Abstract
Small changes of air pressure outside the eardrum of five lizard species led to changes in frequency, level, and peak width of spontaneous otoacoustic emissions (SOAE). In contrast to humans, these changes generally occurred at very small pressures (<20 mbar). As in humans, SOAE amplitudes were generally reduced. Changes of SOAE frequency were both positive and negative, while in humans, they are mostly positive. In addition, in lizards, these effects often showed obvious hysteresis and non-repeatability. The correlation between peak width and height was negative in two species (comparable to humans) and positive in one species. In two other species, no correlation was found. Consequently, a simple oscillator model that explained the negative correlation in humans could not be generally applied to lizards. This presumably reflects the fact that in lizards, the spontaneous otoacoustic emission of sound from the ear consists of a combination of stable oscillations (as in humans), unstable narrow-band oscillations, and broad-band emissions, evident as “plateaus” in emission spectra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous otoacoustic emissions (SOAE) have been reported from all major land-vertebrate groups: frogs, lizards, birds (Manley and van Dijk 2008), and mammals, mostly primates including humans (Lonsbury-Martin and Martin 2008). It is generally accepted that in all cases, the SOAE are manifestations of an active process in the inner ear (Köppl 1995; Manley 2001). Specifically, otoacoustic emissions are believed to be generated by the sensory hair cells themselves (Manley et al. 2001; Hudspeth 2008). These cells have the property of generating vibration at acoustic frequencies, and consequently, they are thought to serve as the source of sound that is detected as an otoacoustic emission at the tympanic membrane.
SOAE are sensitive to several external manipulations. For example, external tones suppress SOAE peaks in a way that is highly frequency selective: tones close to an emission frequency are more efficient in suppressing an SOAE peak than tones farther away. This can be characterized by a suppression tuning curve (e.g., Manley 2004, 2009; Manley and Köppl 2008). In several species, it has been shown that such suppression tuning curves show detailed resemblance to threshold tuning curves of primary auditory neurons (reviews in Manley and van Dijk 2008; Manley and Köppl 2008). This led to the interpretation that the emission-generating mechanism must be closely related to the tonotopically organized frequency selectivity of the inner ear. Specifically, it shows that the generation of a particular emission peak must be topically related to the corresponding tonotopic location of the emission frequency.
A different external manipulation of SOAE is the application of static air pressure to the ear canal. In humans, this typically increases the frequency of SOAE spectral peaks and decreases their amplitude (Schloth and Zwicker 1983; Hauser et al. 1993; Van Dijk et al. 2011). These effects have been interpreted as being the result of a stiffening of the tympanic membrane and oval window membrane. This stiffening reduces middle ear transmission and thereby causes an attenuation of the SOAE in the ear canal. Also, it changes the acoustic load on the inner ear and, in particular, on the inner ear SOAE generators. This is believed to account for the shift of SOAE frequencies (Schloth and Zwicker 1983; Shera 2003; Van Dijk et al. 2011).
Recently, Van Dijk et al. (2011) showed that the attenuation of the SOAE signal can also be interpreted as an intra-cochlear attenuation of the emission source signal. They studied the relationship between amplitude and width of spectral SOAE peaks. By considering a simple oscillator model of emission generation, they showed that SOAE attenuation due to static ear canal pressure must be due to an attenuation of the intra-cochlear source signal. Their study showed that the intra-cochlear mechanics of SOAE generation is directly affected by the properties of the middle ear.
The effect of static ear canal pressure in humans has also been interpreted in terms of the standing-wave model of emission generation in humans. In this model, SOAE peaks correspond to standing waves between the stapes and a tonotopic location close to the SOAE frequency characteristic place (Shera 2003). The effect of manipulations of the middle ear on SOAE frequency depends on the resulting changes of the oval window impedance looking backward out of the cochlea. More specifically, the SOAE frequencies depend on the phase shift introduced by reflection at the oval window of outward-going cochlear waves back into the cochlea. If the static tension on the stapes stiffens the oval window membrane, the phase delay of the reflected wave at the stapes is presumably reduced, and the round-trip travel time within the cochlea is also reduced. Correspondingly, the resonance frequencies that meet the condition required for standing-wave generation increase.
