated twice using the same procedure as in the novel object in home cage test.
Social test
The rabbit’s reaction towards an unfamiliar conspecific was recorded by using a male Gotland rabbit, 10–14 months old and leash trained. The unfamiliar rabbit was leashed and allowed to move outside the experimental enclosure at a distance that prevented physical contact with the focal rabbit (i.e., about 0.5–1.5 m from the enclosure). At the start of the test the focal rabbit could be anywhere in the enclosure, provided that it looked in the direction of where the unfamiliar rabbit was to be placed. The duration of the test ranged from 0.5 to 3.4 min, median duration time was 2.1 min. We used focal animal sampling (Altmann 1974) and recorded frequencies of different behaviors associated with social interactions as well as percentage of time spent performing such behaviors, which are listed in Table S4, electronic supplementary materials. The behaviors recorded in this test have, with some exceptions, previously been observed and documented in social test settings (references are listed in the electronic supplementary materials).
Predator test
The rabbit’s response to an aerial predator attack was tested by a simulation with visual and auditory stimuli. A black painted cardboard bird of prey silhouette with a wingspan of 64 cm was used as the visual stimulus. As the auditory stimulus, we used a peregrine falcon (Falco perigrinus) call which was played back from a Samsung S5230 cellphone. The bird model was attached to a stick and moved over the rabbit in a diving manner 4 times, with the first and the third accompanied by the auditory stimulus. The rabbit could be anywhere in the experimental enclosure at the start of the test, provided that it looked in the direction of the model operator. The duration of the test ranged from 0.5 to 1.1 min, median duration time was 0.7 min. We recorded frequencies of, and percentage of time spent performing different behaviors (listed in Table S5, electronic supplementary materials) associated with predator attacks using focal animal sampling (Altmann 1974). The majority of the behaviors recorded in the predator test have previously been documented in situations associated with threat, in studies of both predator response and social behaviors (references in the electronic supplementary materials). We also defined some behaviors that have not been documented previously, yet were observed frequently in the predator test (i.e. “flight ready” and “flinching”, Table S5, electronic supplementary materials).
Questionnaire for subjective rating
To apply the subjective rating method for personality assessment we constructed a questionnaire for rabbits based on a questionnaire for horses (Fairholm 2007) that was modified after consulting an experienced rabbit breeder for typical rabbit behavior in different situations. Our questionnaire included 68 verb-based descriptions of behaviors or situational events (Appendix, electronic supplementary materials) and was sent out to owners and breeders of Gotland rabbits with issue 1/2010 of the Gotland rabbit club magazine in April 2010. Questionnaires for owners and breeders of the other rabbit breeds were distributed in connection with the tests for behavioral coding. The questionnaire was answered by an observer familiar with the rabbit, who was instructed to base their judgment on their own subjective experience of typical rabbit behavior. When two observers independently evaluated the same rabbit, they were instructed not to discuss their respective judgments.
Data management
For the novel object tests, mean zone position, mean number of object contacts, and mean contact duration per positional observation were calculated to compensate for missed positional observations. A time distribution score for novel arena was calculated from the position recordings as follows. First, the proportion of time spent in each zone was calculated (i.e. the number of observations in each zone divided by the total number of observations). If the rabbit spent equal amounts of time in all four zones, the time proportion spent in each zone was 0.25. If the rabbit did not spend equal amounts of time in all zones, a deviation from the value of 0.25 would be observed. The absolute deviations from 0.25 in each zone were summed up and scored on a 10 point scale, from 1 point for a total deviation of 1.36–1.50 (with 1.50 = the rabbit being in one zone only) to 10 points for a total deviation of 0.00–0.15 (0.00 = the rabbit spending equal amounts of time in all four zones). All behavioral measures and questionnaire items were z-standardized.
Statistical analysis
A factor analysis with orthogonal (varimax) rotation was performed using the measures obtained from behavioral coding to examine the personality dimension structure captured by this method. To determine the number of factors we used parallel analysis of Monte Carlo simulations (Watkins 2006) and scree tests (Cattell 1966). In parallel analysis, factor eigenvalues are generated from 1,000 random data sets with the same number of subjects and items as the actual data set. If the actual eigenvalue for a factor is greater than the simulated one, that factor is retained. The scree test is based on the plot of the eigenvalues of the factors. For each additional factor extracted in the factor analysis, the eigenvalue decreases. Factors with eigenvalues above a point where the decline levels off are retained. We defined absolute item loadings greater than or equal to 0.40 as salient (Bergvall et al. 2011). If an item had salient loadings on more than one factor, we assigned it to the factor on which it had the highest loading.
