Abstract
In this study, assortative mating for different morphological traits was studied in a captive population of house sparrows (Passer domesticus). Males were larger than females. Assortative mating was found for tail length, wing length and general body size. Males with larger badge size mated with females with longer tails. The strongest assortative mating occurred for tail length (r=0.77), and this assortative mating remained significant after controlling for wing length, mass and tarsus length, suggesting that it was not an artefact of assortative mating for body size. The possibility of sexual selection for tail length in the house sparrow is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Females, in general, prefer to pair with high-quality males (review in Andersson 1994). By mating with high-quality males, females may attain benefits in the form of resources or genetic advantages for their offspring (Andersson 1994). In monogamous species, we can expect that females of high quality will mate with high-quality males due to female competition (Amundsen 2000), or because they choose first (Mueller 1995). Then, if a trait (e.g. body size) is positively correlated with quality in both sexes, an assortative mating (i.e., nonrandom mating) for that trait is predicted. Moreover, in monogamous species, males should be as choosy as females when they pair (Johnstone et al. 1996; Amundsen 2000; Kokko and Monaghan 2001). Therefore, high-quality males would mate with high-quality females, leading to assortative mating for the trait used by both sexes to choose mates. Indeed, many bird species show a positive assortative mating for different morphological traits (Olsen et al. 1998; Jawor et al. 2003; Tryjanowski and Šimek 2005).
On the other hand, pairs with a greater number of different phenotypes may use a wider niche and achieve a higher breeding success (e.g., Tryjanowski and Šimek 2005), which would favour an absence of assortative mating. Such niche use by pairs could favour sexual size dimorphism (Mueller 1990). In contrast, sexual size dimorphism may also be favoured by sexual selection (Andersson 1994). In such a case, because fitness is usually positively related with size (Widemo and Sæther 1999), an assortative mating for body size is predicted (see above).
The house sparrow (Passer domesticus) is primarily a socially monogamous species (Summers-Smith 1988; Veiga 1992). This species has sexual dimorphism for plumage coloration: males having a conspicuous breast badge that is displayed to females during courtship (Summers-Smith 1988). This trait is subject to sexual selection, as males with larger badge sizes achieve higher breeding success (Møller 1988a, 1989; Veiga 1993). One possibility is that males with larger badges attain better breeding territories (Veiga 1993). Furthermore, they might pair with higher-quality females. In the present work, I investigated whether positive correlations exist between this sexual trait (badge size) and morphological traits in females. I also analysed the possibility of assortative mating for morphology in this species. For this, I used a population breeding in captivity, thereby excluding the effect of territorial variability.
Materials and methods
The study was performed in 2001 in a population of house sparrows that have been breeding successfully in an aviary since 1999 (Moreno-Rueda and Soler 2002). Sparrows had ad libitum access to nest boxes as well as food, water and nesting material, and were allowed to mate freely. Breeding success was similar to that reported in the literature on field studies (Moreno-Rueda and Soler 2002). Birds were visually isolated from the researcher by curtains. A detailed description of captivity conditions can be found in Moreno-Rueda and Soler (2002). Captivity has the advantage that territorial variability is controlled for. I assumed that mate choice was not affected by the conditions of captivity.
Individuals were colour-ringed for identification. I took morphological measurements of the sparrows before the start of the breeding season. In males, I measured badge height and width with a digital calliper (accuracy 0.01 mm). With these measurements, I calculated badge size using the formula 166.7+0.45×height×width (Møller 1987). For both males and females, I measured tarsus length with the calliper, wing and tail length with a ruler (accuracy 1 mm), and body mass with a spring balance (accuracy 0.5 g). Another subset of individuals was measured twice to calculate the repeatability of the measurements (Lessells and Boag 1987), which proved consistently high (in all cases ≥0.84). With mass and tarsus, wing, and tail lengths, I generated a general body size index as the PC1 factor in a principal components analysis (PCA). During the breeding season, I periodically observed the nests to identify the members of pairs that had formed.
Parametric statistics were used because of the normality of the variables. I used Pearson correlations and partial correlations to examine the relationship between morphology in both pair members. I didn’t use the correction of Bonferroni because it is very restrictive considering the sample size in this study (Moran 2003). Results, therefore, should be taken with some caution. Measurements are given as mean ± standard deviation.
Results
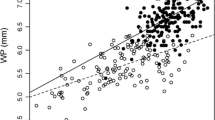
Twelve monogamous pairs were established during the study year in the aviary. The PC1 factor in the PCA explained 54.8% of variance in body size, and the four variables were positively correlated with the body size index (factor loadings for mass, tarsus, wing and tail respectively: 0.71, 0.66, 0.81 and 0.77). Males were larger than females (higher body size index), and they had significantly greater mass and wing and tail length than did females (Table 1). Mates showed assortative mating for body size index and tail length (Table 2). Badge size was significantly correlated only with female tail length (Table 2). Female tail length was also correlated with male body size (Table 2). Male body size and badge size were significantly correlated (r=0.66, P<0.02). Female tail length was not significantly correlated with male body size and badge size when I controlled for male badge (rpartial=0.44, P=0.17) and body (rpartial=0.43, P=0.18) sizes, respectively.