In humans, the termination impedance of the cochlea at the oval window by the middle ear can be modeled as a simple harmonic oscillator (Puria 2003). Stiffening the middle ear by, for example, stretching the eardrum with positive or negative static pressure will increase the resonant frequency of the middle ear (Homma et al. 2010). Correspondingly, the oval window will be stiffer. This reduces the phase shift of reflection at the oval window, which adequately explains the increase of SOAE frequencies as generally observed for an increase and a decrease of the static pressure in the ear canal (Schloth and Zwicker 1983; Hauser et al. 1993; Van Dijk et al. 2011). Note that the slight negative pressure that is usually needed to obtain the lowest SOAE frequency suggests that under normal conditions, the human tympanic membrane is slightly tensed medially (Schloth and Zwicker 1983; Hauser et al. 1993; Van Dijk et al. 2011). In conclusion, the standing-wave model of SOAE (Shera 2003) provides an elegant description of the typical frequency increases due to middle ear stretching, provided that the middle ear incorporated in the model has the appropriate properties (Puria 2003). However, it does not account for SOAE frequency decreases, which were also observed for some SOAE peaks in humans (Hauser et al. 1993; Van Dijk et al. 2011).
It is not known whether static ear canal pressure also affects SOAEs in non-mammalian species, since as yet there is no accepted model for SOAE generation in non-mammals. It would, however, be expected that increases and decreases of ear canal pressure would also produce here a stiffening of the middle ear. This, in turn, may reduce transmission of SOAE signals from the inner to the outer ear. Thus, the relationship between pressure and emission amplitudes is expected to be similar to that in humans. However, the effect of pressure on SOAE center frequency and width would also depend on the details of the inner ear mechanics and the mechanical coupling of the inner ear and the middle ear. Specifically, in lizards, the mechanics of the basilar membrane does not display a travelling wave (Peake and Ling 1980; Manley et al. 1988). Correspondingly, a standing-wave model is presumably not a reasonable option for modeling lizard SOAE. In humans, the standing-wave model predicts an emission frequency increase with changes to static pressure, and this generally, but not always, occurs. Such an increase would not necessarily be expected in lizards.
On the other hand, Bergevin and Shera (2010) showed that a mechanical model of the lizard inner ear, which does not exhibit travelling waves, can model stimulus frequency otoacoustic emissions (SFOAE) in lizards. A further analysis of the model showed that it operates similar to a coherent reflection model of the cochlea. Coherent reflection is suggested as a reflection mechanism in cochlear standing waves (Shera 2003). If the lizard cochlea functions in a way that is similar to a cochlear coherent reflection model, it may be expected that lizard SOAEs, in addition to SFOAEs, behave similar to human SOAEs in response to the application of static ear canal pressure.
Following our brief earlier report (Van Dijk and Manley 2009), this paper is the first to study the effect of ear canal pressure on SOAEs in non-mammalian species. In five lizard species, we measured the effect of static pressure on the amplitude, frequency, and spectral width. As will be shown, the results show similarities and differences to those in humans, which will be discussed in terms of inner ear models of SOAE generation.
Methods
In this study, we examined SOAE from 15 individuals from six species of lizards, all having relatively large papillae. Three species, namely, Cordylus tropidosternum (two individuals, weighing 28.1 and 33 g, Cordylidae), Gerrhosaurus major (3, 190 to 310 g, Gerrhosauridae), and Riopa fernandi (2, 71 and 74 g, Scincidae), belong to closely related lizard families that all have salletal tectorial structures over the hair cells of the higher-frequency region of the papilla (skinks and relatives; see Manley 2009). The other three species, namely, Varanus niloticus (1, 24 g, Varanidae) and the closely related V. exanthematicus (1, 206 g; the data from these two species were analyzed together) and Tupinambis teguixin (4, 60 to 140 g, Teeidae), have papillae in which the tectorial membrane over the higher-frequency hair-cell area is continuous, as in mammals (Manley 1990).
Experiments were carried out in a sound-attenuating chamber (Tegner) using a gas-supported, vibration-isolating table. An Etymotic, ER-10B low-noise microphone system, which had been calibrated using a Bruel and Kjaer 4166 microphone, was attached to a plastic tube that also permitted changes of air pressure via a coupled 50-ml plastic syringe. This assembly was then sealed over the animal's outer ear using Vaseline™. The relative air pressure change was measured using a Greisinger electronic (GMH 3150) electronic manometer. The animal's temperature was controlled via a Harvard animal blanket control unit, where the optimal temperature was sought and set individually. The animal breathed an air/isoflourane (Rhodia) mixture with a flow rate over the nose of 500 ml/min as controlled via a Fortec evaporator and a Platon flow meter. An initial isoflourane concentration of 1.5 ± 0.2 % was used to induce anesthesia, after which the dosage was reduced as low as possible, compatible with the animal not moving (generally 0.7 to 1.0 %). The microphone signal was fed to a spectrum analyzer (Stanford Research Systems, SR 760), and spectra (400 lines, 50 % overlap, Hanning window, 100 to 200 averages) were stored on a computer disk. Spectra were initially measured at 15-Hz bandwidth but thereafter generally at narrower bandwidths depending on the spectral peak distribution. Air pressure was changed in small steps (generally 0.1 mbar), and the resulting spectrum was stored; during measurements, the pressures remained constant. Air pressure was first reduced until the peaks in the SOAE spectra reduced their amplitude to the noise level (but never more than -20 mbar). Pressure was then raised again until at a certain overpressure the Vaseline® seal broke and ended the test run.