Factor analysis for the questionnaire items was performed in the same manner as for the behavioral coding measures, but here we used only the scree test to determine the number of factors, since the number of subjects was too low to run a parallel analysis of Monte Carlo simulations. Prior to factor analysis of the questionnaire items, we estimated the reliability of the observer ratings with two different intra-class correlation coefficients (ICCs; Shrout and Fleiss 1979) using rating data where two observers rated the same rabbit. The first intra-class correlation coefficient, ICC (3,1), indicates the reliability of the two separate ratings. It is calculated by dividing the difference between the between-target (rabbit) mean square (BMS) and the error mean square (EMS) by the sum of the between-target mean square and the product of the number of raters (k) minus 1 and the error mean square (BMS + (k − 1)EMS). The second intra-class correlation coefficient, ICC (3,k), indicates the reliability of the mean ratings of the two observers and is calculated by dividing the difference BMS minus EMS by the BMS. Both inter-rater reliabilities (ICCs) assume that the rater effects are fixed and that target effects are random. Personality traits with ICCs over 0.3 were used in the analysis (Shrout and Fleiss 1979).
Using the behavioral coding data we examined the consistency across situations within identified factors that included behavioral measures from more than one type of test situation, i.e. boldness (novel object and predator situations) and anxiety (novel arena, social, and predator situations). Test specific scores were calculated using unit weighting (as described in the next paragraph) for each test within respective factors, and cross-situation consistency was estimated using Pearson’s correlation coefficient r. In addition, we conducted a k-means cluster analysis (Jain 2010) to explore the structure of the boldness and the anxiety factors, which was done by clustering of individuals to identify groups with similar cross-situational behavior profiles. Clustering of individuals was based on their test-specific scores, which were calculated in the same way as the factor scores.
We also investigated cross-method coherence (Uher and Asendorpf 2008; Uher et al. 2013a). Factor scores were first calculated by transforming item measurements to z-scores and using unit weighting. Z-scores of items with positive and negative salient loadings were weighted +1 and −1 respectively. Z-scores of items that did not have a salient loading were weighted 0. Pearson’s correlation analysis was performed to investigate if the tests for behavioral coding and the questionnaire measured the same dimensions of personality. For this, we only used rabbits that had complete factor scores for all factors derived from the behavior tests and the questionnaire (n = 22).
Variation between groups
We wanted to address the issue of variation between groups of individuals with respect to personality and used the results from the behavioral coding for this purpose. Rabbits that did not have complete factor scores for all three factors were excluded from the analysis. Our data set is relatively small but we compared the following groupings using t-tests: (i) male vs female Gotland rabbits (n = 18 vs n = 16), (ii) adult male vs adult female Gotland rabbits (n = 15 vs. n = 9), (iii) juvenile vs adult Gotland rabbits (n = 10 vs n = 24), (iv) landrace breeds (Gotland and Mellerud rabbits, n = 36) vs selected breeds (the lop breeds and Havana, n = 14), and (v) adult landrace vs adult selected breeds (n = 24 vs n = 14). Effect sizes were calculated as Cohen’s d (Cohen 1988) with pooled weighted standard deviation (Hedges 1981) since the group sizes were unequal. In addition, we performed a post hoc power analysis using G*Power 3 (Faul et al. 2007) due to the small sample sizes. Effect sizes and power estimates are presented in Table 6.
Ethical note
The experiments described comply with the current laws of Sweden. Experimental procedures have been reviewed and approved by the regional ethical committee (Stockholms norra djurförsöksetiska nämnd, Dnr N 317/10).
Results
Behavioral coding
Behavioral coding data aggregated into three distinct personality dimensions (Table 1) which appeared to be associated with explorative and anti-predatory behaviors. We analyzed the 53 measures obtained with the test for behavioral coding using factor analysis, which resulted in 35 measures with absolute loadings greater than or equal to 0.40. The parallel analysis suggested ten factors, while the scree test suggested a five-factor solution. We considered three solutions with five, four, and three factors respectively. The five- and four-factor solutions had significant inter-factor correlations, indicating that there were too many factors. Therefore, three factors explaining 33.8 % of the total variation in the behavioral data were retained (Table 1) since no significant correlations were observed within this solution.
Factor 1 included behavioral measures with salient loadings from novel object in novel arena, while factor 2 comprised measures from novel object in home cage and predator test. Factors 1 and 2 explained 13.6 and 11.4 % of the total variation, respectively. Factor 3 included measures from the predator test, the social test, and novel arena, explaining 8.8 % of the total variation. We labeled factor 1 “exploration”, factor 2 “boldness”, and factor 3 “anxiety”. Repeatability, r, was calculated for novel object in home cage (repetitions I and II) and in novel arena (repetitions III and IV), respectively and for each of the individual behavior measures in those tests (Table S6, electronic supplementary materials) as r = \(s_{A}^{2}\)/(s 2 + \(s_{A}^{2}\)), where \(s_{A}^{2}\) is the among-individual variance component and s 2 is the within-individual variance component. These are calculated from the mean squares (MS) in the analysis of variance as \(s_{A}^{2}\) = (MS A − MS W )/n 0 and s 2 = MS W , where MS A is the among-individual mean square, MS W is the within-individual mean square, and n 0 is a coefficient associated with group sample sizes (Lessells and Boag 1987). As these data are not balanced, we calculated n 0 from the number of individuals in each group (Lessells and Boag 1987). Repeatability for novel object in home cage was r = 0.38, while novel object in novel arena showed a repeatability of r = 0.50. For individual behavioral measures from the novel object in home cage, repeatabilities had the range of r = 0.18–0.61, while repeatabilities for measures from novel object in novel arena ranged between r = 0.23–0.67. In addition, we calculated test–retest reliabilities for the individual behavioral measures using Pearson’s correlation coefficient (Table S6, electronic supplementary materials).
