Abstract
The necessity to preserve the environment and accomplish the rising demand for precious metals has made recycling of spent lithium-ion batteries (LIBs) crucial for conducting business in a sustainable way. An eco-friendly leaching process using ascorbic acid has been suggested in this work to leach critical metals from the spent calcined LIB sample. The optimum leaching of Li (100%) and Co (99.8%) was achieved under optimal conditions, such as 0.8 mol/L ascorbic acid, 60 min of leaching time, 70 °C temperature, and 50 g/L pulp density. To understand the leaching mechanism, detailed leaching kinetics was addressed using several reaction models. In contrast to conventional solvent extraction methods, especially with regard to environmental concerns, the undiluted green solvent Aliquat 336 has been employed in this work to separate critical metals without the use of toxic diluents. The thiocyanate form of Aliquat 336 (Aliquat 336-SCN) was used for the selective extraction of Co without the coextraction of Li. The extraction of cobalt reached 99.9% at 1:1 phase ratio and 2 stages of counter-current extraction. Following the four stages of cross-current stripping with 2.5 mol/L KSCN at a 1/5 O/A ratio, about 99.4% of Co was recovered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world is shifting toward sustainable energy rather than non-renewable energy sources like petroleum and gas due to the globe’s rising population [1]. Automobile companies like Toyota and Volvo announced that by 2050 the mobilities would be entirely or partly replaced by electrical vehicles [2, 3]. Electrification of all mobilities will require rechargeable batteries and lithium-ion batteries (LIBs) in this category which will earn the top position [4]. These batteries have a higher specific energy density, longer life cycles, lower self-discharge, and excellent safety performance [5, 6]. LIB sales are anticipated to reach a global peak in 2030 and a total of 2731 gigawatt hours’ worth of LIBs are expected to be commercially available in 2030. Figure 1 presented the estimated LIB market penetration worldwide in 2020, with projections for 2021–30 [7]. Due to the short lifespan of LIBs, the significant growth in consumption of LIBs may indicate that there will be a large quantity of trash in the form of waste LIBs in the upcoming days [8]. By 2030, it is forecasted that there would be more than 11 million tons of spent LIBs globally [9]. There are more than 200 tons of hazardous electrolytes and 1100 tons of heavy metals in every 4000 tons of discarded LIBs [10]. Landfilling, stabilization, and incineration of used LIBs are unacceptable for environmental reasons as well as the loss of valuable raw materials like lithium, cobalt, manganese, nickel, etc. [11]. Cathodic material recycling from waste LIBs is thus beneficial for the supply of valuable natural resources as well as the reduction of greenhouse gas emissions.
Estimated lithium-ion battery market penetration worldwide in 2020, with projections for 2021–30 [7]
A variety of methods have been used to develop LIB recycling, including pyrometallurgy, bio-hydrometallurgy, and hydrometallurgy [12, 13]. The pyrometallurgical method has the benefit of being straightforward and effective, but it also requires a lot of energy, which makes it challenging to extract precious metals selectively. Additionally, the high-temperature smelting technique generates a lot of toxic gases, which will severely impact the environment [14]. On the other hand, hydrometallurgy is commonly used in industrial sectors as an environmentally and economically compatible technique for extracting valuable metals from discarded LIBs [15, 16]. Numerous studies have been reported on the acid-leaching process of cobalt and lithium using both mineral acids (such as HNO3 [17], HCl [18], H2SO4 [19], and H3PO4 [20]) and organic acids (such as succinic acid [21], lactic acid [22] malic acid [23], ascorbic acid [24], oxalic acid [25] and citric acid [26]). According to the reports, inorganic acids are effective leachants for the separation of critical metal ions from waste LIBs. The leaching process of the cathodic materials was accomplished by Yang et al. using a solution of hydrogen peroxide and sulfuric acid. Nearly 100% of Li, Co, and Ni were leached, whereas manganese's leaching efficiency was 94.0% [27]. H2SO4 was employed by Nayl et al. for the leaching of critical metals from a mixed LIB sample. Only 65% of Cu was leached, compared to more than 97% of Li, Ni, Co, Al, and Mn [28]. But, the leaching with inorganic acids leads to environmental pollution because the leached waste gets into the groundwater and soil that releases poisonous gases such as SO3, NOx, Cl2, and HF [29]. In contrast to inorganic acids, organic acids are more environmentally friendly. Additionally, organic acids are easily biodegradable, rarely release harmful emissions, and the waste generated by leaching is simple to handle. Li et. al. reported that the cathodic materials were leached employing lactic acid and H2O2. The percentage of leaching for cobalt, manganese, lithium, and nickel were found to be 98.9%, 98.4%, 97.7%, and 98.2%, respectively, under ideal conditions [30]. Malic acid was used by Zhou et al. as a leachant and H2O2 as a reductant, and under optimal conditions, 98.13% of lithium and 98.86% of cobalt were leached [31]. In another work, an NCM-type battery sample was leached using citric acid and H2O2 and the leaching rates of Co, Li, Mn, and Ni, were 87%, 96%, 90.5%, and 93%, respectively [32]. According to a detailed literature review, both Co and Li are not efficiently leached in the absence of a reductant, and for the leaching of both metals, H2O2 is the most efficient reducing agent [21, 33, 34]. However, since it is added to the acid solution as an additional reagent, the addition of H2O2 raises the expense of the leaching process. The results need to be reconsidered in order to improve the leaching efficiency with a lower acid consumption and a higher S/L ratio owing to the higher acid content and lower S/L ratio of the previous investigations. Therefore, it is crucial to find a leaching method that consumes less acid, operates under mild conditions, should be cost-effective, and has little influence on the environment while assuring good leaching efficiency [35]. Ascorbic acid can be a choice for lixiviant as well as self-reductant due to its ability to convert metal ions having higher oxidation states to lower ones. Furthermore, the leaching of spent LIBs with ascorbic acid adequate for critical metal selectivity must not be overlooked.
