Abstract
Directional asymmetries in vestibular reflexes have aided the diagnosis of vestibular lesions; however, potential asymmetries in vestibular perception have not been well defined. This investigation sought to measure potential asymmetries in human vestibular perception. Vestibular perception thresholds were measured in 24 healthy human subjects between the ages of 21 and 68 years. Stimuli consisted of a single cycle of sinusoidal acceleration in a single direction lasting 1 or 2 s (1 or 0.5 Hz), delivered in sway (left–right), surge (forward–backward), heave (up–down), or yaw rotation. Subject identified self-motion directions were analyzed using a forced choice technique, which permitted thresholds to be independently determined for each direction. Non-motion stimuli were presented to measure possible response bias. A significant directional asymmetry in the dynamic response occurred in 27% of conditions tested within subjects, and in at least one type of motion in 92% of subjects. Directional asymmetries were usually consistent when retested in the same subject but did not occur consistently in one direction across the population with the exception of heave at 0.5 Hz. Responses during null stimuli presentation suggested that asymmetries were not due to biased guessing. Multiple models were applied and compared to determine if sensitivities were direction specific. Using Akaike information criterion, it was found that the model with direction specific sensitivities better described the data in 86% of runs when compared with a model that used the same sensitivity for both directions. Mean thresholds for yaw were 1.3 ± 0.9°/s at 0.5 Hz and 0.9 ± 0.7°/s at 1 Hz and were independent of age. Thresholds for surge and sway were 1.7 ± 0.8 cm/s at 0.5 Hz and 0.7 ± 0.3 cm/s at 1.0 Hz for subjects <50 and were significantly higher in subjects >50 years old. Heave thresholds were higher and were independent of age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asymmetry in vestibular reflexes has been useful in the diagnosis of vestibular lesions. The initiation of the vestibulo-ocular reflex (VOR) (Halmagyi and Curthoys 1988), caloric irrigation (Bárány 1921), and vestibular evoked myogenic potentials (VEMP) (Welgampola and Colebatch 2005) are now the basis of standard clinical tests whose diagnostic value is based at least partly on the presence of asymmetric responses. The reason that degree of asymmetry is useful in these tests is because the range of normal asymmetry is known. However, there has been very little done to investigate potential vestibular perception asymmetries, even in healthy individuals.
Most of our understanding of vestibular physiology comes by way of understanding the vestibular control of reflexes including the VOR (Halmagyi and Curthoys 1988; Crane and Demer 1998), postural responses (Liaw et al. 2009), and VEMP (Welgampola and Colebatch 2005). However, vestibular reflexes and vestibular perception arise from fundamentally different mechanisms (Merfeld et al. 2005a, b). Thus, it is not surprising that symptoms of vestibular disease including dizziness and vertigo are at best poorly correlated with tests of vestibular reflexes (Kanayama et al. 1995; Perez et al. 2003).
There has been only a hint of perceptual asymmetry in prior studies. Benson and colleagues performed a linear motion perception task in which normal subjects were encouraged to press a button if they were unsure of the direction of movement. These results suggested motion perception thresholds may be direction specific, but no attempts to define direction specific thresholds were made (Benson et al. 1986). A recent review also discusses the possibility of vestibular biases (Merfeld 2011) and mentions that two otherwise normal subjects were excluded from a prior report on vestibular thresholds due to significant asymmetries (Grabherr et al. 2008).
The incidence of balance disturbances increases significantly with each decade beyond the age of 40 years (Agrawal et al. 2009). Similarly, vestibular neurons are progressively lost beyond the age of 40 years (Engstrom et al. 1974; Lopez et al. 1997; Rauch et al. 2001). Vestibular reflexes including the VOR (Stefansson and Imoto 1986; Tian et al. 2001), spinal reflexes (Liaw et al. 2009), and VEMP (Nguyen et al. 2010) also decline with age. Most prior studies of motion perception have focused on a population under age 40 (Walsh 1961; Benson et al. 1986, 1989; Grabherr et al. 2008; MacNeilage et al. 2010; Mallery et al. 2010; Soyka et al. 2011), but when older subjects were included, advanced age was associated with higher perceptual thresholds in fore–aft motion (Kingma 2005).
Forced choice methods have now standardized threshold determination in several fields of psychophysics (Treutwein 1995; Leek 2001; Knill and Pouget 2004), and these techniques are now the standard method of assessing motion perception thresholds (Benson et al. 1989; Grabherr et al. 2008; Gu et al. 2008; MacNeilage et al. 2010; Mallery et al. 2010). These techniques have the advantage of largely eliminating the tendency to bias one choice, but make the assumption that the choices have equal thresholds. If the asymmetry encountered in vestibular reflexes persists in perceptual testing, then perception may be directionally asymmetric. This could be detected using standard psychometric techniques if it was present as a bias (i.e., if the psychometric function was shifted so that the mean was no longer zero). However, a bias implies that when stationary, the subject would perceive motion that does not occur in healthy subjects, and to our knowledge such a bias has not been published.
Asymmetries in the VOR have been demonstrated with asymmetric vestibular function and occur such that the response to stimulation is greater in one direction. This classically occurs with unilateral vestibular lesions in response to a rapid head rotation (Halmagyi and Curthoys 1988) but can also occur in response to whole body rotation (Demer et al. 2001). Caloric testing will also often demonstrate an asymmetry in the absence of spontaneous nystagmus (Bárány 1921; Jacobson and Means 1985). But significant asymmetry in the caloric response is also present in as many as 15–22% of normal individuals (Becker 1979). Interestingly, these tests can demonstrate asymmetries in even when there is no spontaneous nystagmus, such as occurs after compensation. This suggests that the sensitivity of the vestibular system can be direction specific and does not need to occur as a simple offset of the null position.
A goal of this research was to determine the degree of symmetry in vestibular perception. The current paper presents a forced choice technique that allows direction specific self-motion thresholds and the effect of age on these thresholds to be determined.
Materials and methods
Equipment
Motion stimuli were delivered using a 6-degree-of-freedom motion platform (Moog, East Aurora, NY, model 6DOF2000E) similar to that used in other laboratories for human motion perception studies (Grabherr et al. 2008; Fetsch et al. 2009; MacNeilage et al. 2010). Subjects were seated in a padded racing seat (Corbeau, Sandy UT, model FX-1) mounted on the platform (Fig. 1). A four-point racing style harness held the body in place. The head was held in an open-face motorcycle helmet with a chinstrap. Helmets were available in six sizes to allow each subject to be fit appropriately. Once the subject was seated, the helmet was firmly attached to the motion platform using a custom-built structure that allowed adjustment for the subject’s size and comfort. The head was aligned with the midpoint of the platform. The head was anchored via a rigid aluminum structure, which attached to the right side of the helmet and pushed the head back into a head rest attached to the seat to provide further stability. The helmet covered the ears, thus reducing the sound made by the platform.
Experimental setup. The subject was seated in the racing seat on top the motion platform. The body was held in place using a four-point harness and the head was held in place with a motorcycle helmet mounted directly to the motion platform. The button box used to collect the subject’s responses is seen in the subject’s hand.
The platform motion profile in the acceleration (A), velocity (B), and position domains (C). The platform motion calculated from Eq. (3) and a second-order transfer function, which represents the known dynamics of the system by a dotted gray line. The example shown was for a lateral (sway) translation with a 30 cm/s peak velocity and frequency of 1 Hz. This was the most demanding stimuli to reproduce. Acceleration was measured using an analog accelerometer mounted on the motion platform and is shown as a dark solid line in (A). The acceleration signal was numerically integrated to determine velocity and position in (B) and (C) which agreed very closely with the known dynamics.