Subjective rating
For the questionnaire, the intra-class correlation coefficient calculations resulted in 24 personality traits with ICCs greater than 0.30 (Table 2). The scree test suggested a four-factor solution, which was retained, explaining 74.3 % of the total variation (Table 3) with no correlations between the factors within the solution. Factors derived from the questionnaire appeared to reflect mostly social behaviors. Factor 1 included items such as “inventive”, “decisive”, and “active”, explaining 31.9 % of the total variation. Items included in factor 2 were, among others, “affectionate” and “helpful towards rabbits” while items such as “gentle during handling” and “human-directed sociability” constituted factor 3, explaining 20.9 and 12.6 %, respectively. Factor 4 explained 8.9 % of the variation and included the items “unemotional”, “independent”, and “sensitive”. Factor 1 was labeled “confidence”, factor 2 “sociability”, factor 3 “human-directed agreeableness”, and factor 4 “control”.
Consistency across situations and cross-method coherence
The cross-situational consistency of test-specific scores associated with the same factor was low to moderate (r = 0.32, p < 0.05 for boldness and r = 0.35, p = 0.00–0.06 for anxiety; Table 4). For anxiety, cross-situational consistency was calculated as a mean intercorrelation (range r = 0.27–0.40), since that factor included three different test situations. We did not find any significant correlations between the factors derived from the tests for behavioral coding and the questionnaire (Table 5). This indicates that the two methods as applied in this study do not assess rabbit behavior in a manner that can be used to describe behavioral variation as equivalent dimensions of the rabbit personality.
We identified five cross-situational behavior profiles with the cluster analysis for the boldness and anxiety factors (Fig. S1, electronic supplementary materials). Substantial variation in cluster means was observed in the novel object test situation within the boldness factor (range mean = −15.74 to 10.71) and in the predator and social test situations within anxiety (range mean = −1.71 to 12.89 and −1.47 to 14.89, respectively). On the other hand, very little variation in cluster means was observed in the predator test situation and the novel arena test situation in the boldness and anxiety factors, respectively.
Effects of sex, age, and breed
We found that female Gotland rabbits are significantly less explorative and anxious than males (p < 0.01, Table 6), and that juveniles are significantly more anxious than adults (p < 0.01). When only adult Gotland rabbit males and females were compared, the significant difference in anxiety remained (p < 0.05). Also, landrace breeds were significantly more anxious than selected breeds (p < 0.01) when age groups were pooled, although this difference may be due to unequal numbers of juveniles in the breed groups (cf. Table 6). We did not observe any significant differences in anxiety between the breed groups when adults only were compared. Mean-level breed difference for exploration was 3.61, for boldness 1.22 and for anxiety 3.43. No differences were found in boldness in either of the group comparisons. Absolute effect sizes were large (range d = 1.08–1.44) for all significant differences due to sex and age, but only moderate for the difference due to breed (d = 0.57). The t-tests were, however, underpowered due to the small sample sizes, thus we cannot exclude the possibility that there are other differences than those we detected.
Discussion
Our results from the behavioral coding tests indicate that behavioral variation in domestic rabbits captured by these tests can be quantified and described as three distinct personality dimensions. We labeled these three dimensions exploration, boldness, and anxiety. The novel object test in the home cage and in the novel arena, respectively, had a relatively high repeatability, indicating that the behaviors in these situations are stable over time. Reactions towards novel objects differed between the familiar and the novel environment, suggesting that it was context specific. The measures from these tests ended up in the boldness and exploration dimensions, respectively. Moreover, scores associated with predator interactions were explained by the anxiety dimension. This indicates that novel object and anti-predator behavior reflect different personality dimensions in rabbits.
Exploration included all measures from novel object in novel arena (III and IV), but not the measure from novel arena, which was included in the anxiety dimension. A previous behavioral study of rabbit personality, exploring the development of personality traits in young rabbits, measured exploration by one behavior, namely being outside the burrow in a novel arena (Rödel and Monclús 2011). This setup cannot be compared to our novel arena test, since we instead measured the area used by the rabbit, and quantification of this clustered in the anxiety dimension. The bottom substrate in our novel arena test was always free of high vegetation to facilitate the observations, and thus, an open enclosure without overhead cover may have elicited anxiety-driven locomotion aimed to find such cover.
Boldness is one of the most commonly studied personality traits (Carter et al. 2013), and it is often measured by novel object tests (Toms et al. 2010). In our study, boldness included all measures from novel object in home cage (I and II). Since these tests were carried out in the home cage, the novelty of environment was eliminated (Réale et al. 2007). The separation of novel object in home cage and novel object in novel arena indicates that these two test situations measure behaviors clustering in different personality dimensions in rabbits. Thus, it may be erroneous to assume that behavioral patterns that appear similar, in fact reflect the same dimension (Stamps and Groothuis 2010).