It is notable that the highest correlation was between female tail length and male tail length (r=0.77). Two explanations are possible: this could be due only to assortative mating for body size or it could indicate an effective assortative mating for tail length. Therefore, I correlated male and female tail length controlling simultaneously for body size (i.e. male tarsus, wing and mass). The correlation remained significant (rpartial=0.69, P<0.05), suggesting that the mating between females with longer tails and males with longer tails was independent of male body size. Controlling for male badge size also resulted in a significant correlation between male and female tail length (rpartial=0.62, P<0.05). When I controlled for female tarsus, wing and mass, the correlation was almost significant (rpartial=0.65, P=0.057).
Discussion
In this study, I found a positive assortative mating for body size in the house sparrow. Assortative mating for body size is common in different bird species (e.g., Olsen et al. 1998), and can be predicted as a result of mate choice, because body size is frequently correlated with fitness (Widemo and Sæther 1999). In contrast, Tryjanowski and Šimek (2005) did not find an assortative mating for body size in the red-backed shrike (Lanius collurio). In this species, the greater the difference in tarsus length between mates was, the greater the breeding success was (Tryjanowski and Šimek 2005), and this could explain the absence of assortative mating for tarsus length in this species. Male and female house sparrows forage in flocks (Summers-Smith 1988), making a diversification of niches unlikely. Therefore, sexual dimorphism is more likely explained as a sexual selection process in the house sparrow, for example as intrasexual competition in males (see Andersson 1994).
Sexual selection for male badge size exists in this species (Møller 1989; Veiga 1993), and an assortative mating between badge size and female body size was predicted. I did not find it, although there was a trend toward a correlation between female tail length and male badge size. It is probable that a larger sample size is necessary in order to find a significant correlation.
Interestingly, the results showed an assortative mating for tail length in the population studied. This assortative mating might be the result of assortative mating for body size, but after controlling simultaneously for mass, wing and tarsus (traits strongly correlated with body size), the correlation remained significant. Thus, the results consistently show that an assortative mating for tail length exists in the house sparrow, and this assortative mating is not confounded with an assortative mating for body size. The existence of assortative mating does not demonstrate that sexual selection exists for tail length in the house sparrow, but it suggests that sexual selection might mould tail length in one or both sexes in this species. Assortative mating may occur because mutual mate choice for tail length exists in this species (Johnstone et al. 1996), or because one sex (male or female) has a preference for longer tails in the other sex, the assortative mating occurring because intrasexual competition in the chosen sex results in individuals with longer tails mating before those with shorter tails (Mueller 1995). Sexual selection for male tail length has been found in some species (Andersson 1982; Møller 1988b; Palokangas et al. 1992; Romero-Pujante et al. 2002; but see Balmford et al. 1993, for an alternative interpretation). Assortative mating for tail length has also been found, for example, in merlins (Falco columbarius; Warkentin et al. 1992). Sexual selection for female tail length seems to exist in bearded tits (Panurus biarmicus, Romero-Pujante et al. 2002) and barn swallows (Hirundo rustica; Møller 1993; but see Cuervo et al. 1996). Therefore, sexual selection for tail length seems relatively common in birds, but studies on sexual selection for tail length have been performed with species with lengthened tails (Andersson 1982; Møller et al. 1998; Romero-Pujante et al. 2002), and the tail in the house sparrow is not especially lengthened, and the sexual dimorphism of tail length is small (Table 1).
Nevertheless, the absence of a marked sexual dimorphism or a long tail does not imply that tail length is not moulded by sexual selection. If mutual mate choice for tail length exists in this species, as has been shown for other species (e.g., Romero-Pujante et al. 2002), and the strength of sexual selection is similar for both sexes, then only a slight or null sexual dimorphism is predicted. Moreover, tail length is also moulded by natural selection (Evans 1998). Natural selection may counteract the effect of sexual selection, and the optimal tail length would be a result of the difference between the strength of both evolutionary forces. If sexual selection exists only in one sex of this species, but the strength of natural selection is high, the result would be only a slight sexual dimorphism. Therefore, sexual selection may also mould tail length in species with relatively short tails, as in the case of house sparrows. The importance of sexual selection for tail length in species with short tails has been generally ignored, although Balmford et al. (2000) described sexual selection for shorter tails in a species with a short tail, the golden-headed cisticolas (Cisticola exilis).
Sexual selection for tail length in house sparrows seems to be supported by the following observations: (1) males conspicuously display the tail during courtship (Summers-Smith 1988, page 145); (2) flight ability of house sparrows was previously shown to be negatively correlated with tail length (Moreno-Rueda 2003); and (3) data from this study showed an assortative mating for tail length, which could be due to mate choice for tail length. According to the results of the present study, and considering that tail length in house sparrows could be moulded by a selective pressure that makes long tails suboptimal for maneuverability (Moreno-Rueda 2003), tail length might be a handicap (Zahavi and Zahavi 1997) in the house sparrow, as it has been shown to be in the barn swallow (Møller and Nielsen 1997; Møller et al. 1998). Experiments in which tail length is manipulated and mating as well as breeding success are analysed (for example, Andersson 1982) would be necessary in the house sparrow to demonstrate that sexual selection for tail length exists in this species.