After the leaching process, different separation techniques have been studied to recover valuable metals from used LIBs [36, 37]. In comparison to the other methods, the solvent extraction method is becoming more efficient because of its low cost, ease of operation, and environmental friendliness [38]. Ionic liquids are safer and more environmentally friendly alternative extractants in comparison to acidic, basic, and neutral extractants [39,40,41]. For the selective extraction of various metal ions from aqueous media, Bulgariu et al. developed an aqueous two-phase system of polyethylene glycol/ammonia sulfate, with iodide as extracting agent [42]. It has been reported in numerous investigations that fluorinated or functionalized ionic liquids have been utilized to remove metal ions from discarded LIBs [43, 44]. However, there are significant drawbacks with fluorinated ionic liquids, such as the hazardous HF acid that is formed when they are hydrolyzed [45]. Even if some non-fluorinated hydrophobic ionic liquids, including Aliquat 336 [46], Cyphos IL 101 [47], Cyphos IL 102 [48], and Cyphos IL 104 [49] have been employed to extract valuable metals, these liquids are diluted with kerosene, cyclohexane, toluene, and chloroform, etc. Diluents are employed to increase the flow rate and reduce the ionic liquids' viscosity, however, it limits the sustainability of the undiluted extractants which leads to environmental pollution. So more research studies should be emphasized the selective separation of critical metals without the use of toxic diluents.
Using ascorbic acid as the reductant most of the studies reported the leaching of spent NCM, LCO, and mixed-type batteries but using ascorbic acid as a primary leachant limited research works have been performed specially for waste LCO batteries. But, the precise leaching kinetics of the method, as well as the distinct separation of Li and Co from the leach solution were not investigated. As a result, the current study covers an in-depth investigation of the leaching process and removal of essential metal ions from spent LCO batteries. In this work, a completely sustainable attempt for the extraction of critical metals from the spent calcined LIB sample employing ascorbic acid as a self-reductant leaching agent is proposed. Several leaching factors were investigated, including acid concentration, temperature, pulp density (PD), and leaching time. By analyzing the leaching kinetics, it was possible to ascertain the reaction rate and the mechanism underlying the leaching method. The selective extraction of Co and Li from the leach solution using the undiluted thiocyanate form of Aliquat 336 (Aliquat 336-SCN) was investigated. McCabe–Thiele plot and counter-current extractions were carried out for the complete separation of the metals. Stripping studies of Co were also analyzed from the loaded organic phase.
Experimental methods
Materials and reagents
From an authorized waste material provider, the pretreated waste LIB sample was collected. From Merck Life Science Pvt., ascorbic acid (C6H8O6) with a ≥ 99% of purity was purchased. From Sigma Aldrich, trioctylmethylammonium chloride (Aliquat 336) was purchased. Similarly, all additional chemicals were supplied by Merck and were all of the analytical grades, including potassium thiocyanate (KSCN) with a purity of ≥ 98%. Millipore water was used to prepare all solutions.
Analytical methods
The treatment of the LIB sample was carried out in aqua regia and tested using ICP-OES (inductively coupled plasma optical emission spectrometry, iCAP PRO, Thermo scientific) to determine the total metal concentrations in the spent LIB sample. Before and after calcination and leaching, the LIB samples were examined using XRD (X-ray diffraction, of Rigaku Ultima IV), SEM (scanning electron microscopy), and EDS (energy-dispersive X-ray spectroscopy, EVO-18, Carl Zeiss). UV–Visible double beam spectrophotometer (Systronics, 2202) was used for the analysis of Aliquat 336-SCN before and after extraction.
Leaching process
The waste LIB sample was leached using ascorbic acid in a three-necked flat-bottomed flask (500 mL) fitted with a magnetic stirrer, a vapor condenser, and a temperature monitor. A specific amount of the LIB sample was measured as solid agents while ascorbic acid solutions of varying concentrations were generated as the leaching agents. After the leaching process, the mixture was separated through filtration. ICP-OES was used to determine the metal values that were present in the leach solution. The following equation (Eq. 1) was used to calculate the leaching efficiency for various metals.
Extraction process
Shaking equal volumes of the organic and aqueous phases in a Borosil separating funnel (Capacity 125 ml) for 15 min at 30 ± 0.5 °C was performed in the extraction trials. The raffinate solution (aqueous phase) was collected after phase separation, and the concentration of metal ions was measured. Equation 2 was used to estimate the distribution ratio (D), and Eq. 3 was used to determine the extraction efficiency (%E).
where Vaq = volume of aqueous phase solution, Vorg = volume of organic phase solution, Ci = concentration of the metal ions before extraction, and Cf = concentration of the metal ions after extraction.