Anxiety was labeled as such since it included most flight related behaviors from the predator test, all of which had high, positive item loadings. Fear can be expressed not only through flight and defense behaviors, but also through social communication, i.e. alarm calls. Stamping is such a behavior in rabbits (Mykytowycz and Hesterman 1975) and it was included in the anxiety factor as well. It might be argued that domestic animals have lost their anti-predatory behaviors since they are no longer exposed to their natural predators (Price 1984); however, these behaviors can be elicited by environmental changes and humans (Boissy 1995). Several studies have shown that anti-predatory behaviors have an underlying genetic basis (Magurran 1990; Petersson and Järvi 2006; Håkansson et al. 2007).
Consistency across situations was low to moderate within the boldness and anxiety dimensions. This is in accordance with several personality studies on primates (Stevenson-Hinde et al. 1980; Uher et al. 2008, 2013a), reporting low to moderate cross-situational consistency at the population level but higher consistency of behavioral patterns across situations within individuals over time (e.g. Uher et al. 2008). However, higher cross-situational consistencies at the population level have been reported for other species. For example, Kralj-Fišer et al. (2007) report high correlations (r = 0.60–0.93) for “aggressiveness” across different social situations in graylag geese. Similarly, correlations for predator exposure with presence and absence of predator cues in streamside salamander larvae were moderate to high (r = 0.43–0.80; Sih et al. 2003). According to Uher (2011), individual behavioral differences of interest in personality research must be stable at least for some time, and vary between individuals. Thus, if cross-situational consistency is high in the population, the variation between individuals is limited and may reflect behaviors associated with fitness. We could not investigate the temporal stability of behavioral patterns across situations of individual rabbits since we lacked repetitions in the predator and social tests. However, the consistency across situations at the population level was low to moderate, which is often the case despite high temporal consistency of individual behavioral patterns across situations (Uher et al. 2008, 2013a), indicating higher levels of variation among the individuals. This, in turn, may suggest that the major part of the behavioral variation observed in this study is likely to be individual differences in personality.
The cluster analysis identified five clusters, each of which represents a mean cross-situational behavior profile (Tables S7 and S8, electronic supplementary materials). The greatest variation in cluster means was observed in the novel object test situation within the boldness factor, as well as in the predator and social test situations within the anxiety factor (Fig. S1, electronic supplementary materials). In contrast, variation in cluster means was limited in the predator and the novel arena test situations within the boldness factor and the anxiety factor, respectively (Fig S1, electronic supplementary materials). The patterns observed in these situations may be explained by the presence of a genotype x environment interaction, that is, lack of phenotypic and genetic variance in this particular situation due to past selection pressure (Réale et al. 2007). Thus, behaviors in these particular situations clustering in the boldness and anxiety factors may be associated with fitness. The patterns displayed by the different cross-situational behavior profiles indicate that individuals differ substantially in their behavioral response to different situations associated with the same personality dimension. Additionally, groups of individuals seem to react to these situations in a similar manner, suggesting the presence of personality type structures in the material.
Comparing the behavioral coding and subjective rating methods
Our study, along with Carter et al. (2012b) and Uher et al. (2013b), is one of relatively few which directly compare the behavioral coding and the subjective rating methods for assessing personality dimensions using the same animal material (29 of the 35 rabbits assessed with the subjective ratings method were also assessed in the behavioral coding tests). However, we detected no correlation between personality dimensions identified using the two separate methods. The factor analysis of the questionnaire items resulted in four dimensions labeled confidence, sociability, human-directed agreeableness and control. The first dimension included positive loadings on “inventive” and “decisive”. Therefore, we labeled this factor confidence, as the item composition reflects activity and enterprise. Stevenson-Hinde and Zunz (1978) report a similar bipolar personality dimension (confidence-fearfulness) in rhesus monkeys. The sociability dimension included positively loaded “affectionate” and “helpful to rabbits”, reflecting behaviors associated with social interactions with individuals of the same species, and has been reported in spotted hyenas (Gosling 1998). Human-directed agreeableness included several items associated with human-directed social behaviors. The last dimension consisted of positively loaded “unemotional” and “independent” and negatively loaded “sensitive”, therefore labeled as control.
Previous studies describing rabbit personality dimensions and not only single measurements, have used the subjective rating method only (Gosling and Bonnenburgh 1998; Mullan and Main 2007). The lack of significant correlations between the personality dimensions identified by behavioral coding and subjective rating in our present study indicate that these methods capture different dimensions of the rabbit personality. While the questionnaire seems to reflect dimensions in the social spectrum, the behavioral coding method captures dimensions in the non-social spectrum. In contrast to our results, Carter et al. (2012b) report a significant correlation between behavioral tests and observer ratings of boldness in baboons. The observers followed the baboons for several months prior to their ratings, and were thus familiar with the whole behavior repertoire of the animals. The rabbits in our study were housed as farm stock; indoors, in cages or pens, with daily human contact but less than if they were housed as companion pets in a human residence. Thus, observers that rated the rabbits based their judgments on a limited variety of situations that may not reflect explorative and fear-related behaviors. On the other hand, the behavioral test battery may not capture social behaviors because one single observation occasion may not be sufficient to obtain perception of how the rabbit functions in a social situation. However, the lack of correlation between the behavioral test and the questionnaire dimensions is supported by the findings of Uher et al. (2013b), who report a lack of correlation between trait ratings and behavioral measures of “social orientation to group members” in crab-eating macaques. The authors suggest that these results could be due to the anthropocentric biases (in terms of age and sex differences) of the raters, who perceived young individuals as more curious and spontaneous than older ones, and males as more sexually active than females. No such differences were found in the behavioral measures; in fact, young macaques appeared to be more anxious than old ones. Likely, these findings reflect age- and sex-based stereotypes of human individuals, that are socio-culturally shared (Uher et al. 2013b). Thus, the observers in our study may have rated the rabbits based on a preconceived anthropocentrically biased personality concept of those individuals, explaining the lack of cross-method correlation.