Data from a single block of trials for subject #24 who had an asymmetric threshold. In this example, the platform motion was surge (forward/backward) at 0.5 Hz. Forward responses are represented by left-pointing filled triangles. Backward responses are represented by right-pointing, open triangles. The size of the plot symbols is proportional to the number of stimulus presentations at a given velocity and direction. Data from the same block of trials is plotted in both panels. Ranges in parentheses represent the 95% confidence interval of the curve fit parameters. A Peak velocity on a linear scale plotted against the fraction of backward responses. The dashed line represents the single sigma (SS) Gaussian function best fit to the data, and the solid line represents the best fit using a hybrid dual sigma (DS) function in which the sigma can be varied independently on both sides of the mean. The distribution of the data points suggests that a SS Gaussian function cannot accurately describe the responses in both directions. The Bayesian Information Criteria was −406 for the single sigma method and −530 for the dual sigma method indicating the data is better characterized by the dual sigma method. B A method of determining thresholds using log velocity (LV). The log of the absolute value of peak velocity plotted against the fraction of correct responses for the same data. The dashed line represents the single sigma Gaussian function best fit to the backward data, and the solid line represents the best fit to the forward data.
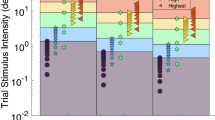
Directional thresholds by subject. Filled symbols represent motion in one direction, and open symbols represent motion in the opposite direction. The error bars represent the 95% confidence interval for the fit of the cumulative distribution function to the responses observed. An asterisk indicates that a threshold was not shown in that subject because it the subject could not reliably identify the largest stimulus.
Response to null stimuli as the fraction of responses in one direction (right, up, or forward) as a proportion of the total responses. Each motion type is represented by a different plot symbol shape. In two trial blocks, the 95% confidence interval did not include 0.5, these blocks are marked by an asterisk. The gray symbols indicate the AI was significantly different from zero at p <0.05, and the black symbols indicate a significant different at p <0.01. There was no correlation between the AI and the response to null stimuli (R = 0.02, p = 0.4).
The noise from the platform was similar regardless of motion direction. Tests were completed in darkness to see if the platform direction could be determined from the sound during the fastest (largest) stimuli used in this experiment. This was completed with the subject stationary. The subject was not able to determine the motion direction in this sound only condition better than would be expected by chance (data not shown).
Sounds from the platform were further masked using a white noise stimulus reproduced from two platform-mounted speakers on either side of the subject. Other studies of vestibular perception using a similar type of motion platform have also used masking noise which was presented at 60 dB with the addition of ear plugs (Grabherr et al. 2008) or via headphones (MacNeilage et al. 2010). The intensity of the masking noise used in the current study varied with time as a half-sine wave so that the peak masking noise occurred at the same time the peak velocity was reached. This created a masking noise similar to the noise made by the platform. Sound levels at the location of the subject were measured using a Quest Technologies, model 1900 sound level meter (Quest Technologies, Oconomowoc, WI, USA). Average sound pressure level (SPL) of the ambient sound was 58 dB, with a peak level of 68 dB when no motion was delivered. The masking noise had a peak of 92 dB. The motion platform had a peak noise level of 84 dB for velocities of 30° or cm/s for movements in the horizontal plane (yaw, surge, and sway) and 88 dB for heave. At 15 cm/s, the peak noise of the platform was 74 dB for horizontal plane motion and 78 dB for heave. The masking sound intensity was the same for every stimulus independent of the stimulus velocity. At 7.5 cm/s, the peak platform noise was 70 dB for horizontal plane motion and 71 dB for heave. Below 7.5 cm/s, the peak noise of the platform was lower than the peak of the ambient noise in the room (68 dB). The platform noise was similar at each peak velocity for both 1.0 and 0.5 Hz stimuli. No masking noise was used between stimuli. We found this type of masking much more effective than a continuous masking noise of constant intensity. The experiment was conducted in complete darkness. At the request of a reviewer, in two subjects (#1 and #10) who had a large amount of baseline data, the experiment was repeated using head phones (Fig. 7A, day 7, and Fig. 7C, days 3 and 4) to apply the masking noise rather than using the speakers. This was done to eliminate the potential of using masking sound reflecting off fixed objects in the room to identify the movement direction. Additionally in these two subjects, there were four additional trials in each subject using conditions known to produce significant asymmetries (Fig. 7A, days 8 through 11, Fig. 7C days 5 and 6, and Fig. 7D days 4 and 5). These results were similar to the prior results indicating that the masking noise did not provide a cue to movement direction.
Thresholds across trial blocks for subjects and conditions with multiple sessions all with 0.5-Hz stimuli. Days shown were consecutive but occurred over as long as an 18-month period. Error bars represent a 95% confidence interval. In most trials, auditory masking was delivered from speakers mounted on the platform. Trials in which there was masking delivered via head phones (†) or no masking was delivered (*) were performed in subjects #1 and #10. The C column represents the data combined from all sessions and fit with a single function.
Responses were collected using a three-button control box that the subject held. The center button was pressed by the subject to initiate each stimulus. The two buttons at either end were used to identify the perceived direction of motion. The subject was instructed to hold the box in an orientation appropriate for the experiment, i.e., so that the end buttons were aligned with the direction of motion.
Stimuli
The stimulus consisted of a sine wave in acceleration, which lasted 1 or 2 s (1 or 0.5 Hz) (Fig. 2 and Eq. 1) and occurred in a single direction. Four types of motion were tested including yaw (rotation about an earth vertical axis), sway (lateral translation), surge (anterior/posterior translation), and heave (up/down translation). Each frequency and motion type was tested in separate block of trials, which consisted of two staircases with 55 stimuli each (110 stimuli total). The stimulus can be described in the acceleration [a(t)], velocity [v(t)], or position [d(t)] domains given the frequency in hertz (f) and total displacement ( D ) (Eqs. 1–3). The motion platform required a position signal [d(t)] at a resolution of 60 Hz (Fig. 2 and Eq. 3). Prior work on threshold determination has shown that peak velocity (2Df) is the most consistent measurement of rotation threshold (Benson et al. 1989; Grabherr et al. 2008); therefore, we have consistently specified the stimulus in terms of peak velocity. These motion profiles were chosen because they contain no discontinuities in acceleration, velocity, or position, and they have previously been used for threshold determination (Benson et al. 1989; Grabherr et al. 2008). Stimuli were designed to have a similar peak velocity across frequencies. The peak velocity and peak acceleration for each stimulus type is shown in Table 1.
Three checks were made to ensure the intended stimulus was accurately delivered to the subject. First, the position was independently measured using a laser mounted on the platform, which marked position on a ruler mounted to the wall. Using this method, the position of the platform was extremely reproducible and accurate to the resolution of this technique (about 0.5 mm for translation and 0.1° for rotation) for the range of movements tested. Second, the acceleration was measured using a three-axis linear accelerometer (Model ADXL05EM-3, ±4 G range; Analog Devices, Norwood, MA, USA) mounted on the motion platform 1 m behind the center of rotation, which permitted direct measurement of translational acceleration and calculation of angular acceleration. Low pass filtration of the command signal by the platform dynamics (Gu et al. 2006) led to a peak velocity that was 94% of that predicted by the command signal alone. Measured acceleration was compared to the predicted movement of the motion platform after accounting for filtration of the command signal by the platform dynamics. It was found that the acceleration and velocity determined from the accelerometer agreed with the calculated trajectory (Fig. 2). Finally, data was collected with a three-axis accelerometer attached to a bite bar so that the acceleration experienced by the head could be directly compared with the stimulus delivered, and measured on the platform. This confirmed that the stimulus delivered to the head was directionally symmetric and similar to platform motion with no significant decoupling for the types of stimuli used (Fig. 3).