The separation factor (β) was evaluated using the following equation (Eq. 4),
where D1 = partition coefficient of the more extracted metal ion and D2 = partition coefficient of the less extracted metal ion.
Preparation of Aliquat 336-SCN
20 g of Aliquat 336 was weighed out and stirred for 15 to 20 min with 25 mL 2.0 mol/L of HCl. Following the decantation of the aqueous solution, 50 mL of Millipore water was used to wash the organic phase. White turbidity developed and it was allowed to settle for 4 to 5 h. The water was then decanted and the organic phase was mixed with 25 mL of 2.0 mol/L KSCN solution. After decanting the aqueous phase and filtering the organic phase with 1PS paper, the undiluted thiocyanate form of Aliquat 336 was stored for the experiment.
Regeneration of Aliquat 336-SCN/stripping process
Back extraction of Co from the loaded organic phase is performed using 2.5 mol/L KSCN as a stripping agent. A sufficient amount of loaded Aliquat 336-SCN was generated prior to stripping. The Co-loaded organic phase was then equilibrated with 2.5 mol/L of KSCN using an O/A ratio of 1/5. The aqueous phase was removed, and the organic phase was treated with hot water (70 °C) to get pure Aliquat 336-SCN and remove any residual Co and impurities.
Characterization of the spent LIB sample
The discarded LIB sample was calcined for five hours at 700 °C, and the findings of the aqua regia test before and after the calcination are presented in Table 1. Both before and after calcination, the XRD study using a CuK (λ = 1.5406 Å) radiation source was performed with the 2θ range of 10° to 80° at 25 °C. The reference data from the JCPDS database (LiCoO2 = 00-050-0653, C = 00-008-0415, Co3O4 = 01-076-1802, and CuO = 03-065-2309) were compared with the diffraction patterns. Additionally, characterization of the LIB samples was carried out using SEM and EDS analysis at various magnifications.
Results and discussion
Leaching of spent LIB sample
Table 1 revealed that the concentration of metal ions has been enriched after calcination due to the removal of carbon from the sample. XRD patterns showed the presence of Co and Li in the form of LiCoO2 (Fig. 2). Before calcination, Fig. 2a displayed two intense carbon peaks in the XRD pattern, which disappeared after calcination (Fig. 2b). The SEM–EDS results clearly showed the carbon’s presence before calcination (Fig. 3a, b) and its disappearance after calcination (Fig. 3c, d).
Impact of ascorbic acid concentration
At 30 °C, the impact of ascorbic acid concentrations varying from 0.05 to 1.0 mol/L on the leaching rates of Li and Co from the waste LIB sample was examined at 10 g/L of PD, and a leaching period of 60 min. The leaching rates of Li and Co reached 29.6% and 20.8%, respectively, when the ascorbic acid concentration was 0.05 mol/L, as displayed in Fig. 4a. The concentration of ascorbic acid was increased from 0.05 to 0.8 mol/L, and this resulted in an increase in the leaching rates of Li and Co from 29.6% to 88.3% and 20.8% to 84.1%, respectively. The leaching efficiency of these metal ions was not noticeably increased by a further increase in ascorbic acid concentration. The ICP-OES results indicated that Al and Cu were not dissolved by ascorbic acid. To facilitate further experiments, 0.8 mol/L was chosen as the optimal concentration of ascorbic acid.
Impact of various parameters on leaching efficiency of Li and Co, a ascorbic acid concentration (temperature = 30 °C, PD = 10 g/L, leaching time = 60 min), b leaching time ([ascorbic acid] = 0.8 mol/L, temperature = 30 °C, PD = 10 g/L), c temperature ([ascorbic acid] = 0.8 mol/L, PD = 10 g/L, leaching time = 60 min) and d pulp density ([ascorbic acid] = 0.8 mol/L, temperature = 70 °C, leaching time = 60 min)
Impact of leaching time
The impact of leaching time ranging from 10 to 90 min on Li and Co's leaching rates is depicted in Fig. 4b. With a leaching time of 10 min, only 13.8% of Co and 27.2% of Li were dissolved. The percentage of leaching for Li and Co was increased to 88.3% and 84.1%, respectively when the leaching period was increased to 60 min. However, after 60 min the leaching efficiency remained steady despite the progressively growing trend. The 60 min leaching time was therefore continued throughout the experiments.
Temperature impact on leaching
The impact of temperature on the leaching rate of Li and Co was studied, by varying the temperature from 30 to 70 °C. As depicted in Fig. 4c, only 84.1% of Co and 88.3% of Li were leached at 30 °C. The solute diffusivity increased with temperature, which caused the increase in the leaching rate. The primary cause of this is that the proportion of successful collisions is inversely related to activation energy and directly proportional to temperature. Higher temperature accelerates the reaction's rate by raising kinetic energy, resulting in reduced activation energy, which favors the enhancement of leaching efficiency. The optimum leaching of Co (99.8%) and Li (100%) was achieved at 70 °C.