Since temperature can affect SOAE frequencies, it was important to hold the body temperature steady. To do this, we kept the animal covered and reduced air flow incurred through door openings, etc., to a minimum. Although it was not always possible to hold the temperature within ±0.1 °C and especially Varanus has a large temperature coefficient (Manley 2004), none of the frequency changes measured during pressure changes were found to correlate clearly with any small temperature drift.
The analysis of the spectra was carried out off-line. The frequencies and amplitudes of the SOAE peaks (identified by their temperature sensitivity and suppressibility by external tones) were read off for each pressure step. Peak widths at 3 dB below the peak level were measured by a least-squared fit of a Lorenzian curve to the spectra (Van Dijk et al. 2011). If multiple SOAE peaks were partly merged, a model function consisting of multiple peaks was used.
Results
All species showed SOAE peaks in their spectra, but not all ears showed good SOAE amplitudes. The number of SOAE peaks per ear varied, with Varanus, Cordylus, and Riopa only having two to three peaks, whereas Gerrhosaurus and Tupinambis generally had five or more. Thus, the amount of data from the different species varied.
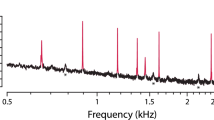
Since the lizard's mouth cavity is always open both to the outside and widely to both middle ears, the manometer reading gave directly the pressure difference across the tympanum. SOAE could change both their center frequency and their amplitude as the result of changes in air pressure. The spectra were quite sensitive to pressure, and except in three cases, no peaks were measureable below a pressure of -20 mbar (-200 daPa = approx. 20 cm H2O, i.e., the pressure felt at the ear when the head is 20 cm below a water surface) outside the eardrum. The breaking of the pressure seal at positive pressures, however, generally occurred before all SOAE had reduced to noise amplitudes. The effects of pressure were not the same in all cases; frequencies could rise or fall, as could peak amplitudes. Generalizations were difficult, except that for decreasing pressures outside the eardrum, the amplitudes tended to fall and, if anything, become larger for positive pressures (Figs. 1 and 2). Except in Cordylus, negative pressure led to a frequency increase in approximately 60 % of peaks, whereas positive pressure led to a frequency decrease in 60 % of peaks. In general, the effects were larger for lower-frequency SOAE peaks (compare Fig. 3).
Waterfall diagrams of spectra measured during changes in air pressure outside the eardrum in A Gerrhosaurus major and B Tupinambis nigropunctatus. Note the different frequency and amplitude scales. Beginning at the top trace at 0 pressure difference, the spectra are arbitrarily shifted down for each pressure step (pressure values shown on the RIGHT). In A and B, respectively, two and four SOAE peaks were analyzed. C, D “Plateau” emission energy. For each species, the first and last traces from A and B, respectively, are plotted on an absolute amplitude scale to illustrate the fall in sound levels at and between SOAE peaks.
Frequency (LEFT COLUMN) and amplitude (RIGHT COLUMN) of SOAE as a function of pressure difference across the eardrum in four species of lizard. A, B Tupinambis nigropunctatus; C, D Varanus exanthematicus; E, F Cordylus tropidosternum; G, H Gerrhosaurus major. In each case of negative or positive pressure change, the starting frequency or level is the reference point.
Illustration of a large hysteresis effect and a change in relative frequency as a function of pressure difference for three repeated measurements of three SOAE peaks in one Cordylus tropidosternum. For the lower two SOAE frequencies, the first measurement (CONTINUOUS LINE in each case) showed much larger frequency excursions than the later measurements 2 and 3 (DASHED and DOTTED LINES, respectively). The SOAE frequencies for each emission peak at the start of the experiment are indicated in panel A.