Experimental procedure
Subjects were instructed that during each block of trials they would move in one of two directions. Following stimulus delivery, they were prompted to push one of two buttons to select the direction of perceived movement. Subjects were encouraged to guess if they did not know the direction of motion. To discourage biased guesses towards one direction, subjects were informed that there would be an equal number of movements in each direction. The experiment was practiced a few times in the light to ensure comprehension of the task prior to data collection in darkness.
Prior to stimulus delivery, the subject heard a 500-Hz, 0.125-s single tone to signal that the next stimulus was ready. The stimulus was delivered immediately after the subject pressed the start button. After the stimulus was delivered, two 0.125-s tones were played in rapid succession to indicate the stimulus had been delivered and suggest that one of two response buttons should be pressed. These tones were played from speakers mounted to the motion platform to eliminate any potential auditory localization cues. When a response button was pressed, a key click sound was played which did not depend on the accuracy of the response, but indicated that the subject’s selection had been recognized by the program. If no response was entered within 2 s, a “timeout sound” was played (a low frequency buzz). After either a response or timeout, the platform returned to the center starting position using a motion profile similar to the stimulus but taking 0.5 s longer. This lead to a lower velocity return movement designed to avoid giving cues to the initial motion direction.
Stimuli were chosen from two independent staircases, with each staircase representing a single direction of motion (e.g., one right and one left). As subjects answered correctly, they would “step-down” the staircase, meaning that stimuli were presented at lower peak velocities. Similarly, as subjects answered incorrectly, stimuli were presented at higher peak velocities, indicating a “step-up” on the staircase. Prior to each stimulus, one directional staircase was randomly chosen, making the direction unpredictable to the subject. The algorithm that chose the staircase tended to favor the staircase with more stimulus presentations remaining (similar to pulling marbles of two colors out of a bag) and would allow no more than five stimuli in a row in one direction.
The initial stimulus was at the maximum peak velocity (30°/s or cm/s at 1 Hz, and 15°/s or cm/s at 0.5 Hz, Table 1). The maximum stimulus was limited by the maximum distance the device could move, which was 15 cm of translation and 15° of rotation. Although the device was capable of moving slightly further than these figures, these were the maximums used because motion was less smooth near the limits. After the direction was identified correctly twice in succession, the stimulus was made more difficult by decreasing its magnitude by 50%. Each single time the direction was identified incorrectly, the magnitude was doubled up to a maximum value (two up, one down). Fifty-five stimuli were given in each staircase so there were a total of 110 stimulus presentations (including null stimuli if given) in each block of trials. Having direction specific staircases permitted independent adjustment of the stimulus magnitude in each direction.
A potential problem with this method was that if the thresholds were asymmetric, the subject might be aware from other cues (such as noise or vibration) that the magnitude of the stimuli was greater in one direction than the other. It would then be possible for perception of the stimulus magnitude alone to provide cues to which staircase they were on and hence their direction of motion. Preliminary experiments using this method in four subjects found one subject who was able to use this technique to decrease the asymmetry of his thresholds. To address this issue, the algorithm allowed each staircase to present a stimulus in the opposite direction (e.g., the right staircase would sometimes present a stimulus to the left) when the level of the two staircases differed in magnitude. If the staircases were one level apart, the probability of delivering a stimulus in the opposite direction was 30%. If the staircases were separated by two or more levels, the probability of delivering an opposite direction stimulus was 50%. This made it so subjects could not identify from which staircase a stimulus was presented using magnitude alone. A maximum of two opposite direction stimuli were delivered consecutively. The accuracy of responses entered for the opposite direction stimuli were not used to adjust the level of the staircase, so that the stimulus magnitude would continue to reflect only the accuracy of responses in the primary direction of the staircase.
To determine if asymmetries were due to biased guessing in one direction, we introduced stimulus presentations where no motion was delivered (i.e., null stimuli). Because the platform has some amount of vibration and sound associated with motion, preliminary experiments revealed that stimulus presentations in which no motion occurred were obvious to the subject. Instead, a null stimulus was designed to be the same duration as the other stimuli but formed from a sum of three sine waves at 1.2, 2, and 5 Hz, each of which had a magnitude of 0.3 mm. Multiplying this sum of sine by half a sine wave ensured that the motion would begin and end at the origin. Thus, the maximum displacement of the motion was less than 1 mm, and the net displacement was zero. A similar stimulus of appropriate duration was given after the response was entered to simulate a return to the origin. The null stimulus only occurred after at least 10 stimuli had been presented in a staircase. Thus, these null stimuli would only be included when the motion was near the subject’s threshold to avoid alerting the subject that a null stimulus was given. The null stimulus occurred at a 20% probability starting with the 10th stimulus presentation so that on average 18 null stimuli were delivered during the experiment.
In nine subjects, at least one and often several blocks of trials were repeated using similar testing conditions on a different testing session and day. This was done to test the stability of responses over multiple testing sessions. The exact number of repetitions for a given testing condition depended largely on the availability of the subject, but conditions that demonstrated larger directional differences in thresholds tended to be repeated. To prevent biasing, the statistics on population were calculated using only a single mean value when same condition was tested multiple times in a single subject.
Subjects
A total of 24 subjects participated in the experiment. Sixteen were male and eight female. Ages ranged from 21 to 68 (39 ± 17, mean ± standard deviation). Experimental time was limited to 90 min in each session to maintain subject alertness. A block of trials usually took 15 min to complete. Most subjects participated in multiple sessions. Informed consent was obtained from all participants. The protocol was approved by the University of Rochester Research Science Review Board.
Subjects were screened prior to participation. The screening included caloric testing, an audiogram, visual acuity testing, and screening questions to rule out any known history of vestibular disease or cognitive deficit. Based on these results, the subjects had normal peripheral vestibular function and hearing.
Analysis
The data was analyzed to determine thresholds using three methods. Initially, responses were analyzed by plotting the peak stimulus velocity against the fraction of responses in the positive direction (e.g., right, forward, up). Using this technique, the data could be fit to a Gaussian cumulative distribution function specified by a sigma (σ) and mean (μ). This method was known as the single sigma (SS) cumulative Gaussian function described by the following equation:
This Eq. (4) can describe asymmetries using only an offset of the mean or bias. However, this function frequently did not fit the observed data well, such as that shown in Figure 4A.
A second method was devised to explore the possibility that the responses might be better described using a hybrid dual sigma (DS), psychometric function with a separate sigma on the positive (σ p ), and negative (σ n ) side of the mean (σ):
Although such a function is continuous because the mean (μ) is the same on both sides, the derivative is not continuous because the slope is different on each side of the mean. This limited the methods that could be used to fit parameters of this function to the data.