Impact of pulp density
With increasing the PD from 10 g/L to 70 g/L, the impact of PD on the efficiency of Li and Co leaching was studied. Other leaching parameters remain unchanged. Figure 4d revealed that when the PD was increased from 10 to 50 g/L, the leaching rates of both metals remained constant, while the leaching rates declined as the PD was increased further. The 100% of Li and 99.8% of Co were leached at PD of 50 g/L, as depicted in Fig. 4d. At PD of 60 g/L, the maximum Li (3.41 g/L) and Co (21.012 g/L) concentrations in the leach solution were obtained, and as PD was increased further, the contents of metal ions were not appreciably increased. For optimum dissolution of Co and Li, the PD of 60 g/L should be set. But, the PD was fixed at 50 g/L for the maximum leaching because the residue still contained a significant amount of Co and Li. Under optimum conditions, such as 0.8 mol/L acid concentration, 60 min, 70 °C, and 50 g/L PD, the leaching rates of Li and Co reached 100% and 99.8%, respectively.
Leaching kinetics
Kinetic studies are usually employed to understand the rate of the reaction and the leaching mechanism. A detailed discussion of the models with corresponding equations is provided in the supporting information. At various temperatures (30–70 °C) and leaching times (10–60 min), detailed kinetics of the leaching method was investigated. The leaching process of Co and Li from wasted LIB was found not to follow the diffusion control model (Fig. S1) and Avrami reaction model (Fig. S2) due to the lower R2 values than the surface chemical reaction model (Fig. 5). Other leaching factors continue to be at their ideal values. The R2 values for the surface chemical reaction model were > 0.98 which predicted that the ascorbic acid leaching system was controlled by this model. The surface chemical reaction model was used to calculate the specific rate constants (k) for Li and Co (Fig. 5a, b). From the Arrhenius equation k and T (leaching temperature) can be related as (Eq. 5).
where Ea (kJ/mol) is the apparent activation energy, and A is the pre-exponential factor. The Arrhenius equation's simplified form (Eq. 6) can be used to determine the apparent activation energies for the different metals.
Table 2 showed that raising the temperature is advantageous for increasing the leaching rates of Li and Co by showing that the rate constants increased as the temperature was increased. For the leaching of Li and Co, higher correlation coefficient values (R2 > 0.96) indicated that the surface chemical reaction model correlated with the results better (Fig. S3). According to Fig. S3, Li and Co have activation energies of 11.07 kJ/mol and 12.01 kJ/mol, respectively. Li showed a lower Ea than Co, suggesting that Li might be more easily leached. The variations in ascorbic acid concentration data (From Fig. 4a) were examined to support whether or not the leaching process was controlled by the surface chemical reaction model. The plot of ln k vs ln [ascorbic acid] (Fig. S4c) and the higher R2 values (> 0.99) for Li and Co (Figs. S4 a, b) validated the fitting of the surface chemical reaction model for the leaching of both metals.
Leaching mechanism
Deprotonation of one of the hydroxyl groups of ascorbic acid produces the ascorbate ion and the stabilization of that ascorbate ion by the two primary resonance structures is the reason why the hydroxyl groups of ascorbic acid are considerably more acidic than other hydroxyl compounds. Dehydroascorbic acid \(({\mathrm{C}}_{6}{\mathrm{H}}_{6}{\mathrm{O}}_{6})\), which functions as a reducing agent, is created by the oxidation of ascorbic acid with the loss of two electrons. Ascorbic acid is thus referred to as a self-reductant organic acid. Ascorbic acid dissociation can be illustrated as follows,
The surface chemical reaction model is used to predict the leaching mechanism of spent LiCoO2 batteries. The leaching kinetics revealed that Li was more easily leached than Co because of the trivalent cobalt ions. These trivalent ions need to be converted into soluble divalent ions employing ascorbic acid as a self-reductant in order to enhance the leaching percentage of Co. The two most probable and stable products in terms of thermodynamics are \({\mathrm{C}}_{6}{\mathrm{H}}_{6}{\mathrm{O}}_{6}{\mathrm{Li}}_{2}\) and \({\mathrm{C}}_{6}{\mathrm{H}}_{6}{\mathrm{O}}_{6}\mathrm{Co}\) [24]. The reaction of LiCoO2 with \({\mathrm{C}}_{6}{\mathrm{H}}_{8}{\mathrm{O}}_{6}\) may be represented as,
Figures S5a and S5b depict the SEM images of the waste calcined LIB samples both before and after leaching. Before leaching, irregular and agglomerated geometry were found (Fig. S5a). The larger particles with agglomerated shapes vanished after leaching (Fig. S5b). Peaks of CuO and Co3O4 were seen in the XRD patterns of the leached residue in Fig. 6. It implied that when ascorbic acid was utilized as the leachant, the Cu and Al were not dissolved in the leaching process which was also confirmed by the ICP-OES and EDS analysis. It was also informed that as Co3O4 was unable to leach, it remained in the residue [29, 50].
Separation of Li and Co employing ionic liquid
After the complete dissolution of the metal ions in the aqueous phase, it is necessary to separate them. The solvent extraction method was used for the separation of Li and Co. Acidic extractants can be used for the extraction of cobalt but co-extraction of lithium will inhibit clean separation [22]. The use of volatile diluents has a negative impact on both human health and the environment. Hence undiluted ionic liquids could be a better choice and some of the authors reported the use of undiluted IL for metal extraction and separation [45, 51]. Though several quaternary phosphonium ionic liquids have been examined for the extraction of Li [52, 53], the efficient extraction process for Co and Li separation using Aliquat 336 has not been explored. Thus, the selective extraction of Li and Co from the leach solution using the undiluted thiocyanate form of Aliquat 336 was investigated in this study. Considering the optimum leaching conditions, 500 mL of leached solution with a composition of 3.085 g/L of lithium and 20.61 g/L of cobalt was generated and it was 10 times diluted ([Li] = 0.3085 g/L and [Co] = 2.061 g/L) for further investigations.