In many cases, the results showed a hysteresis, such that the changes observed toward reduced pressure were not necessarily quite the same as those recorded at the same pressures on the return path to zero pressure difference (Fig. 3). The deviations in frequency were, however, generally small (<~1.5 %). In addition, in several cases, there was a clear quantitative non-repeatability—when the same measurement series was repeated on the same ear, the pressure effects became smaller. This was seen in some ears for two additional measurements (Fig. 3). For these reasons, in all cases, we only analyzed in detail data for the first negative-going pressure change.
One major complicating factor in lizards is the inconsistent presence (inconsistent between ears) or absence of a “plateau” in the SOAE spectrum. Such plateaus have been reported previously in, for example, Tiliqua (Köppl and Manley 1993), in other skink-like lizard families (Manley 2009), and in geckos (Manley et al. 1996; Bergevin et al. 2010), where they were also named “baseline emissions.” In the present data, whereas in Varanus and Cordylus there was generally no or only a small plateau (Fig. 1C), both Tupinambis and Gerrhosaurus showed plateaus in some of the ears, and in some cases, these were greater than 10 dB in amplitude above the estimated microphone noise level (Fig. 1D). In general, equipment-noise baselines were rarely seen within spectra; in quite a number of spectra, SOAE peaks “rode” upon a high plateau. The sound energy in such plateau frequencies also decreased with changes in air pressure (Fig. 1C, D), and when nearby tone frequencies were added, suppression of the plateau confirmed that the plateaus are in fact emission energy.
The relationship between peak width and peak height showed significant hysteresis for individual peaks. By pooling results within a species, however, significant trends and differences between species became obvious (Fig. 4). In Varanus and Cordylus, peak width was negatively correlated with peak height. In Tupinambis and Gerrhosaurus, peak width was uncorrelated with peak height. Finally, in Riopa, there was a positive correlation between peak width and peak height.
Relationship between relative width and relative amplitude of SOAE spectral peaks in five lizard genera. The VERTICAL AXIS displays the ratio Δf/Δf 0, where Δf is the width of a spectral peak for a particular ear canal pressure and Δf 0 is the width of the corresponding peak at the start of the experiment (i.e., Δf 0 was determined at an initial ear canal pressure equal to 0 mbar). Similarly, the HORIZONTAL AXIS is the peak level relative to the initial amplitude. A Varanus exanthematicus, two animals, four ears, and seven SOAE peaks, B Tupinambis teguixin, 3 animals, 4 ears, and 18 SOAE peaks, C Cordylus tropidosternum, two animals, two ears, and three SOAE peaks, D Gerrhosaurus major, two animals, two ears, and three SOAE peaks, E Riopa fernandi, one animal, two ears, and six SOAE peaks. The LEGEND for these five panels: CLOSED SYMBOLS lizard data, OPEN SYMBOLS human data adapted from Van Dijk et al. (2011). SOLID CURVE least-square fits to lizard data, y = a × x n, where y is the relative peak width and x the relative peak amplitude (sound pressure in Pa). The resulting parameter n is indicated in the panels. DASHED CURVE predicted relationship for a simple oscillator model (Van Dijk et al. 2011). The human data closely follow the model, whereas the lizard data are not described by the model. F Results for three representative SOAE peaks in three lizards, respectively. The data points are connected to reflect the successive ear canal measurements.
Discussion
In our lizard data, small pressure differences across the tympanic membrane led to decreases in the amplitude of SOAE peaks and to shifts in their center frequency. There are qualitative similarities and differences to effects seen in human ears. Previous measurements in humans of the effects of pressure changes across the tympanum on SOAE have generally shown that (a) frequency rises for both an increase and a decrease of pressure, (b) the pressures required are large (a 40-mbar pressure results in an amplitude reduction of only about 6 dB), (c) the lowest frequency is generally found at a slightly negative pressure, and (d) the peak bandwidth changes systematically with peak amplitude, and high amplitude peaks are narrower (Schloth and Zwicker 1983; Van Dijk et al. 2011). In lizards, (a) frequency changes are both increases and decreases and vary between species (Fig. 2), (b) the pressures required are much smaller than in humans (a 5–10-mbar pressure often resulted in a 6-dB change), (c) the lowest frequency is not consistently found at any particular pressure, and (d) peak bandwidths show no consistent changes across species.