Variance of behavioral and rating data explained by the personality factors
The percentage of the total variance explained by the factors constructed for the behavioral data was quite low (33.8 %). This may be caused by variation in the test situations, since the behavioral tests were performed outside of a standardized laboratory environment. Fairly low percentages of explained variance are not uncommon in animal personality research; several studies report variances explained by constructed factors below 50 % (e.g. Mater and Anderson 1993; Svartberg and Forkman 2002; Bergvall et al. 2011). Of the total variance, 74.3 % was explained by the factors constructed for the rating data. Gosling (1998) assessed personality in spotted hyenas using subjective rating of traits and reported that 75.0 % of the total variance was explained by the factors constructed, a percentage similar to ours. Other studies using the subjective rating method report lower percentages (around 50 %; Gold and Maple 1994; Svartberg 2005). The percentage of variance accounted for in factor or principal component analysis appears to be affected by sample size and number of variables analyzed (Peterson 2000). The study showed that the percentage of variance explained by the factors decreases as the sample size and number of variables increases. In the present study, the sample size for behavioral coding was n = 61 and 53 variables were analyzed, while the sample size for subjective rating was n = 35 with 24 variables analyzed. The relationship between explained variance, sample size, and number of variables that was shown by Peterson (2000), might explain the difference in variance explained by the factors in the behavioral and rating data, respectively.
Comparing groups of rabbits
When comparing groups of rabbits we found some differences in the anxiety and exploration factors, but not in the boldness factor. Male and juvenile Gotland rabbits were more anxious than females and adults, respectively, and landrace breeds were more anxious than selected breeds. The anxiety dimension reflects anti-predatory behaviors, and several studies report that young rabbits react more strongly to predators (Vitale 1989; Pongrácz and Altbäcker 2000). The response to predatory threat becomes more specific with time and experience and is referred to as “quantitative response” (Inglis 1979), which could explain the differences between adult and juvenile Gotland rabbits. The difference in anxiety remained when adult male and female Gotland rabbits only were compared, with males being more anxious than females. This may be explained by the higher testosterone levels in the males, because it is possible that the sexes might have reacted differently towards the unfamiliar conspecific, which was an unneutered male. However, when comparing the test-specific score for the social and predator test situations between unneutered adult male and female Gotland rabbits, we found that males were significantly more anxious than females in both situations. Thus, we cannot state that the difference in anxiety between males and females is caused by the sex of the unfamiliar rabbit in the social test alone, or that it did not affect the results at all. The difference between landrace and selected breeds in anxiety may have been influenced by the quantitative response or by testosterone level differences, since several of the males in the selected breeds group were neutered. However, given that differences in the anxiety dimension were significant between both age and sex groups, it is most likely that differences between breeds are caused by the interaction of those two. We found that male Gotland rabbits had a negative mean factor loading on both boldness and anxiety, while females showed the opposite. This is in accordance with the theory that boldness and anxiety reflect different personality dimensions. Also, we observed a difference between male and female Gotland rabbits in the exploration factor. However, when adults only were compared, this difference was no longer significant and may likely be due to age.
Conservation of biological diversity
It is important to conserve biological variation, including behavioral variation; however, personality or other measures of behavioral diversity are often not considered in conservation and management programs (e.g. McDougall et al. 2006). For instance, many traditional landrace breeds of domestic animals are identified as of conservation concern because they have not been a target of selective breeding and possess many valuable traits as a result of long-term adaptation to their specific environments (Sponenberg and Bixby 2007; FAO 2007b). Efforts have been made to evaluate the genetics of such breeds (e.g. Tapio et al. 2005), but studies on personality are largely lacking. Since many personality traits have a genetic basis (Poissant et al. 2013; van Oers et al. 2004), it could be assumed that breeds or populations that exhibit greater genetic variation also would have greater variation in such personality traits. Different personality types are associated with several life history characteristics such as survival (Bremner-Harrison et al. 2004) and fitness (see Smith and Blumstein 2008), resilience in habitats under fragmentation, and dispersal (Gherardi et al. 2012), and should thus also be considered in conservation and management programs. Although our study is a pilot, we find indications that the Gotland rabbit breed, which is identified as of conservation concern in Sweden (Swedish Board of Agriculture 2009), exhibits behavioral variation that can be screened with the behavioral coding method designed here. Further, our results suggest potential behavioral differences between Gotland rabbits and rabbits representing breeds under selection for primarily physical appearance.