It would be expected that the DS function would fit any data set better because it has three parameters instead of two for the SS function. The best fit to the data collected for a subject’s responses was determined for both functions using a general purpose numerical method previously described (Coleman and Li 1994, 1996). To determine the relative quality of the two models, we applied the Akaike information criterion (AIC) (Akaike 1992)and Bayesian information criterion (BIC) (Schwarz 1978) both of which consider the accuracy of the fit and assign a penalty for including extra parameters. They were computed using the number of data points (n), sum squared error (SSE), and number of free parameters (k) as follows; with more negative values indicating the model yields a better characterization of the data:
Because the number of data points in each condition was always more than 7, the BIC always gave a bigger penalty to the model with more parameters.
The third method of measuring response asymmetry was also investigated by separately fitting functions to data collected in each direction using the log of velocity (LV) as shown in Figure 4B and similar to that suggested in a recent review (Merfeld 2011). For responses well below the threshold, it would be expected that the accuracy of response would be due to chance alone or 50% (assuming an unbiased response, which the prior methods supported). Thus, the Gaussian function was fit over 0.5 to 1.0 (Fig. 4B). The threshold was defined as the mean of the best-fitting cumulative Gaussian when plotting the log of the absolute value of velocity against the percent of correct responses. The mean of such a function occurs at the point where 75% of responses are correct. Using the previously described SS and DS methods, the sigma identifies the 84% correct point; thus, the LV method tended to estimate slightly lower thresholds. This method allowed fitting of a standard Gaussian function which had a continuously defined derivative and permitted use of an established Monte Carlo maximum-likelihood criteria allowing for a small lapse rate as previously described (Wichmann and Hill 2001a, b) and used by others (Fetsch et al. 2009; MacNeilage et al. 2010). The bootstrapping method of this technique permitted fits to be repeated several times to determine the range of uncertainty in the curve fit parameters which was not possible using the fitting method applied to the SS/DS techniques.
The asymmetry of responses was calculated using an asymmetry index (AI), which was defined as the log base 2 of the threshold in the positive direction (i.e., right, forward, or up) divided by the threshold in the negative direction (left, backward, or down) using the LV data. Thus, if the two thresholds were equal, the AI would be zero; if the threshold to the right were twice that of the left, the AI would be 1; and if the threshold to the left were four times, the threshold to the right the AI would be −2.
To determine if an AI was significant for a given block of trials, it was calculated using the bootstrapping method as described above (Wichmann and Hill 2001a, b). Thresholds from 2,000 pairs of curve fits were compared to psychometric functions for opposite direction responses so that 2,000 AIs were determined for each run. After the mean value was found, the number of AIs on the opposite side of zero was used to determine a p value. Thus, if in 20 sets of fits (1%) the AI were on the opposite side of zero, the p value would be 0.01.
Student’s t test was used to compare continuous data, and statistical significance was defined as p <0.05. Differences in frequencies of responses for null stimuli were compared using the Pearson chi-square test. Spearman’s rank-order coefficient was used to determine correlation between variables. To determine reproducibility of thresholds in the subset of subjects in whom a run of trials was repeated on multiple days, ANOVA was used. Confidence intervals for responses to null stimuli were calculated assuming a normal distribution (Macmillan and Creelman 2005) where H is the fraction of responses of one type and N is the number of responses:
Subjects were separated into two groups to determine the potential effects of age. Subjects were divided by the age of 50 years. This age was chosen to create a significant age gap between the two groups with an older group representative of ages where balance disorders typically occur and the younger group more representative of the ages of subjects used in prior studies of vestibular perception. Sixteen were younger than 50 and eight were older than 50.
Results
Motion perception thresholds were measured for yaw, sway, surge, and heave. Stimuli were delivered at both 0.5 and 1.0 Hz in separate blocks of trials. Because of the time involved and the availability of subjects, not all conditions could be tested in all subjects. There were four types of motion at two frequencies, or a total of eight test types. All subjects completed at least four test types with the average being 5.8 ± 1 test types completed (mean ± SD). Data from a single subject in one test type (surge at 0.5 Hz) is shown in Figure 4. As displayed in panel A (Fig. 4A), the responses suggest the sigma of the psychometric function may be different in the forward and backward directions. Thus, a DS Gaussian function may better characterize the responses in both directions. In panel B (Fig. 4B), the same data is plotted as the fraction of correct responses with the stimulus plotted on a logarithmic scale, again depicting the asymmetry in this subject’s responses.
Direction specific perceptual thresholds determined using the LV method are plotted for each test type by subject number in Figure 5. In subjects where the test was repeated in multiple sessions, the average for the sessions was reported. The DS method yielded direction specific sigmas that were qualitatively similar to the means found using the LV method. For the positive direction, there was a significant correlation (R = 0.70, p < 0.001) with the mean from the LV method averaging 43% of the sigma from the DS method. A similar correlation existed in the negative direction (R = 0.67, p < 0.001) with the mean from the LV method averaging 41% of the sigma from the DS fit. Because the LV and DS method produced qualitatively similar direction specific thresholds, asymmetry was reported using the LV method because it offered the advantage of being able to use bootstrapping to produce confidence intervals.
In a few subjects and conditions, the subject could not reliably determine the direction of the stimulus even at the maximum stimulus for that condition. During heave (up–down) at 0.5 Hz, the threshold could not be determined for two subjects (#22 and #14) in either direction, and in a single direction in an additional four subjects (#4, #5, #6, and #9). In one of these subjects (#22), motion thresholds for backwards motion could also not be determined at 0.5 or 1.0 Hz. All of these subjects were older than 50, with the exception of one 21-year-old subject (#23) who could not reliably identify sway towards the right at 1 Hz. Thus, results for these conditions are not reported, although, it can be assumed that the threshold was beyond that which could be measured using the current apparatus and experimental design (20 cm or °/s for 1 Hz, and 10 cm or °/s at 0.5 Hz).
Potential bias
The bias was determined from the mean of both SS and DS Gaussian fits such as that shown in Figure 4A. The mean tended to be larger in blocks of trials when the sigma was larger, but when expressed as a fraction of sigma, the absolute value of the mean was 21% of the standard deviation for a SS Gaussian fit and 26% of the averaged of the positive and negative standard deviations for a DS Gaussian fit. The small difference in the means between the two techniques was significant (p = 0.01, t test), although both methods demonstrated only slight deviation from zero.
To further test the possibility that some subjects may have a bias, null stimuli were included in trial blocks completed by 12 of the 24 subjects for a total of 46 blocks of trials. A mean of 18 ± 4 (mean ± SD, range 10 to 27) null stimuli were delivered during the average trial block that included null stimuli.
In three subjects who were familiar with the design of the experiment, trial blocks were conducted to determine their ability to differentiate null stimuli from motion stimuli near their vestibular threshold. These stimuli could not be differentiated better than by chance. The remaining subjects were not aware at the time of testing that some stimuli did not include any net motion.
In only two of the 46 blocks of trials that included null stimuli was 0.50 outside the 95% confidence interval for the subject’s responses, no different than that expected by chance. In two subjects (#1 and #10), test conditions that were known to have significant perceptual asymmetries (sway at 0.5 Hz for #1 and heave at 0.5 Hz #10) were repeated multiple times with the presence of null stimuli to improve statistical power, but data from these combined blocks of trials were also not significantly different from 50 to 50 even though the asymmetry persisted. These results confirm that the asymmetries noted cannot be explained by biased guessing in one direction as this bias was not seen with null stimulus presentations.
The possibility that asymmetry might be correlated with guessing one response more frequently than the other was investigated by plotting the AI against the fraction of positive responses (i.e., right, up, or forward) (Fig. 6), but these measures were poorly correlated (R = 0.25) and the trend was not significant (Spearman’s rank–order correlation coefficient, p = 0.13).