The fact that only a very small pressure is needed to observe pronounced effects on SOAEs is presumably related to characteristics of the lizard middle ear, which differs from that of humans in several respects. Not only has it effectively only one ossicle (columella and extracolumella are continuous), but the eardrum curvature is convex outward. In addition, the steady tension on the eardrum is much smaller. Presumably because of this, equivalent sound pressures induce larger displacement amplitudes of the ossicle: below 1 kHz, a mean of 4.8 times; above 1 kHz, a mean of 2.5 times higher amplitudes in geckos than in guinea pigs (data from Manley 1972 and Manley and Johnstone 1974 collected using the same apparatus). These numbers refer to tympanic membrane vibrations at acoustic frequencies. It is, however, expected that similar differences apply to static displacements of the ear drum.
The reasons behind this flexible middle ear lie presumably in the wide middle ear–buccal cavity connection and thus in its wide exposure both to the outside and to the inside. Not only is the eardrum exposed to potential damage from outside, but also a lizard eating is likely to have food penetrating the middle ear, and the system needs to be flexible to absorb these impacts without damage. For these reasons, even a small change in air pressure can deform the eardrum, and it is less surprising that a hysteresis was observed, presumably related to the eardrum not immediately returning to its original position or tension after displacement. Note that a hysteresis in the position of the tympanic membrane due to pressure application has also been reported in gerbils (Von Unge et al. 1993). It apparently reflects a rather common property of the tympanic membrane, which may also account for the hysteresis observed in human data on ear canal pressure (e.g., Van Dijk et al. 2011)
A further difference between the lizard and human middle ears relates to their connection to the outside air. In lizards, the middle ear is open to the ambient air through the mouth cavity and nasal passages. Thus, changing the pressure in front of the tympanic membrane will not change the air pressure behind the tympanic membrane. In contrast, in humans, the middle ear cavity is normally not connected to the outside air. Hence, a pressure applied at the lateral surface of the tympanic membrane will also change the pressure in the middle ear cavity (Dirckx et al. 1998). However, in the human study by Schloth and Zwicker (1983), subjects were required to swallow when the static pressure was applied in order to vent the middle ear. Hence, the circumstances for which the SOAEs were recorded were similar to those in lizards, with a middle ear pressure that equals that of the ambient air. Thus, the difference in the connection of the middle ear to the ambient air is unlikely to account for the differences that were observed between humans and lizards.
Although the pressure effects in lizards occur for much smaller pressures that in humans, both human and lizard emission amplitudes generally tend to reduce when pressure is applied. This may be due to a reduction of the transmission of acoustic energy from the inner ear to the outer ear. Also, stiffening of the tympanic membrane may lead to a concomitant stiffening of the oval window. Under those conditions, the inner ear emission generators are loaded by a modified impedance, which may attenuate their oscillation amplitude.
For positive pressure, the SOAE amplitude increased. Due to the breaking of the Vaseline seal of the measurement probe, it was not possible to study the SOAE behavior at pressures above +10 mbar. However, many SOAE peaks showed a tendency to decrease above about +5 mbar. Thus, it is reasonable to assume that these emissions would further attenuate for larger pressures. Consequently, lizard SOAEs are presumably similar to human emission with respect to the attenuation and eventual disappearance of SOAEs for large negative (shown here) and positive (extrapolated from current results) static pressures.
The pressure effect in humans and lizards, however, clearly differs with respect to changes in the frequency of SOAEs and in the width of SOAE peaks. While in humans the dominant effect is an increase of the frequency, in most lizard SOAE, both increases and decreases are about equally common. Recall that in humans, the effect of stiffening of the oval window on the standing wave may explain the increase in frequency. The difference between humans and lizards suggests that in lizards, the standing-wave model of SOAEs (Shera 2003; see “Introduction”) does not apply. In fact, though, the effect in humans is also variable (Hauser et al. 1993; Van Dijk et al. 2011), which suggests that in humans, the standing-wave model may not apply to all SOAE peaks.
The effect of stiffening of the cochlear boundary has also been investigated in a model consisting of a chain of active oscillators (Wit and Van Dijk 2012, Fig. 8). This model consists of a chain of coupled oscillators and intends to represent a portion of the cochlear partition. The longitudinal coupling between the oscillators in the chain could represent fluid coupling as is presumably present in the mammalian cochlea or coupling via the basilar membrane or tectorial membrane, as present in both mammals and lizards. Note that stiffening of the coupling of the highest frequency oscillator to the adjacent boundary results in an increase of the frequency of the spontaneous oscillation produced by the model. The stiffening of the highest-frequency basal oscillator may correspond to stiffening of the oval window due to the application of static pressure to the ear canal. In other words, the model predicts that an increase of the ear canal pressure results in an increase of SOAE frequencies. Thus, both the standing-wave model (Shera 2003) and the oscillator chain model (Wit and Van Dijk 2012) account for an increase of SOAE frequencies due to static pressure application. Neither of these models describe the decreases in frequency that have also been observed, both in humans (Hauser et al. 1993; Van Dijk et al. 2011) and lizards (this work).