Final note
To summarize, the behavioral variation we observe in domestic rabbits using behavioral coding can be described as three personality dimensions, which we labeled exploration, boldness, and anxiety. Similar dimensions have been suggested for several other species. Methods for mapping and quantifying biological variation, including behavior, in the old Swedish Gotland rabbit breed have been identified as of value for conservation purposes in Sweden. We suggest that the behavioral tests used here to assess personality traits in rabbits, are helpful in this respect. A shortcoming of our study is the lack of repeatability estimations for all but the novel objects tests (which were repeated over a relatively short time span of 15–20 min) to address the consistency in behavior reactions of separate rabbits. Further, our sample sizes were relatively limited. Therefore, we recommend an extended study covering larger number of individuals and repeating all tests at separate points in time. Such an extended study should be complemented with additional tests to explore social behaviors.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–265
Arrington LR, Kelley KC (1976) Domestic rabbit biology and reproduction. The University Press of Florida, Gainesville
Barnett CA, Thompson CF, Sakaluk SK (2012) Aggressiveness, boldness and parental food provisioning in male house wrens (Troglodytes aedon). Ethology 118:984–993
Baumung R, Simianer H, Hoffman I (2004) Genetic diversity studies in farm animals: a survey. J Anim Breed Genet 121:361–373
Bell AM (2011) Personality in the wild. Nature 491:341–342
Bergvall UA, Schäpers A, Kjellander P, Weiss A (2011) Personality and foraging decisions in fallow deer, Dama dama. Anim Behav 81:101–112
Boissy A (1995) Fearfulness in animals. Q Rev Biol 70:165–191
Bremner-Harrison S, Prodohl PA, Elwood RW (2004) Behavioural trait assessment as a release criterion: boldness predicts early death in a reintroduction programme of captive-bred swift fox (Vulpes velox). Anim Conserv 7:313–320
Brown C, Braithwaite VA (2004) Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim Behav 68:1325–1329
Bruford MW, Bradley DG, Luikart G (2003) DNA markers reveal the complexity of livestock domestication. Nat Rev Genet 4:900–910
Cardellino RA, Boyazoglu J (2009) Research opportunities in the field of animal genetic resources. Livest Sci 120:166–173
Carere C, Eens M (2005) Unraveling animal personalities: how and why individuals consistently differ. Behaviour 142:1149–1157
Carter AJ, Marshall HH, Heinsohn R, Cowlinshaw G (2012a) How not to measure boldness: novel object and antipredator responses are not the same in wild baboons. Anim Behav 84:603–609
Carter AJ, Marshall HH, Heinsohn R, Cowlinshaw G (2012b) Evaluating animal personalities: do observer assessments and experimental tests measure the same thing? Behav Ecol Sociobiol 66:153–160
Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R (2013) Animal personality: what are ecologists measuring? Biol Rev 88:465–475
Cattell RB (1966) The scree test for the number of factors. Multivar Behav Res 1:245–276
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum, Hillsdale
EEA (European Environment Agency) (2009) Progress towards the European 2010 biodiversity target. EEA report no 4/2009, Copenhagen
Fairholm E (2007) Horses and ponies: more than just a size difference? M.A. Honours thesis, University of Edinburgh
FAO (2007a) Global plan of action for animal genetic resources and the interlaken declaration adopted by the International Technical Conference on Animal Genetic Resources for Food and Agriculture, Interlaken, Switzerland, 3–7 September 2007. Commission on Genetic Resources for Food and Agriculture of the Food and Agriculture Organization of the United Nations, FAO, Rome, Italy. (http://www.fao.org)
FAO (2007b) The state of the world’s animal genetic resources for food and agriculture—in brief, edited by Dafydd Pilling & Barbara Rischkowsky for Food and Agriculture of the Food and Agriculture Organization of the United Nations, FAO, Rome. (http://www.fao.org)
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Fine AH (2010) Handbook on animal-assisted therapy. Elsevier Science, San Diego
Fox RA, Millam JR (2010) The use of ratings and direct behavioural observation to measure temperament traits in cockatiels (Nymphicus hollandicus). Ethology 116:59–75
Fox RA, Ladage LD, Roth TC II, Pravosudov VV (2009) Behavioural profile predicts dominance status in mountain chickadees, Poecile gambeli. Anim Behav 77:1441–1448
Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU (2007) Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc R Soc B 274:333–339
Gherardi F, Aquiloni L, Tricarico E (2012) Behavioural plasticity, behavioural syndromes and animal personality in crustacean decapods: an imperfect map is better than no map. Curr Zool 58:567–579
Gold KC, Maple TL (1994) Personality assessment in the gorilla and its utility as a management tool. Zoo Biol 13:509–522
Gosling SD (1998) Personality dimensions in spotted hyenas (Crocuta crocuta). J Comp Psychol 112:107–118