Direction specific thresholds
The hypothesis that the data was best characterized by asymmetries in directional sensitivity was tested by comparing the fit of the SS Gaussian to that of the DS Gaussian. The average sigma using the SS technique was 2.41. The average of the sigma in each direction using the DS technique was 2.56. Applying the t test to the SS and mean DS sigma, there was no significant difference (p > 0.1). Because the SS fit is a special case of the DS fit when sigma is the same in both directions, it would be expected that the DS method would always provide an equal or better fit to the data when characterized by goodness-of-fit measures such as sum-squared error. The AIC and BIC were applied to determine whether this improvement was sufficient to justify the addition of an extra parameter. In cases where a single subject was tested in multiple runs using the same conditions across multiple sessions, the average value of these runs was used. In 86% of runs, the AIC was improved and in 75% of runs the BIC was improved using the DS model. As expected, the DS model was more likely to be better when the direction specific sigmas were further apart. The larger sigma was 82% larger than that of the opposite direction when the DS model was favored but only 7% larger when the SS model was favored by the BIC. The difference between direction specific sigmas was more likely to be larger when the DS model was favored (p < 0.001).
Response variability
Thresholds were re-measured in a subset of subjects during different sessions. Frequently, one testing condition would be repeated from a prior session as a test of consistency. In nine subjects (#1, #8, #10, #11, #12, #17, #19, #20, and #21), blocks of trials were repeated in later sessions with similar conditions on a different day, and data from five of these subjects are shown in Figures 7 and 8. Repeated blocks of trials are shown as separate data points in Figure 9.
The combined data fit to a single psychometric function. The combined trials for each panel are the same trials analyzed individually in the corresponding panel of Figure 7. The symbol size is proportional to the number of stimulus presentations in each panel. The largest symptoms represent as few as 26 stimuli (B) to more than 120 (A). The means (75% correct) of these psychometric functions are given in the column marked C in Figure 7.
Asymmetry index (AI) by subject. Only data in which thresholds could be determined in both directions are show. In subjects in whom same condition was tested multiple times, multiple data points are shown. Gray data points indicate that the threshold for that block of trials were significantly directionally asymmetric at the p <0.05 level, black data points indicate a significance of p <0.01. A positive AI indicates that the right, upward, or backward threshold was larger than the threshold in the opposite direction.
The greatest number of repeat trial blocks was in subject #1 who had a moderate asymmetry in right–left sway. Thresholds were independently determined on 11 sessions on 11 different days over an 18-month period. The thresholds remained stable in this subject and others with a consistent asymmetry on every day the subjects were tested (Fig. 7). Trials from different days could be combined allowing a single curve to be fit to the data as shown in Figure 8.
The issue of how reproducible the thresholds were when runs were repeated during subsequent testing sessions was addressed using ANOVA. The most number of repetitions (11) occurred in subject #1 for sway at 0.5 Hz. Applying a two-way ANOVA in this subject revealed a highly significant difference between rightward and leftward thresholds (p < 0.0001) with the direction of motion accounting for 86% of the variance. The day of testing accounted for only 4% of the variance with a p value similar to chance (p = 0.52) indicating the thresholds were highly reproducible over time in this subject. In seven subjects, a block of trials was repeated on at least 3 days. A two-way ANOVA was repeated in this subset of subjects using the thresholds determined during first 3 days of testing. In this larger group of subjects, there was a significant effect of movement direction (p = 0.03). This occurred even though some subjects in whom thresholds were repeated did not have a significant asymmetry (i.e., subject #8 in yaw as shown in Figs. 7 and 8). However, the direction of motion accounted for 24% of the total variance with the day of testing accounting for only 0.2% of the variance. The day of testing had no significant effect on direction specific threshold (p = 0.87).
In a total of four subjects (#1, #8, #10, and #11), preliminary trial blocks were conducted that did not include opposite direction stimuli. In all of these subjects, the asymmetry in one or more conditions was equal or larger when opposite direction stimuli were included. Because of this difference, only blocks of trials including opposite direction stimuli were included in the experiment, and the preliminary trials are not reported. Data from repeated test conditions in all the subjects who had repeat trial blocks are shown using separate symbols in Figure 9.
Directional asymmetry in thresholds
An asymmetry index (AI) was calculated to quantify the degree of directional asymmetry present in each subject. This index is the log-base-2 of the threshold in one direction relative to the opposite direction, such that a perfectly symmetric response would yield an AI of zero, and threshold twice as high in the positive direction when compared with the other would be 1, and four times as high would be 2. Negative values represent asymmetry in the other direction. These values were highly variable between subjects, but remained relatively consistent when re-tested in subsequent sessions (Fig. 9). In these cases, the sessions were analyzed separately and the p values/AIs were the average from all sessions. When stimuli from multiple test sessions in the same subject and test condition were combined in this way, 14% of subjects for each test condition had a significant asymmetry at the p <0.01 level and 27% had a significant asymmetry at p <0.05 (Table 2). Across the eight test conditions for each subject (i.e., if there were multiple sessions for one subject, only the average value for that subject was included), the average AI was −0.06 ± 1.28 (mean ± SD) with a root mean squared (RMS) of 1.37. Only two subjects demonstrated no significant asymmetries on any of the conditions that were tested. Thus, 92% of subjects demonstrated a significant asymmetry in at least one test type. When the p values for the differences in opposite directions were averaged during repeated tests of the same type within a subject, the average p value remained p <0.05 in 28% (range 0 to 75%, SD 23%) of test conditions within each subject. Thus, threshold asymmetries tended to be limited to a specific direction of motion rather than being ubiquitous within a given subject.
To evaluate if some test conditions produced a higher AI than others, the absolute value of the AI was determined for each subject and test condition. To prevent biases from subjects who completed a test type multiple times, only the average for each subject for that frequency and motion type was used. ANOVA revealed that neither frequency (0.5 or 1.0 Hz) nor motion type had a significant effect on AI (p > 0.1 for both).
Directional asymmetries occurred in some individuals and were generally not consistent across the population. The one possible exception to this is heave at 0.5 Hz when the mean threshold for upward motion was 5.7 ± 2.3 cm/s and the threshold for down was 3.8 ± 2.0 cm/s. These were of borderline significance (p = 0.03). However, the difference may actually be overestimated because four subjects could not reliably identify the maximum stimulus in one direction during heave at 0.5 Hz, and this was in the down direction for three of these subjects. For the other testing conditions, the mean thresholds for the population were similar in both directions (p > 0.05 for all).
A mode of self-motion that tended to produce an asymmetry at 0.5 Hz in a subject also tended to be asymmetric at 1.0 Hz in that subject with a significant correlation (p = 0.004) (Fig. 11) and a slope of 1.07 (95% CI 0.77–1.63). This demonstrates that asymmetries can be consistently observed using these methods, even across different frequencies.
The possibility that asymmetry might be correlated with guessing one direction more frequently than the other was investigated by plotting the AI against the fraction of positive responses (i.e., right, up, or forward) (Fig. 5), but these measures were poorly correlated (R = 0.25) and the trend was not significant (Spearman’s rank-order correlation coefficient, p = 0.13). In the two runs when the response to null stimuli was significantly different (p < 0.05) than 50–50 (Fig. 5, marked with asterisk), responses both showed an AI whose absolute value was less than unity so this bias was not the cause of a significant directional asymmetry.