The relationship between peak height and width was highly variable between lizard species and even within species (Fig. 4). This contrasts with the negative correlation between width and height in humans. In humans, this relationship is accounted for by a simple oscillator model, which models the broadening of the peaks (i.e., the width) as resulting from internal cochlear noise to which the emission generator is exposed. If the amplitude of the emission decreases due to static ear canal pressure, the emission becomes weaker relative to the noise, and its spectral peak broadens. The majority of SOAE peaks in the lizard do not behave according to this simple pattern. Why would lizard SOAEs be so different from human SOAE in this respect?
Lizard emissions display a number of characteristics that have not been shown in humans. First, in addition to the well-defined peaks observed in lizard SOAE spectra, many lizard spectra also contain plateaus on which the spectral peaks “sit” (e.g., Manley et al. 1996; Stewart and Hudspeth 2000). These have also been termed “baseline emissions” (Manley et al. 1996; Bergevin et al. 2010). The frequencies at the edges of these plateaus are temperature sensitive, and they can also be suppressed by external tones (e.g., in gecko SOAE; Manley et al. 1996). Consequently, they are considered to be otoacoustic emissions from many hair cells that presumably reside at the tonotopic locations in the ear corresponding to a broad range of frequencies. Apparently, their output energy is not bundled into narrow peaks but rather exists as a broad-band emission of acoustic energy from the ear. In other words, lizard ears manifest different degrees of synchronization of emissions, ranging from broad-band plateaus to narrow-band signals.
A second difference between human and lizard emissions relates to the statistical properties of the emission signals that correspond to narrow-band spectral peaks. Van Dijk et al. (1996) reported that 7 out of 16 lizard emissions showed amplitude statistics that were consistent with an essentially sinusoidal emission signal. The other emissions were dominated by Gaussian noise statistics, even for emissions that well exceeded the noise to the recording equipment. This behavior clearly differs from that in humans (Bialek and Wit 1984) and frogs (Van Dijk et al. 1989). Van Dijk et al. (1998) concluded that these Gaussian emissions must exhibit large amplitude fluctuations. In other words, in these cases, the emission generators are not able to maintain a stable inner ear oscillation.
Thus, in summary, lizard SOAE show two characteristics that have not been described in humans: (1) SOAE spectra often exhibit broad-band emission plateaus, and (2) a portion of the emission peaks corresponds to fluctuating narrow-band signals with Gaussian statistics. In humans, the sinusoidal nature of emission signals has led to the suggestion that their behavior can be described by self-sustained oscillators (Johannesma 1980; Bialek and Wit 1984). A number of studies showed that the characteristics of such oscillators well describe emission properties (e.g., statistical properties, Bialek and Wit; phase-lock to an external stimulus, Van Dijk and Wit 1990). In response to static pressure applied to the ear canal, an oscillator model well explained the negative correlation between SOAE peak height and width (Van Dijk et al. 2011; OPEN SYMBOLS in Fig. 4). In the current paper, we carefully analyzed the spectral characteristics of peaks in emission spectra. This showed that lizard SOAE often do not show the negative correlation between peak width and height that was observed in human SOAE. Van Dijk et al. (1998) observed a sinusoidal behavior in most larger SOAEs of the monitor lizard Varanus exanthematicus. Correspondingly, the relationship between width and height in this species as shown in the present paper most closely corresponded to that in humans (Fig. 4A). In other words, SOAEs in Varanus are human-like in the sense that they appear to behave as self-sustained oscillators, with a relationship between peak height and width that is qualitatively similar to that in humans. In other lizard species (with the possible exception of Cordylus, Fig. 4C), the synchronization of SOAE energy appears to be too poor, and an individual peak in the SOAE spectrum cannot be described by a single relatively simple non-linear oscillator model previously proposed (e.g., a Van der Pol or Rayleigh oscillator; Johannesma 1980; Bialek and Wit 1984; Van Dijk et al. 2011).