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86
Gosling SD, Bonnenburgh AV (1998) An integrative approach to personality research in anthrozoology: ratings of six species of pets and their owners. Anthrozoos 11:148–156
Groothuis TGG, Carere C (2005) Avian personalities: characterization and epigenesis. Neurosci Biobehav Rev 29:137–150
Håkansson J, Bratt C, Jensen P (2007) Behavioral differences between two captive populations of red jungle fowl (Gallus gallus) with different genetic backgrounds, raised under identical conditions. Appl Anim Behav Sci 102:24–38
Hedges LV (1981) Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat 6:106–128
Inglis IR (1979) Visual bird scarers: an ethological approach. In: Wright EN, Inglis IR, Feare CJ (eds) Bird problems in agriculture. MAFF, London, pp 121–143
Jain AK (2010) Data clustering: 50 years beyond k-means. Pattern Recognit Lett 31:651–666
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhius HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23:925–935
Kralj-Fišer S, Scheiber IBR, Blejec A, Moestl E, Kotrschal K (2007) Individualities in a flock of free-roaming greylag geese: behavioral and physiological consistency over time and across situations. Horm Behav 51:239–248
Kurvers RHJM, Eijkelenkamp B, Van Oers K, Van Lith B, Van Vieren SE, Ydenberg RC, Prins HHT (2009) Personality differences explain leadership in barnacle geese. Anim Behav 78:447–453
Kurvers RHJM, Nolet BA, Prins HHT, Ydenberg RC, van Oers K (2012) Boldness affects foraging decisions in barnacle geese: an experimental approach. Behav Ecol 23:1155–1161
Laikre L (2010) Genetic diversity is overlooked in international conservation policy implementation. Conserv Genet 11:349–354
Lee CM, Ryan JJ, Kreiner DS (2007) Personality in domestic cats. Psychol Rep 100:27–29
Lehmann M (1991) Social behavior in young domestic rabbits under semi-natural conditions. Appl Anim Behav Sci 32:269–292
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lopez P, Hawlena D, Polo V, Amo L, Martín J (2005) Source of individual shy-bold variations in anti-predator behaviour of male Iberian rock lizards. Anim Behav 69:1–9
Magurran AE (1990) The inheritance and development of minnow anti-predator behavior. Anim Behav 39:834–842
Mater JA, Anderson RC (1993) Personalities of octopuses (Octopus rubescens). J Comp Psychol 107:336–340
McDougall PT, Réale D, Sol D, Reader SM (2006) Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Anim Conserv 9:39–48
Monclús R, Rödel HG (2008) Different forms of vigilance in response to presence of predators and conspecifics in a group-living mammal, the European rabbit. Ethology 114:287–297
Mullan SM, Main DCJ (2007) Behaviour and personality of pet rabbits and their interactions with their owners. Vet Rec 160:516–520
Müller R, Schrader L (2005) Behavioural consistency during social separation and personality in dairy cows. Behaviour 142:1259–1312
Mykytowycz R, Hesterman ER (1975) An experimental study of aggression in captive European rabbits, Oryctolagus cuniculus (L.). Behaviour 52:92–120
Peterson RA (2000) A meta-analysis of variance accounted for and factor loadings in exploratory factor analysis. Mark Lett 11:216–275
Petersson E, Järvi T (2006) Anti-predator response in wild and sea-ranched brown trout and their crosses. Aquaculture 253:218–228
Poissant J, Réale D, Martin JGA, Festa-Bianchet M, Coltman DW (2013) A quantitative trait locus analysis of personality in bighorn sheep. Ecol Evol 3:474–481
Pongrácz P, Altbäcker V (2000) Ontogeny of the responses of European rabbits (Oryctolagus cuniculus) to aerial and ground predators. Can J Zool 78:655–665
Price EO (1984) Aspects of animal domestication. Q Rev Biol 59:1–32
Réale D, Festa-Bianchet M (2003) Predator induced natural selection on temperament in bighorn ewes. Anim Behav 65:463–470
Réale D, Gallant BY, Leblanc L, Festa-Bianchet M (2000) Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim Behav 60:589–597
Réale D, Reader SM, Sol D, McDougall PT, Dingermanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Reed TE (1994) The domestic rabbit. Literature resource for 4-H projects, University of Florida. Accessible at florida4h.org/projects/rabbits/Files/TheDomesticRabbit.pdf
Reyes-Meza V, Hudson R, Martinez-Gomez M, Nicolas L, Rödel HG, Bautista A (2011) Possible contribution of position in the litter huddle to long-term differences in behavioral style in the domestic rabbit. Physiol Behav 104:778–785
Rödel HG, Monclús R (2011) Long-term consequences of early development on personality traits: a study in European rabbits. Behav Ecol 22:1123–1130
Rödel HG, von Holst D (2009) Features of the early juvenile development predict competitive performance in male European rabbits. Physiol Behav 97:495–502
Rödel HG, Monclús R, von Holst D (2006) Behavioural styles in European rabbits: social interactions and responses to experimental stressors. Physiol Behav 89:180–188
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Sih A, Kats LB, Maurer EF (2003) Behavioural correlations across situations and the evolution of anti-predator behaviour in a sunfish–salamander system. Anim Behav 65:29–44