Thresholds of translation perception
Mean thresholds for translation measured as a function of stimulus peak velocity at 0.5 Hz and averaged across subjects and directions (with repeated conditions in one subject treated as a single value per individual) were 2.3 ± 1.4, 2.3 ± 1.7, and 4.8 ± 1.4 cm/s for sway, surge, and heave, respectively (Fig. 10). The thresholds at 1.0 Hz were significantly lower than those at 0.5 Hz (p < 0.01 for all three) at 0.8 ± 0.5, 0.9 ± 1.0, and 2.6 ± 3.0 cm/s for sway, surge, and heave, respectively. The thresholds for sway and surge were not significantly different from each other (t test, p > 0.1 for both). Comparison between types of motion showed that thresholds for surge and sway were significantly lower than those for heave at both 0.5 and 1.0 Hz (p < 0.01 for all).
When expressed in terms of peak acceleration, the influence of stimulus frequency was diminished. At 0.5 Hz, the thresholds were 3.6 ± 2.2, 3.7 ± 2.7, and 7.5 ± 3.8 cm/s/s for sway, surge, and heave, respectively. At 1.0 Hz, these thresholds were similar at 2.6 ± 1.6, 3.0 ± 3.2, and 8.4 ± 9.3 cm/s/s. In the acceleration domain, the differences by frequency were only significant for sway (p < 0.01) and not for surge (p > 0.1) or heave (p = 0.07).
Thresholds of rotation perception
For yaw rotation, the mean thresholds were 1.3 ± 0.9°/s at 0.5 Hz and 0.9 ± 0.7°/s for 1.0 Hz (p > 0.1). When expressed as angular acceleration, the mean threshold at 0.5 Hz was 2.0 ± 1.4°/s/s and at 1.0 Hz was 3.0 ± 2.3°/s/s, which was a statistically significant difference (p = 0.01).
Age effects
Examination of the data indicated that in many conditions, a subset of subjects older than age 50 demonstrated higher thresholds (Fig. 12). For the purposes of analysis, patients were divided into two groups: under 50 and over 50.
Only one subject over age 50 performed the rotation thresholds at 1 Hz so it was not possible to form a useful comparison, although this subject had the highest threshold for that condition (2.5°/s). At 0.5 Hz, the group under 50 had a mean threshold of 1.2 ± 0.7°/s and the other group’s mean threshold was 1.5 ± 0.7°/s; however, this difference was not significant (p > 0.1).
Mean translational thresholds were higher in the group over 50 for both frequencies in every category (Table 3), although the difference was not significant for surge at 1 Hz. This supports the idea that otolith function deteriorates with age and shows that the decline can be seen in perceptual testing as well as physiologic studies.
There was no evidence that the AI was correlated with the subject’s age. There was a poor correlation between age and the fraction of asymmetric trial blocks (R = 0.04), which was not statistically significant (Spearman’s rank-order correlation coefficient, p = 0.25). There was also a poor correlation when the average asymmetry for each subject was considered (R = 0.18), which was also not significant (Spearman’s rank-order correlation coefficient, p > 0.5). Thus, although thresholds are higher in older individuals, the system is affected similarly in both directions and no increase in asymmetric responses is noted.
Other potential effects
Gender had no significant effect on thresholds or the asymmetry index for any of the conditions tested. The potential effect of handedness was considered, but because only one subject was left handed (#17), it was not possible to make a meaningful comparison.
Discussion
The primary motivation for this study was to establish norms of human vestibular perception that could be useful for diagnosis when compared to patients with vestibulopathy. There have been several prior studies that have established vestibular perceptual thresholds of healthy humans (Walsh 1961; Clark 1967; Gundry 1978; Melvill Jones and Young 1978; Benson and Brown 1989; Gianna et al. 1996; Grabherr et al. 2008; MacNeilage et al. 2010; Mallery et al. 2010; Soyka et al. 2011). However, these studies have not provided two important details: possible effects of aging and possible directional asymmetries. Prior work in the field has focused on a young population that almost never included subjects beyond the age of 40, even though it has been shown that age does effect perception of steady-state translation (Kingma 2005). Because the prevalence of vestibular disorders is much higher after the age of 50 (Agrawal et al. 2009), there is a need for normative data in older individuals. Prior studies have not investigated possible dynamic asymmetries in normal perception beyond an observation that subjects were more likely to report uncertainty in direction when moving upward relative to downward (Benson et al. 1986). Other tests of vestibular function, such as the VOR and VEMP, consider asymmetry outside the normal range as an important diagnostic clue.
In experiments using psychophysics, there is often a tradeoff between the number of stimulus presentations (i.e., time required to complete an experiment) and the precision of the result. The method described here is relatively rapid compared to some that have been described previously. Because a goal of this research was to develop a technique that could be applied to clinical populations, maximizing efficiency was important. Typically more than 55 stimulus presentations per psychometric function are required to obtain good quality psychometric fits (Wichmann and Hill 2001a). However, our aim was to develop a technique that would be feasible in a clinical setting. In order to reduce testing time, we limited the number of stimulus presentations. The consequence, in terms of variability in threshold measures obtained, can be observed in the data from subjects who were tested multiple times (Figs. 7 and 8) and in the data collected at both frequencies (Fig. 11). Based on repeated measurements and ANOVA of the direction specific thresholds in a subset of subjects (Figs. 7, 8, and 12), we found that thresholds determined by the technique varied little between testing sessions.
There is now some consensus on the range of normal perceptual thresholds (Merfeld 2011). The threshold for yaw rotation in the range of 0.5 to 1.0 Hz has recently been reported near 0.7°/s (Grabherr et al. 2008). The mean values at 0.3 Hz were previously reported as 1.5°/s (Benson et al. 1989). In our cohort, the mean thresholds for rotation were 1.3 ± 0.9°/s at 0.5 Hz, and 0.9 ± 0.7°/s for 1.0 Hz (p > 0.1), which is within the range of these prior reports. The slightly higher thresholds in our study relative to that found by Grabherr et al. may be because our cohort includes individuals significantly older than those in the earlier study, making these thresholds more generalizable to the population.
Most recent studies have reported thresholds of linear motion perception in acceleration. At 0.3 Hz, the threshold of 15.4 cm/s/s for supine patients about a dorsal–ventral axis and 5.7 cm/s/s in sway were reported (Benson et al. 1986). At 0.5 Hz, the thresholds in this study were near 8 cm/s/s for heave and 4 cm/s/s surge and sway. MacNeilage et al. reported thresholds of 9.7 and 6.3 cm/s/s in heave and sway, respectively, which is also similar to the current findings (MacNeilage et al. 2010). The MacNeilage et al. paper looked for asymmetries at the population level and did not find them, although individual asymmetries were not reported.
Perceptual asymmetries
A common assumption of perceptual psychophysics is that the psychometric function is symmetric about a mean value. This assumption has been successfully challenged for contrast sensitivity (Burkhardt et al. 1984; Garcia-Perez and Alcala-Quintana 2009) and time perception (Wackermann and Spati 2006). However, symmetry is still assumed in the experimental design and analysis of most psychophysical experiments (Treutwein 1995; Leek 2001; Macmillan and Creelman 2005). In many prior vestibular perception studies, it has been assumed in both the design of the experiment and analysis of the responses that perceptual thresholds are similar in opposite directions of movement (Melvill Jones and Young 1978; Benson et al. 1989; Grabherr et al. 2008; MacNeilage et al. 2010).