Selective separation of co using Aliquat 336-SCN
The removal of metals from leach liquor was studied using both undiluted Aliquat 336 and Aliquat 336-SCN. The results showed that only 10.5% of Co was extracted when undiluted Aliquat 336 was used as the extractant, whereas the efficiency of Co extraction reached 94.3% when undiluted Aliquat 336-SCN was used (Fig. 7a). Figure 7b showed the image of two phases after extraction with Aliquat 336-SCN and the bluish-green color suggested that cobalt was extracted as cobalt thiocyanate. Less than 5 mg/L of Li was extracted to the organic phase with a higher separation factor (βCo/Li) of 1253.5. Aliquat 336-SCN performed admirably in the selective extraction of Co from leach liquor containing Li. It was also reported that the thiocyanate form of Aliquat 336 was preferable for cobalt extraction rather than other forms such as sulfate and chloride [54]. The UV–visible spectra of the neat and loaded Aliquat 336-SCN were taken and presented in Fig. 8. The neat form showed no absorption peak whereas the loaded Aliquat 336-SCN showed two distinct absorption peaks at 589 and 628 nm. The λmax was observed at 628 nm, indicating that the cobalt was extracted as cobalt thiocyanate into the organic phase. Also, it was reported that the spectra of the cobalt-loaded organic phase showed a broad peak between 550 and 700 nm, which is a characteristic indication of a tetrahedral complex of Co(II) [48, 55].
McCabe–Thiele plot for Co extraction
McCabe–Thiele plot was required for the complete separation of Co from the leach solution containing Li. Maintaining the total volume of phases fixed, the leach liquor was equilibrated with undiluted Aliquat 336-SCN at an O/A ratio ranging from 5/1 to 1/5 and the findings were presented in Fig. 9. Two stages of counter-current extraction with an O/A ratio of 1:1 was suggested for the complete separation of Co (Fig. 9). The theoretical prediction was verified by performing the counter-current extraction. The outcomes revealed that 2.059 g/L of Co was extracted into the organic phase along with 0.004 g/L of Li and that the raffinate contained 0.002 g/L of Co and 0.3045 g/L of Li.
Regeneration of Aliquat 336-SCN
To get pure cobalt oxide as well as to reuse the extractant, reverse extraction of Co from the loaded organic phase is required. To avoid disrupting the thiocyanate form of Aliquat 336, KSCN was employed as the stripping agent. Enough loaded Aliquat 336-SCN was generated by performing 2-stages of counter-current extraction with a 1:1 phase ratio. The recovery of Co from the loaded organic phase containing 2.059 g/L Co was investigated by varying the KSCN concentration from 1 to 2.5 mol/L at an O/A ratio of 1/5. It has been observed that only 57.8% of Co was stripped with 2.5 mol/L KSCN. Then the cross-current method was adopted for a complete stripping of Co. About 99.4% of Co was recovered after 4 stages of successive cross-current stripping (Table 3). Then the organic phase was washed with hot water (70 °C) to obtain pure Aliquat 336-SCN and remove any residual Co and impurities. After stripping, the precipitation of Co was carried out employing sodium hydroxide as a precipitation agent and also Li was precipitated as Li2CO3 from the Li-rich solution.
Comparative study
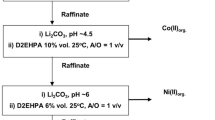
There has not been much research on the leaching of spent LIBs using ascorbic acid as a leaching agent rather than a reducing agent. The results of all ascorbic acid-based leaching and separation techniques that do not completely separate Li and Co are summarized in Table 4. The findings need to be reviewed in order to improve the leaching efficiency with minimal acid consumption and a higher S/L ratio. Additionally, the clean separation of Li and Co was also not studied to propose a complete flow sheet. In this research, the complete process for selective separation of Li and Co from spent LiCoO2 type batteries is represented in a flow sheet (Fig. 10).
Conclusions
The XRD, SEM, and EDS studies have been performed for the waste LIB both before and after the calcination. The presence of Co and Li as LiCoO2 was identified in XRD patterns. The results from XRD, and SEM–EDS clearly demonstrated the carbon's presence before calcination and its disappearance after calcination. With 0.8 mol/L ascorbic acid, Al and Cu were not leached by ascorbic acid, whereas the leaching rates of Li and Co reached 88.3% and 84.1%, respectively. The optimum leaching efficiencies of 99.8% Co and 100% Li were achieved with 0.8 mol/L ascorbic acid, 70 °C, 50 g/L PD, and 60 min of leaching time. Kinetic studies have been analyzed to understand the reaction rate underlying the leaching process. Higher R2 values indicated an ideal match of the surface chemical reaction model. The two thermodynamically stable and most likely products, \({\mathrm{C}}_{6}{\mathrm{H}}_{6}{\mathrm{O}}_{6}{\mathrm{Li}}_{2}\) and \({\mathrm{C}}_{6}{\mathrm{H}}_{6}{\mathrm{O}}_{6}\mathrm{Co}\) is predicted from the leaching mechanism. The selective extraction of Co was carried out using undiluted Aliquat 336-SCN and according to the results, 94.3% of Co was extracted with a higher separation factor (βCo/Li) of 1253.5. 99.9% of Co was extracted employing 2-stages of counter-current extraction with an O/A ratio of 1:1. The λmax of cobalt-loaded Aliquat 336-SCN was observed at 628 nm, indicating that the cobalt was extracted as cobalt thiocyanate into the organic phase. 99.4% of the Co was recovered after the four stages of cross-current stripping with 2.5 mol/L KSCN at a 1/5 O/A ratio.