References
Amundsen T (2000) Why are female birds ornamented? Trends Ecol Evol 15:149–155
Andersson M (1982) Female choice selects for extreme tail length in a widowbird. Nature 299:818–820
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Balmford A, Thomas ALR, Jones IL (1993) Aerodynamics and the evolution of long tails in birds. Nature 361:628–631
Balmford A, Lewis MJ, Brooke M de L, Thomas ALR, Johnson CN (2000) Experimental analyses of sexual and natural selection on short tails in a polygynous warbler. Proc R Soc Lond B 267:1121–1128
Cuervo JJ, de Lope F, Møller AP (1996) The function of long tails in female barn swallows (Hirundo rustica): an experimental study. Behav Ecol 7:132–136
Evans MR (1998) Selection on swallow tail streamers. Nature 394:233–234
Jawor JM, Linville SU, Beall SM, Breitwisch R (2003) Assortative mating by multiple ornaments in northern cardinals (Cardinalis cardinalis). Behav Ecol 14:515–520
Johnstone RA, Reynolds JD, Deutsch JC (1996) Mutual mate choice and sex differences in choosiness. Evolution 50:1382–1391
Kokko H, Monaghan P (2001) Predicting the direction of sexual selection. Ecol Lett 4:159–165
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
Moreno-Rueda G (2003) The capacity to escape from predators in Passer domesticus: an experimental study. J Ornithol 144:438–444
Moreno-Rueda G, Soler M (2002) Cría en cautividad del Gorrión Común Passer domesticus. Ardeola 49:11–17
Møller AP (1987) Variation in badge size in male house sparrows Passer domesticus: evidence for status signalling. Anim Behav 35:1637–1644
Møller AP (1988a) Badge size in the house sparrow Passer domesticus: effects of intra- and intersexual selection. Behav Ecol Sociobiol 22:373–378
Møller AP (1988b) Female choice selects for male sexual tail ornaments in the monogamous swallow. Nature 332:640–642
Møller AP (1989) Natural and sexual selection on a plumage signal of status and on morphology in house sparrows, Passer domesticus. J Evol Biol 2:125–140
Møller AP (1993) Sexual selection in the barn swallow Hirundo rustica. III. Female tail ornaments. Evolution 47:417–431
Møller AP, Nielsen JT (1997) Differential predation cost of a secondary sexual character: sparrowhawk predation on barn swallows. Anim Behav 54:1545–1551
Møller AP, Barbosa A, Cuervo JJ, de Lope F, Merino S, Saino N (1998) Sexual selection and tail streamers in the barn swallow. Proc R Soc Lond B 265:409–414
Mueller HC (1990) The evolution of reversed sexual dimorphism in size in monogamous species of birds. Biol Rev 65:553–585
Mueller HC (1995) Correlation coefficients as evidence of female preference for male size of mate. Condor 97:284–284
Olsen P, Barry S, Baker GB, Mooney N, Cam G, Cam A (1998) Assortative mating in falcons: do big females pair with big males? J Avian Biol 29:197–200
Palokangas P, Alatalo RV, Korpimäki E (1992) Female choice in the kestrel under different availability of mating options. Anim Behav 43:659–666
Romero-Pujante M, Hoi H, Blomqvist D, Valera F (2002) Tail length and mutual mate choice in bearded tits (Panurus biarmicus). Ethology 108:885–895
Summers-Smith JD (1988) The sparrows, a study of the genus Passer. Poyser, Calton, UK
Tryjanowski P, Šimek J (2005) Sexual size dimorphism and positive assortative mating in red-backed shrike Lanius collurio: an adaptive value? J Ethol 23:161–165
Veiga JP (1992) Why are house sparrows predominantly monogamous? A test of hypotheses. Anim Behav 43:361–370
Veiga JP (1993) Badge size, phenotypic quality, and reproductive success in the house sparrow: a study on honest advertisement. Evolution 47:1161–1170
Warkentin IG, James PC, Oliphant LW (1992) Assortative mating in urban breeding merlins. Condor 94:418–426
Widemo F, Sæther SA (1999) Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol Evol 14:26–31
Zahavi A, Zahavi A (1997) The handicap principle: a missing piece of Darwin’s puzzle. Oxford University Press, New York
Acknowledgements
Lola G. López de Hierro, Rubén Rabaneda and Juan Diego Ibáñez helped me during the care of animals in the aviary. Comments from José Javier Cuervo helped me to put my ideas in order, and comments by anonymous referees greatly improved the manuscript. David Nesbitt improved the English.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Moreno-Rueda, G. Sexual size dimorphism and assortative mating for morphological traits in Passer domesticus. J Ethol 24, 227–230 (2006). https://doi.org/10.1007/s10164-005-0183-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-005-0183-1