Directional asymmetries in vestibular reflexes are the basis of clinically useful tests such as caloric responses (Bárány 1921; Becker 1979; Jacobson and Means 1985), head thrust (Halmagyi and Curthoys 1988), vertical axis rotation (Baloh et al. 1977), and VEMP (Welgampola and Colebatch 2005). Measurement of subjective visual vertical during static testing and off axis rotation has also been used to detect asymmetries in otolith function (Kingma 2006). This testing measures the static response to a constant acceleration such as gravity.
Perceptual asymmetries may occur due to either bias or directional differences in dynamic sensitivity. Response bias is a common problem in forced choice experiments, and several methods of measuring it have been proposed (Macmillan and Creelman 2005). A recent review of vestibular psychophysics has hypothesized that asymmetries in motion perception may be due to bias (Merfeld 2011), but the only data cited are two subjects who were excluded from the author’s prior study and have not been published. Most calculations of response bias make an assumption that the underlying psychometric function is symmetric about the mean, which is not appropriate given the current evidence provided in this study. To measure response bias as directly as possible, we decided to measure the response in the absence of a stimulus. Preliminary results demonstrated that subjects were able to easily determine when no stimulus was delivered due to the absence of subtle vibration and sound cues that typically occurred with stimulus delivery. Subsequently, a null stimulus was designed that produced similar vibration and sound but no net motion. These results indicated that subjects tended to guess both directions about equally, and when bias was present, it was not associated with an asymmetry (Fig. 6).
There is additional evidence that these asymmetries are not due to response bias alone. All subjects tended to have asymmetries only during a subset of test conditions. If these asymmetries were due to preferential guessing, they would be likely to occur in all test conditions for that subject. When subjects were tested with the same parameters in multiple sessions, ANOVA indicated that the day of testing accounted for a minimal amount of variation in the threshold determinations. Thus, thresholds even when asymmetric tended to be consistent across multiple days when the same condition was tested, so this phenomenon is unlikely due to chance (Figs. 7, 8, and 12).
Finally, a bias implies that subjects perceive they are in motion even when they are stationary, which is, in essence, vertigo. Although this occurs pathologically, it is not commonly perceived in normal subjects.
In the current data, the BIC indicates that directional specific DS Guassian better explains the responses than a SS Guassian in three quarters of trial blocks, and the rate is even higher when the AIC is used. Even when a SS Guassian is used to fit data that is clearly asymmetric (Fig. 4), the best fit includes only a small bias.
This paper presents a method for determining direction specific vestibular thresholds by using separate direction specific staircases and fitting separate psychometric functions to responses in each direction, which is novel to the vestibular field. These results indicate that vestibular perception thresholds are direction specific in a fraction of healthy people (Fig. 6) and that, when present, asymmetries are consistently found during subsequent sessions (Figs. 7 and 8). However, when responses are averaged over the study population, most responses are symmetric (Fig. 10). Interestingly, the one condition where a systematic asymmetry was present was for heave at 0.5 Hz, where most subjects were more sensitive to downward motion. This is a similar finding with a comparable marginal level of significance as previously reported using a different technique (Benson et al. 1986).
A significant directional asymmetry in threshold was found 27% of the trial blocks and in at least one stimulus type in 92% of the subjects tested. These results indicate that such asymmetries are common and do not indicate vestibular pathology. The true incidence of directional asymmetries probably depends on how hard one looks for them. Increasing the number of stimulus presentations in each run and decreasing the step size in the staircase would allow more statistical power and perhaps demonstrate that smaller asymmetries are actually significant.
The origin of these perceptual asymmetries is unclear. Directionally asymmetric responses in the semicircular canals are well known as Ewald’s second law (Ewald 1892). However, these asymmetries have been studied almost exclusively during high velocity rotation, well above the threshold of perception. In addition, the asymmetry in the vestibulo-ocular reflex is minimal for low velocity rotation (Baloh et al. 1977; Katsarkas et al. 1995).
Some evidence exists that the otolith-based vestibular reflexes may be asymmetric. The medial area of the utricle is larger than the lateral area in humans (Rosenhall 1972) and three quarters of the neurons respond preferentially to ipsilateral tilt in monkeys (Fernandez and Goldberg 1976). Transient linear motion produces a linear VOR (LVOR) which is commonly asymmetric at high accelerations in healthy humans (Lempert et al. 1998; Crane et al. 2003) and more asymmetric after an acute unilateral vestibular lesion, although symmetry returns to the normal range over time (Lempert et al. 1998).
It is possible that the asymmetries seen in this study originate in the peripheral semicircular canals and otolith organs. Such asymmetries are likely well tolerated during daily activities because the vestibular system does not need a high degree of accuracy when responding to very low velocity stimuli such as those used in this study. It is likely that, when present, factors such as vision and proprioception play the predominate role in this domain.
The effect of aging
Age-related changes have previously been described for other tests of otolith function such as the LVOR in which age increases the latency of the response and decreases the sensitivity (Tian et al. 2002). In the study by Tian et al., subjects in their sixth decade had an LVOR sensitivity 56% of that seen in subjects in their twenties. The VEMP, which uses audio clicks to stimulate the sacculus, also declines with age (Nguyen et al. 2010). In comparing cervical (cVEMP) and ocular (oVEMP) results between subjects in their twenties and those older than 50, the amplitude of both responses was decreased by half in the older group. Perception of sinusoids in surge has been shown to decline with age, but no effect of age was seen with lateral motion (Kingma 2005). These prior axis dependent effects have been difficult to interpret because the thresholds found by Kingma et al. were significantly lower than those found by others (Benson et al. 1986; Gianna et al. 1996). In the current study, translational thresholds doubled on average after the age of 50 (Table 3) for all three directions of translation, which is similar to the studies of the LVOR and VEMP and consistent with utricular and saccular function decreasing by half.
The gain of the angular VOR in response to rapid whole body rotation decreases to an average of 87% that achieved in the second decade by the sixth decade (Tian et al. 2001). Another study of the angular VOR in response to yaw stimuli of 60 to 100°/s found responses in subjects older than 75 years to be 89% of the response of people age 18 to 39, but the difference was not significant (Baloh et al. 1993). Dynamic visual acuity with head rotation, which is test of the angular VOR as well as vision, also declines with age (Viciana et al. 2010). Thus, although angular VOR function declines with age, the decline is small compared with that seen in the LVOR and VEMP. In the current study, the perceptual thresholds of yaw rotation mirrored the effects of age for vestibular reflexes. The younger subjects had thresholds 80% of those in the older group, an effect of age that was not significant.