In this context, it should be remembered that in general, the anatomy of the lizard ear clearly differs from that of mammals. The number of hair cells involved in SOAE generation in lizards is often small. Even in large papillae, such as the 2-mm-long papilla of the bobtail skink Tiliqua rugosa, the maximum number of hair-cell generators involved per SOAE peak would be approximately 115 (1,600 hair cells in the basal segment (Köppl 1988) divided by 14 SOAE peaks (Köppl and Manley 1993)). In smaller papillae, SOAE have been measured that originated from two to three hair cells (Manley and Gallo 1997). In addition to the small numbers of hair cells, the coupling between the hair cells varies widely in strength, from continuous tectorial membranes similar to those in mammals, through sallets, which couple only a small localized group of cells, to a complete lack of a tectorial membrane over higher-frequency hair cells (Manley 1997). It is likely that in Varanus species, which have large papillae in which the hair cells are coupled by a mammalian-like tectorial membrane, the two to three SOAE peaks are each generated by an unusually large number of hair cells and thus most closely resemble human SOAE. We still understand too little about the mechanics of emission synchronization to understand how these facts will influence the behavior of SOAE under all conditions.
In conclusion, our data show that static pressure at the eardrum affects otoacoustic emission generation in lizards. As in human data, emission amplitudes in lizards mostly tend to reduce for both negative and presumably positive static pressures. However, these amplitude changes already occur at very small static pressures. Unlike in humans, emission frequencies shift up and down with approximately equal number of cases. Finally, the relationship between peak height and width could not be accounted for by a simple oscillator model. This presumably relates to the fact that many SOAE peaks do not represent stable oscillations (Van Dijk et al. 1998). In the single species that produces sharp and strong SOAE peaks (Varanus), however, the emissions do show a behavior that is qualitatively similar to that in humans that can be modeled as a self-sustained oscillator.
References
Bialek W, Wit HP (1984) Quantum limits to oscillator stability: theory and experiments on acoustic emissions from the human ear. Phys Let 104A:173–178
Bergevin C, Shera CA (2010) Coherent reflection without traveling waves: on the origin of long-latency otoacoustic emissions in lizards. J Acoust Soc Am 127:2398–2409
Bergevin C, Velenovsky DS, Bonine KE (2010) Coupled, active oscillators and lizard otoacoustic emissions. In: Shera CA, Olsen ES (eds) What fire is in mine ears: progress in auditory biomechanics. Melville, American Institute of Physics, pp 453–460
Dirckx JJ, Decraemer WF, Von Unge M, Larsson C (1998) Volume displacement of the gerbil eardrum pars flaccida as a function of middle ear pressure. Hear Res 118:35–46
Hauser R, Probst R, Harris FP (1993) Effects of atmospheric pressure variation on spontaneous, transiently evoked, and distortion product otoacoustic emissions in normal human ears. Hear Res 69:133–145
Homma K, Shimizu Y, Kim N, Du Y, Puria S (2010) Effects of ear-canal pressurization on middle-ear bone- and air-conduction responses. Hear Res 263:204–15
Hudspeth AJ (2008) Making an effort to listen: mechanical amplification in the ear. Neuron 59:530–545
Johannesma PIM (1980) Narrow band filters and active resonators. In: G. van den Brink and F.A. Bilsen (Eds.) Psychophysical, physiological and behavioural studies in hearing. Delft University Press, pp. 62–63.