Data availability
Not applicable.
References
Chen H, Gu S, Guo Y, Dai X, Zeng L, Wang K, He C, Dodbiba G, Wei Y, Fujita T (2021) Leaching of cathode materials from spent lithium-ion batteries by using a mixture of ascorbic acid and HNO3. Hydrometallurgy 205:105746. https://doi.org/10.1016/j.hydromet.2021.105746
Cars Volvo, Volvo Cars to go all electric, (2017). https://www.media.volvocars.com/global/en-gb/media/pressreleases/210058
Toyota motor corporation, environmental report 2018. (2018). https://global.toyota/pages/global_toyota/sustainability/report/er/er18_07-13_en.pdf
Nishi Y (2001) Lithium ion secondary batteries; past 10 years and the future. J Power Sources 100:101–106. https://doi.org/10.1016/S0378-7753(01)00887-4
Chen M, Ma X, Chen B, Arsenault R, Karlson P, Simon N, Wang Y (2019) Recycling end-of-life electric vehicle lithium-ion batteries. Joule 3:2622–2646. https://doi.org/10.1016/j.joule.2019.09.014
Sun S, Jin C, He W, Li G, Zhu H, Huang J (2021) Management status of waste lithium-ion batteries in China and a complete closed-circuit recycling process. Sci Total Environ 776:145913. https://doi.org/10.1016/j.scitotenv.2021.145913
Wang W, Zhang Y, Zhang L, Xu S (2020) Cleaner recycling of cathode material by in-situ thermite reduction. J Clean Product 249:119340. https://doi.org/10.1016/j.jclepro.2019.119340
Li J, Lai Y, Zhu X, Liao Q, Xia A, Huang Y, Zhu X (2020) Pyrolysis kinetics and reaction mechanism of the electrode materials during the spent LiCoO2 batteries recovery process. J Hazard Mater 398:122955. https://doi.org/10.1016/j.jhazmat.2020.122955
Wang K, Zhang G, Luo M (2022) Recovery of valuable metals from cathode—anode mixed materials of spent lithium-ion batteries using organic acids. Separations 9:259. https://doi.org/10.3390/separations9090259
Roy JJ, Rarotra S, Krikstolaityte V, Zhuoran KW, Cindy YD, Tan XY, Carboni M, Meyer D, Yan Q, Srinivasan M (2022) Green recycling methods to treat lithium-ion batteries E-waste: a circular approach to sustainability. Adv Mater 34:2103346. https://doi.org/10.1002/adma.202103346
Xiao J, Niu B, Xu Z (2021) Highly efficient selective recovery of lithium from spent lithium-ion batteries by thermal reduction with cheap ammonia reagent. J Hazard Mater 418:126319. https://doi.org/10.1016/j.jhazmat.2021.126319
Xiao J, Niu B, Song Q, Zhan L, Xu Z (2021) Novel targetedly extracting lithium: an environmental-friendly controlled chlorinating technology and mechanism of spent lithium ion batteries recovery. J Hazard Mater 404:123947. https://doi.org/10.1016/j.jhazmat.2020.123947
Zhao J, Zhang B, Xie H, Qu J, Qu X, Xing P, Yin H (2020) Hydrometallurgical recovery of spent cobalt-based lithium-ion battery cathodes using ethanol as the reducing agent. Environ Res 181:108803. https://doi.org/10.1016/j.envres.2019.108803
Mohanty A, Sahu S, Sukla LB, Devi N (2021) Application of various processes to recycle lithium-ion batteries (LIBs): a brief review. Mater Today Proc 47:1203–1212. https://doi.org/10.1016/j.matpr.2021.03.645
Nayaka GP, Zhang Y, Dong P, Wang D, Zhou Z, Duan J, Li X, Lin Y, Meng Q, Pai KV, Manjanna J, Santhosh G (2019) An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J Environ Chem Eng 7:102854. https://doi.org/10.1016/j.jece.2018.102854
Li G, Rao M, Jiang T, Huang Q, Peng Z (2011) Leaching of limonitic laterite ore by acidic thiosulfate solution. Miner Eng 24:859–863. https://doi.org/10.1016/j.mineng.2011.03.010
Wang R-C, Lin Y-C, Wu S-H (2009) A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 99:194–201. https://doi.org/10.1016/j.hydromet.2009.08.005
Medić DV, Sokić MD, Nujkić MM, Đorđievski SS, Milić SM, Alagić SČ, Antonijević MM (2023) Cobalt extraction from spent lithium-ion battery cathode material using a sulfuric acid solution containing SO2. J Mater Cycles Waste Manage. https://doi.org/10.1007/s10163-022-01580-w
Chen X, Ma H, Luo C, Zhou T (2017) Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J Hazard Mater 326:77–86. https://doi.org/10.1016/j.jhazmat.2016.12.021
Li L, Qu W, Zhang X, Lu J, Chen R, Wu F, Amine K (2015) Succinic acid-based leaching system: a sustainable process for recovery of valuable metals from spent Li-ion batteries. J Power Sources 282:544–551. https://doi.org/10.1016/j.jpowsour.2015.02.073
Sahu S, Devi N (2022) Effective leaching of spent lithium-ion batteries using DL-lactic acid as lixiviant and selective separation of metals through precipitation and solvent extraction. Environ Sci Poll Res. https://doi.org/10.1007/s11356-022-24560-x