References
Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB (2009) Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med 169:938–944
Akaike H (1992) Data analysis by statistical models. No To Hattatsu 24:127–133
Baloh RW, Honrubia V, Konrad HR (1977) Ewald’s second law re-evaluated. Acta Otolaryngol 83:475–479
Baloh RW, Jacobson KM, Socotch TM (1993) The effect of aging on visual-vestibuloocular responses. Exp Brain Res 95:509–516
Bárány R (1921) Diagnose von Krankheitserscheinungen im Bereiche des Otolithenapparates. Acta Otolaryngol:434-437
Becker GD (1979) The screening value of monothermal caloric tests. Laryngoscope 89:311–314
Benson AJ, Brown SF (1989) Visual display lowers detection threshold of angular, but not linear, whole-body motion stimuli. Aviat Space Environ Med 60:629–633
Benson AJ, Spencer MB, Stott JR (1986) Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med 57:1088–1096
Benson AJ, Hutt EC, Brown SF (1989) Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med 60:205–213
Burkhardt DA, Gottesman J, Kersten D, Legge GE (1984) Symmetry and constancy in the perception of negative and positive luminance contrast. J Opt Soc Am A 1:309–316
Clark B (1967) Thresholds for the perception of angular acceleration in man. Aero Med 38:443–450
Coleman TF, Li Y (1994) On the convergence of reflective Newton methods for large-scale nonlinear minimization subject to bounds. Math Program 67:189–224
Coleman TF, Li Y (1996) An interior, trust region approach for nonlinear minimization subject to bounds. SIAM J Optim 6:418–445
Crane BT, Demer JL (1998) Human horizontal vestibulo-ocular reflex initiation: effects of acceleration, target distance, and unilateral deafferentation. J Neurophysiol 80:1151–1166
Crane BT, Tian J, Wiest G, Demer JL (2003) Initiation of the human heave linear vestibulo-ocular reflex. Exp Brain Res 148:247–255
Demer JL, Crane BT, Tian JR, Wiest G (2001) New tests of vestibular function. Ann N Y Acad Sci 942:428–445
Engstrom H, Bergstrom B, Rosenhall U (1974) Vestibular sensory epithelia. Arch Otolaryngol 100:411–418
Ewald EJR (1892) Physiologische untersuchungen über das endorgan des nervs octavus. Bergmann, Wiesbaden
Fernandez C, Goldberg JM (1976) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39:970–984
Fetsch CR, Turner AH, Deangelis GC, Angelaki DE (2009) Dynamic re-weighting of visual and vestibular cues during self-motion perception. J Neurosci 29:15601–15612
Garcia-Perez MA, Alcala-Quintana R (2009) Fixed vs. variable noise in 2AFC contrast discrimination: lessons from psychometric functions. Spat Vis 22:273–300
Gianna C, Heimbrand S, Gresty M (1996) Thresholds for detection of motion direction during passive lateral whole-body acceleration in normal subjects and patients with bilateral loss of labyrinthine function. Brain Res Bull 40:443–447, discussion 448–449
Grabherr L, Nicoucar K, Mast FW, Merfeld DM (2008) Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186:677–681
Gu Y, Watkins PV, Angelaki DE, DeAngelis GC (2006) Visual and nonvisual contributions to three-dimensional heading selectivity in the medial superior temporal area. J Neurosci 26:73–85
Gu Y, Angelaki DE, Deangelis GC (2008) Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci 11:1201–1210
Gundry AJ (1978) Thresholds of perception for periodic linear motion. Aviat Space Environ Med 49:679–686
Halmagyi GM, Curthoys IS (1988) A clinical sign of canal paresis. Arch Neurol 45:737–739
Jacobson GP, Means ED (1985) Efficacy of a monothermal warm water caloric screening test. Ann Otol Rhinol Laryngol 94:377–381
Kanayama R, Bronstein AM, Gresty MA, Brookes GB, Faldon ME, Nakamura T (1995) Perceptual studies in patients with vestibular neurectomy. Acta Otolaryngol Suppl 520(Pt 2):408–411
Katsarkas A, Galiana HL, Smith HL (1995) Vestibulo-ocular reflex (VOR) biases in normal subjects and patients with compensated vestibular loss. Acta Otolaryngol 115:476–483
Kingma H (2005) Thresholds for perception of direction of linear acceleration as a possible evaluation of the otolith function. BMC Ear Nose Throat Disord 5:5
Kingma H (2006) Function tests of the otolith or statolith system. Curr Opin Neurol 19:21–25
Knill DC, Pouget A (2004) The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci 27:712–719
Leek MR (2001) Adaptive procedures in psychophysical research. Percept Psychophys 63:1279–1292
Lempert T, Gianna C, Brookes G, Bronstein A, Gresty M (1998) Horizontal otolith-ocular responses in humans after unilateral vestibular deafferentation. Exp Brain Res 118:533–540
Liaw MY, Chen CL, Pei YC, Leong CP, Lau YC (2009) Comparison of the static and dynamic balance performance in young, middle-aged, and elderly healthy people. Chang Gung Med J 32:297–304
Lopez I, Honrubia V, Baloh RW (1997) Aging and the human vestibular nucleus. J Vestib Res 7:77–85
Macmillan NA, Creelman CD (2005) Detection theory: a user’s guide, 2nd edn. Psychology Press, East Sussex
MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE (2010) Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci 30:9084–9094
Mallery RM, Olomu OU, Uchanski RM, Militchin VA, Hullar TE (2010) Human discrimination of rotational velocities. Exp Brain Res 204:11–20
Melvill Jones GM, Young LR (1978) Subjective detection of vertical acceleration: a velocity-dependent response? Acta Otolaryngol 85:45–53
Merfeld DM (2011) Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Exp Brain Res 210:389–405
Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S (2005a) Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt&Translation. J Neurophysiol 94:199–205
Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S (2005b) Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during Translation and Tilt. J Neurophysiol 94:186–198
Nguyen KD, Welgampola MS, Carey JP (2010) Test–retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol 31:793–802
Perez N, Martin E, Garcia-Tapia R (2003) Dizziness: relating the severity of vertigo to the degree of handicap by measuring vestibular impairment. Otolaryngol Head Neck Surg 128:372–381
Rauch SD, Velazquez-Villasenor L, Dimitri PS, Merchant SN (2001) Decreasing hair cell counts in aging humans. Ann N Y Acad Sci 942:220–227
Rosenhall U (1972) Vestibular macular mapping in man. Ann Otol Rhinol Laryngol 81:339–351
Schwarz GE (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Soyka F, Robuffo Giordano P, Beykirch K, Bulthoff HH (2011) Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Exp Brain Res 209:95–107
Stefansson S, Imoto T (1986) Age-related changes in optokinetic and rotational tests. Am J Otol 7:193–196
Tian JR, Shubayev I, Baloh RW, Demer JL (2001) Impairments in the initial horizontal vestibulo-ocular reflex of older humans. Exp Brain Res 137:309–322
Tian JR, Crane BT, Wiest G, Demer JL (2002) Effect of aging on the human initial interaural linear vestibulo-ocular reflex. Exp Brain Res 145:142–149
Treutwein B (1995) Adaptive psychophysical procedures. Vision Res 35:2503–2522
Viciana D, Ferrer J, Palma MJ, Zapata C, Lopez-Escamez JA (2010) Dynamic visual acuity during head-thrust test in canal planes in healthy subjects and patients with vestibular neuritis. Acta Otolaryngol 130:1260–1266
Wackermann J, Spati J (2006) Asymmetry of the discrimination function for temporal durations in human subjects. Acta Neurobiol Exp (Wars) 66:245–254
Walsh EG (1961) Role of the vestibular apparatus in the perception of motion on a parallel swing. J Physiol 155:506–513
Welgampola MS, Colebatch JG (2005) Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology 64:1682–1688
Wichmann FA, Hill NJ (2001a) The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys 63:1314–1329
Wichmann FA, Hill NJ (2001b) The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys 63:1293–1313
Acknowledgments
This research was supported by a clinician-scientist grant from the American Otological Society and NIDCD K23 DC011298-01. We would also like to thank Justin Y. Chan for technical support and Greg deAngelis for his insight that was useful in designing these experiments and for reviewing a pre-publication version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roditi, R.E., Crane, B.T. Directional Asymmetries and Age Effects in Human Self-Motion Perception. JARO 13, 381–401 (2012). https://doi.org/10.1007/s10162-012-0318-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-012-0318-3