Köppl C (1995) Otoacoustic emissions as an indicator for active cochlear mechanics: a primitive property of vertebrate auditory organs. In: Manley GA, Klump GM, Köppl C, Fastl H, Oeckinghaus H (eds) Advances in hearing research. World Scientific Publishing, Singapore, pp 207–218
Köppl C (1988) Morphology of the basilar papilla of the bobtail lizard Tiliqua rugosa. Hear Res 35:209–228
Köppl C, Manley GA (1993) Spontaneous otoacoustic emissions in the bobtail lizard. I: General characteristics. Hear Res 71:157–169
Lonsbury-Martin BL, Martin GK (2008) Otoacoustic emissions: basic studies in mammalian models. In: Manley GA, Fay RR, Popper A (eds) Active processes and otoacoustic emissions in Hearing. Springer, New York, pp 261–303
Manley GA (1972) The middle ear of the tokay gecko. J Comp Physiol 81:239–250
Manley GA (1990) Peripheral hearing mechanisms in reptiles and birds. Springer, New York
Manley GA (1997) Diversity in hearing-organ structure and the characteristics of spontaneous otoacoustic emissions in lizards. In: Lewis ER, Long GR, Lyon RF, Narins PM, Steele CR (eds) Diversity in auditory mechanics. World Scientific Publishing Co, Singapore, pp 32–38
Manley GA (2001) Evidence for an active process and a cochlear amplifier in non-mammals. J Neurophysiology 86:541–549
Manley GA (2004) Spontaneous otoacoustic emissions in monitor lizards. Hear Res 189:41–57
Manley GA (2009) Spontaneous otoacoustic emissions in lizards: a comparison of the skink-like lizard families Cordylidae and Gerrhosauridae. Hear Res 255:58–66
Manley GA, Köppl C (2008) What have lizard ears taught us about auditory physiology? Hear Res 238:3–11
Manley GA, Gallo L (1997) Otoacoustic emissions, hair cells and myosin motors. J Acoust Soc Amer 102:1049–1055
Manley GA, Johnstone BM (1974) Middle-ear function in the guinea pig. J Acoust Soc Amer 56:571–576
Manley GA, Van Dijk P (2008) Otoacoustic emissions in amphibians, lepidosaurs and archosaurs. In: Manley GA, Fay RR, Popper A (eds) Active processes and otoacoustic emissions in hearing. Springer, New York, pp 211–260
Manley GA, Yates G, Köppl C (1988) Auditory peripheral tuning: evidence for a simple resonance phenomenon in the lizard Tiliqua. Hear Res 33:181–190
Manley GA, Gallo L, Köppl C (1996) Spontaneous otoacoustic emissions in two gecko species, Gekko gecko and Eublepharis macularius. J Acoust Soc Amer 99:1588–1603
Manley GA, Kirk D, Köppl C, Yates GK (2001) In-vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proc Nat Acad Sci USA 98:2826–2831
Peake WT, Ling A (1980) Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency selectivity. J Acoust Soc Amer 67:1736–1745
Puria S (2003) Measurements of human middle ear forward and reverse acoustics: implications for otoacoustic emissions. J Acoust Soc Amer 113:2773–2789
Schloth E, Zwicker E (1983) Mechanical and acoustical influences on otoacoustic emissions. Hear Res 11:285–293
Shera CA (2003) Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves. J Acoust Soc Amer 114:244–262
Stewart CE, Hudspeth AJ (2000) Effects of salicylates and aminoglycosides on spontaneous otoacoustic emissions in the tokay gecko. PNAS 97:454–459
Van Dijk P, Manley GA (2009) The effect of ear canal pressure on the spectral width of spontaneous otoacoustic emissions: comparison between human and lizard ears. In: Cooper NC, Kemp DT (eds) Concept and challenges in the biophysics of hearing. World Scientific, Hackensack, pp 196–202
Van Dijk P, Wit HP (1990) Synchronization of spontaneous otoacoustic emissions to a 2f 1–f 2 distortion product. J Acoust Soc Amer 88:850–856
Van Dijk P, Wit HP, Segenhout JM (1989) Spontaneous otoacoustic emissions in the European edible frog Rana esculenta: spectral details and temperature dependence. Hear Res 42:273–282
Van Dijk P, Manley GA, Gallo L, Pavusa A, Taschenberger G (1996) Statistical properties of spontaneous otoacoustic emissions in one bird and three lizard species. J Acoust Soc Amer 100:2220–2227
Van Dijk P, Manley GA, Gallo L (1998) Correlated amplitude fluctuations of spontaneous otoacoustic emissions in five lizard species. J Acoust Soc Amer 104:1559–1564
Van Dijk P, Maat B, De Kleine E (2011) The effect of static ear canal pressure on human spontaneous otoacoustic emissions: spectral width as a measure of the intra-cochlear oscillation amplitude. J Assoc Res Otolaryngol 12:13–28
Von Unge M, Decraemer WF, Bagger-Sjöbäck D, Dirckx JJ (1993) Displacement of the gerbil tympanic membrane under static pressure variations measured with a real-time differential moire interferometer. Hear Res 70:229–242
Wit HP, Van Dijk P (2012) Are human spontaneous otoacoustic emissions generated by a chain of coupled nonlinear oscillators? J Acoust Soc Am 132:918–926
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to GAM. The research of PvD is supported by the Heinsius Houbolt Foundation and is part of the research program of the Groningen Department: Healthy Ageing and Communication. We would like to thank the students and technical staff of the Department of Zoology, T.U. München, who collected some of these data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Dijk, P., Manley, G.A. The Effects of Air Pressure on Spontaneous Otoacoustic Emissions of Lizards. JARO 14, 309–319 (2013). https://doi.org/10.1007/s10162-013-0385-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-013-0385-0