de OliveiraDemarco J, StefanelloCadore J, da Silveira F, de Oliveira E, HiromitsuTanabe D (2019) Assumpção Bertuol recovery of metals from spent lithium-ion batteries using organic acids. Hydrometallurgy 190:105169. https://doi.org/10.1016/j.hydromet.2019.105169
Sahu S, Devi N (2023) Two-step leaching of spent lithium-ion batteries and effective regeneration of critical metals and graphitic carbon employing hexuronic acid. RSC Adv 13:7193–7205. https://doi.org/10.1039/D2RA07926G
Verma A, Johnson GH, Corbin DR, Shiflett MB (2020) Separation of lithium and cobalt from LiCoO2: a unique critical metals recovery process utilizing oxalate chemistry. ACS Sustain Chem Eng 8:6100–6108. https://doi.org/10.1021/acssuschemeng.0c01128
Chen X, Fan B, Xu L, Zhou T, Kong J (2016) An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J Clean Prod 112:3562–3570. https://doi.org/10.1016/j.jclepro.2015.10.132
Yang Y, Lei S, Song S, Sun W, Wang L (2020) Stepwise recycling of valuable metals from Ni-rich cathode material of spent lithium-ion batteries. Waste Manage 102:131–138. https://doi.org/10.1016/j.wasman.2019.09.044
Nayl AA, Elkhashab RA, Badawy SM, El-Khateeb MA (2017) Acid leaching of mixed spent Li-ion batteries. Arab J Chem 10:S3632–S3639. https://doi.org/10.1016/j.arabjc.2014.04.001
Chen M, Wang R, Qi Y, Han Y, Wang R, Fu J, Meng F, Yi X, Huang J, Shu J (2021) Cobalt and lithium leaching from waste lithium ion batteries by glycine. J Power Sources 482:228942. https://doi.org/10.1016/j.jpowsour.2020.228942
Li L, Fan E, Guan Y, Zhang X, Xue Q, Wei L, Wu F, Chen R (2017) Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain Chem Eng 5:5224–5233. https://doi.org/10.1021/acssuschemeng.7b00571
Zhou S, Zhang Y, Meng Q, Dong P, Fei Z, Li Q (2021) Recycling of LiCoO2 cathode material from spent lithium ion batteries by ultrasonic enhanced leaching and one-step regeneration. J Environ Manage 277:111426. https://doi.org/10.1016/j.jenvman.2020.111426
Islam A, Roy S, Khan MA, Mondal P, Teo SH, Taufiq-Yap YH, Ahmed MT, Choudhury TR, Abdulkreem-Alsultan G, Khandaker S, Awual MdR (2021) Improving valuable metal ions capturing from spent Li-ion batteries with novel materials and approaches. J Mol Liquids 338:116703. https://doi.org/10.1016/j.molliq.2021.116703
Yang J, Jiang L, Liu F, Jia M, Lai Y (2020) Reductive acid leaching of valuable metals from spent lithium-ion batteries using hydrazine sulfate as reductant. Trans Nonferrous Metals Soc China 30:2256–2264. https://doi.org/10.1016/S1003-6326(20)65376-6
Yu M, Zhang Z, Xue F, Yang B, Guo G, Qiu J (2019) A more simple and efficient process for recovery of cobalt and lithium from spent lithium-ion batteries with citric acid. Sep Purif Technol 215:398–402. https://doi.org/10.1016/j.seppur.2019.01.027
Masias A, Marcicki J, Paxton WA (2021) Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett 6:621–630. https://doi.org/10.1021/acsenergylett.0c02584
Raj T, Chandrasekhar K, Kumar AN, Sharma P, Pandey A, Jang M, Jeon B-H, Varjani S, Kim S-H (2022) Recycling of cathode material from spent lithium-ion batteries: challenges and future perspectives. J Hazard Mater 429:128312. https://doi.org/10.1016/j.jhazmat.2022.128312
Mossali E, Picone N, Gentilini L, Rodrìguez O, Pérez JM, Colledani M (2020) Lithium-ion batteries towards circular economy: a literature review of opportunities and issues of recycling treatments. J Environ Manage 264:110500. https://doi.org/10.1016/j.jenvman.2020.110500
Zhou Z, Fan J, Liu X, Hu Y, Wei X, Hu Y, Wang W, Ren Z (2020) Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent. Hydrometallurgy 191:105244. https://doi.org/10.1016/j.hydromet.2019.105244
Sahu S, Mohapatra M, Devi N (2022) Effective hydrometallurgical route for recovery of energy critical elements from E-wastes and future aspects. MaterToday Proc. https://doi.org/10.1016/j.matpr.2022.05.491
Zeng X, Li J (2014) Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries. J Hazard Mater 271:50–56. https://doi.org/10.1016/j.jhazmat.2014.02.001
Zante G, Braun A, Masmoudi A, Barillon R, Trébouet D, Boltoeva M (2020) Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents. Minerals Eng 156:106512. https://doi.org/10.1016/j.mineng.2020.106512
Bulgariu L, Bulgariu D (2013) Selective extraction of Hg (II), Cd (II) and Zn (II) ions from aqueous media by a green chemistry procedure using aqueous two-phase systems. Sep Purif Technol 118:209–216. https://doi.org/10.1016/j.seppur.2013.07.007