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Smith BR, Blumstein DT (2008) Fitness consequences of personality: a meta-analysis. Behav Ecol 19:448–455
Southern HN (1947) Sexual and aggressive behavior in the wild rabbit. Behaviour 1:173–194
Spencer-Booth Y, Hinde RA (1969) Tests of behavioural characteristics for rhesus monkeys. Behaviour 33:180–211
Sponenberg DP, Bixby DE (2007) Managing breeds for a secure future: strategies for breeders and breed associations. The American Livestock Breeds Conservancy, Pittsboro, pp 9–14
Stamps J, Groothuis TG (2010) The development of animal personality: relevance, concepts and perspectives. Biol Rev 85:301–325
Stevenson-Hinde J, Zunz M (1978) Subjective assessment of individual rhesus monkeys. Primates 19:473–482
Stevenson-Hinde J, Stillwell-Barnes R, Zunz M (1980) Individual differences in young rhesus monkeys. Primates 21:498–509
Svartberg K (2005) A comparison of behaviour in test and in everyday life: evidence of three consistent boldness-related personality traits in dogs. Appl Anim Behav Sci 91:103–128
Svartberg K, Forkman B (2002) Personality traits in the domestic dog (Canis familiaris). Appl Anim Behav Sci 79:133–155
Swedish Board of Agriculture (2009) Action plan for long-term sustainable use of Swedish domestic animal genetic resources. Report from the Swedish Board of Agriculture to the Swedish Ministry of Agriculture. (Handlingsplan för långsiktigt uthållig förvaltning av svenska husdjursgenetiska resurser, Jordbruksverkets redovisning av regeringsuppdrag 17 juni 2009) (In Swedish)
Tapio M, Tapio I, Grislis Z, Holm L-E, Jeppsson S, Kantanen J, Miceikiene I, Olsaker I, Viinalass H, Eythorsdottir E (2005) Native breeds demonstrate high contributions to the molecular variations in northern European sheep. Mol Ecol 14:3951–3963
Toms CN, Echevarria DJ, Jouandot DJ (2010) A methodological review of personality-related studies in fish: focus on the shy–bold axis of behaviour. Int J Comp Psychol 23:1–25
Uher J (2011) Individual behavioral phenotypes: an integrative meta-theoretical framework. Why ‘behavioral syndromes’ are not analogues of ‘personality’. Dev Psychobiol 53:521–548
Uher J, Asendorpf JB (2008) Personality assessment in the great apes: comparing ecologically valid behavior measures, behavior ratings, and adjective ratings. J Res Pers 42:821–838
Uher J, Asendorpf JB, Call J (2008) Personality in the behaviour of great apes: temporal stability, cross-situational consistency and coherence in response. Anim Behav 75:99–112
Uher J, Addessi E, Visalberghi E (2013a) Contextualised behavioural measurements of personality differences obtained in behavioural tests and social observations in adult capuchin monkeys (Cebus apella). J Res Pers 47:427–444
Uher J, Werner CS, Gosselt K (2013b) From observations of individual behaviour to social representations of personality: developmental pathways, attribution biases, and limitations of questionnaire. J Res Pers 47:647–667
United Nations framework Convention on Biological Diversity (1992). http://www.cbd.org. Accessed April 2013
van Oers K, de Jong G, Drent PJ, van Noordwijk AJ (2004) A genetic analysis of avian personality traits: correlated, response to artificial selection. Behav Genet 34:611–619
van Overveld T, Matthysen E (2013) Personality and information gathering in free-ranging great tits. PLoS One 8:1–9
Vitale AF (1989) Changes in anti-predatory responses of wild rabbits, Oryctolagus cuniculus (L.), with age and experience. Behaviour 110:47–61
Watanabe NM, Stahlman WD, Blaisdell AP, Garlick D, Fast CD, Blumstein DT (2012) Quantifying personality in the terrestrial hermit crab: different measures, different inferences. Behav Process 91:133–140
Watkins WM (2006) Determining parallel analysis criteria. J Mod Appl Stat Methods 5:344–346
Wilson ADM, Stevens ED (2005) Consistency in context specific measures of shyness and boldness in rainbow trout, Oncorhynchus mykiss. Ethology 111:849–862
Acknowledgments
We thank Kristoffer Andersson and Helena Sundsgård for assistance in the field and Anders Lekander, Nils Ryman, and Peter Guban for helpful advice. Thanks also to Torekällberget, Vallby, and Fredriksdal open air museums, Stora Skuggan, Akalla, and Eolshäll 4-H farms, Christina Arup, Carina and Magnus Bood, Thor and Ingrid Ringström, and all rabbit owners and breeders who provided animals and data for this study. Financial support from the Swedish Research Council Formas (L.L.), the Swedish Research Council (U.A.B.), and the Swedish Environmental Protection Agency (L.L., U.A.B.) is acknowledged. We also would like to thank three anonymous reviewers for their valuable and insightful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Andersson, A., Laikre, L. & Bergvall, U.A. Two shades of boldness: novel object and anti-predator behavior reflect different personality dimensions in domestic rabbits. J Ethol 32, 123–136 (2014). https://doi.org/10.1007/s10164-014-0401-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-014-0401-9