Zheng H, Dong T, Sha Y, Jiang D, Zhang H, Zhang S (2021) Selective extraction of lithium from spent lithium batteries by functional ionic liquid. ACS Sustain Chem Eng 9:7022–7029. https://doi.org/10.1021/acssuschemeng.1c00718
Zheng H, Huang J, Dong T, Sha Y, Zhang H, Gao J, Zhang S (2022) A novel strategy of lithium recycling from spent lithium-ion batteries using imidazolium ionic liquid. Chinese J Chem Eng 41:246–251. https://doi.org/10.1016/j.cjche.2021.09.020
Vander Hoogerstraete T, Wellens S, Verachtert K (2013) Binnemans Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid separations relevant to rare-earth magnet recycling. Green Chem 15:919–927. https://doi.org/10.1039/C3GC40198G
Tran TT, Liu Y, Lee MS (2021) Separation of cobalt, nickel, and copper metal using the mixture of HCl in ethylene glycol and Aliquat 336 in kerosene. J Market Res 14:2333–2344. https://doi.org/10.1016/j.jmrt.2021.07.139
Xu L, Chen C, Fu M-L (2020) Separation of cobalt and lithium from spent lithium-ion battery leach liquors by ionic liquid extraction using Cyphos IL-101. Hydrometallurgy 197:105439. https://doi.org/10.1016/j.hydromet.2020.105439
Dhiman S, Gupta B (2019) Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. J Clean Prod 225:820–832. https://doi.org/10.1016/j.jclepro.2019.04.004
Inman G, Nlebedim IC, Prodius D (2022) Application of ionic liquids for the recycling and recovery of technologically critical and valuable metals. Energies 15:628. https://doi.org/10.3390/en15020628
Santhosh G, Nayaka GP (2021) Cobalt recovery from spent Li-ion batteries using lactic acid as dissolution agent. Clean Eng Technol 3:100122. https://doi.org/10.1016/j.clet.2021.100122
SobekovaFoltova S, VanderHoogerstraete T, Banerjee D (2019) Binnemans Separation and purification technology samarium/cobalt separation by solvent extraction with undiluted quaternary ammonium ionic liquids. Sep Purific Technol. https://doi.org/10.1016/j.seppur.2018.07.069
Zante G, Masmoudi A, Barillon R, Trébouet D, Boltoeva M (2020) Separation of lithium, cobalt and nickel from spent lithium-ion batteries using TBP and imidazolium-based ionic liquids. J Ind Eng Chem 82:269–277. https://doi.org/10.1016/j.jiec.2019.10.023
Othman EA, van der Ham AGJ, Miedema H, Kersten SRA (2020) Recovery of metals from spent lithium-ion batteries using ionic liquid [P8888][Oleate]. Sep Purific Technol 252:117435. https://doi.org/10.1016/j.seppur.2020.117435
Nayl AA (2010) Extraction and separation of Co (II) and Ni (II) from acidic sulfate solutions using aliquat 336. J Hazard Mater 173:223–230. https://doi.org/10.1016/j.jhazmat.2009.08.072
Rybka P, Regel-Rosocka M (2012) Nickel (II) and cobalt (II) extraction from chloride solutions with quaternary phosphonium salts. Sep Sci Technol 47:1296–1302. https://doi.org/10.1080/01496395.2012.672532
Refly S, Floweri O, Mayangsari TR, Sumboja A, Santosa SP, Ogi T, Iskandar F (2020) Regeneration of LiNi1/3Co1/3Mn1/3O2 cathode active materials from end-of-life lithium-ion batteries through ascorbic acid leaching and oxalic acid coprecipitation processes. ACS Sustain Chem Eng 8:16104–16114. https://doi.org/10.1021/acssuschemeng.0c01006
Li L, Lu J, Ren Y, Zhang XX, Chen RJ, Wu F, Amine K (2012) Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J Power Sourc 218:21–27. https://doi.org/10.1016/j.jpowsour.2012.06.068
Lie J, Liu J-C (2021) Closed-vessel microwave leaching of valuable metals from spent lithium-ion batteries (LIBs) using dual-function leaching agent ascorbic acid. Sep Purific Technol 266:118458. https://doi.org/10.1016/j.seppur.2021.118458
Acknowledgements
For providing the research facilities, the authors gratefully acknowledge S'O'A (Deemed to be University).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Sibananda Sahu (First author): writing-original draft, visualization, data curation, formal analysis, investigation, and software. Niharbala Devi (Corresponding author): conceptualization, methodology, supervision, writing—review and editing, and validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflicts of interest.
Consent for publication
This paper has been read by all authors and given their approval for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahu, S., Devi, N. Hydrometallurgical treatment of spent lithium ion batteries using environmentally friendly leachant and extractant. J Mater Cycles Waste Manag 25, 3303–3315 (2023). https://doi.org/10.1007/s10163-023-01754-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01754-0