Abstract
Background
The accumulation of glucose degradation products (GDPs) during peritoneal dialysis (PD) can lead to immature angiogenesis in the peritoneum. However, the effect of GDPs on angiogenesis, at concentrations observed in dialysate effluent, has not been widely investigated. We do not know how the inflammation observed in PD-related peritonitis affects angiogenesis of the peritoneum.
Methods
Human umbilical vessel endothelial cells (HUVEC) and human umbilical aortic smooth muscle cells (HUASMC) were used to examine the response to the three main GDPs found in peritoneal dialysate (methylglyoxal (MGO), 3-deoxyglucosone (3-DG), and 5-hydroxymethylfurfural (5-HMF). Supernatant from lipopolysaccharide (LPS)-activated murine macrophage cell lines (RAW 264.7 cells) were used to stimulate angiogenesis in the peritoneum. Changes in the expression of vascular endothelial growth factor-A (VEGF-A) and platelet-derived growth factor B (PDGFB) in HUVEC, and PDGF-receptor beta (PDGF-Rβ) in HUASMC, were examined by real-time PCR, Western blot, and ELISA.
Results
In HUVECs, the expression of PDGFB mRNA and protein were decreased by exposure to MGO, 3-DG, and 5-HMF at concentrations observed in dialysate effluent. A subsequent decrease in secreted PDGF-BB was observed. In HUASMCs, MGO and 5-HMF increased the expression of VEGF-A mRNA and protein, while 5-HMF decreased the expression of PDGF-Rβ. VEGF-A is upregulated, and PDGF-Rβ is downregulated, by conditioned medium of LPS-stimulated macrophages in HUASMCs.
Conclusions
The GDPs of PD effluent cause an imbalance of angiogenic factors in endothelial cells and vascular smooth muscle cells that may lead to immature angiogenesis in the peritoneum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
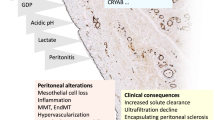
Encapsulated peritoneal sclerosis (EPS) is a severe complication of long-term peritoneal dialysis (PD) that is characterized by intraperitoneal inflammation and fibrosis, which at times results in the encasement of bowel loops. Fibrosis and angiogenesis are reported to be characteristic features of chronic PD-related peritoneal damage [1]. Previous research has shown that angiogenesis in the peritoneal membrane is related to ultrafiltration failure that might lead to EPS in PD patients [2]. Physiological angiogenesis needs pericyte recruitment and covering endothelial cells (ECs) [3]. Impaired angiogenesis without pericyte has been reported in diabetic retinopathy, where it causes vascular leakage and increased permeability [4]. The glucose degradation product (GDP), methylglyoxal (MGO) is a reactive di-carbonyl compound. GDPs are produced during heat sterilization or storage of peritoneal dialysis fluid (PDF) [5]. Recently, we found that MGO increases production of vascular endothelial growth factor-A (VEGF-A) and suppresses the expression of platelet-derived growth factor-BB (PDGF-BB) in cultured ECs and that cumulative exposure of MGO in the peritoneum of PD patients was significantly associated with immature angiogenesis without pericyte coverage [6]. However, the concentration of MGO used in the prior studies was higher than that observed in dialysate effluent used in clinical applications [7]. Moreover, the effect of other GDPs that are commonly found in dialysate effluent (e.g., 3-deoxyglucosone (3-DG) and 5-hydroxymethylfurfural (5-HMF)) on impaired angiogenesis remains poorly understood. Thus, the aim of this study was to evaluate whether GDPs effect angiogenesis, not only in ECs but also in vascular smooth muscle cells (VSMCs).
Materials and methods
Primary cells and culture
Human umbilical vessel endothelial cells (HUVEC) (Lonza, Walkersville,
MD, USA) were cultured in endothelial cell growth medium with 5% fetal bovine serum (FBS) (EBM-2; Lonza) and human umbilical aortic smooth muscle cells (HUASMC) (Takara Bio Inc, Otsu, Japan) were incubated in DMEM (Nacalai Tesque, Kyoto, Japan) with 10% FBS (Biowest, Nuaillé, France), 100 U/mL penicillin and 100 mg/mL streptomycin (Life Technologies, Carlsbad, CA), in a humidified atmosphere with 5% CO2 at 37 °C. Confluent cells were starved for 12 h in serum free medium with MGO, 3-DG, or 5-HMF (Sigma-Aldrich Japan, Tokyo, Japan) for 12 or 48 h. Among the 2.5% glucose PD dialysate excluding the acidic solution, we chose the highest concentrations of each MGO (0.6 µM), 3-DG (130 µM), and 5-HMF (14.7 µM) based on the previous report [7]. A schema of GDPs in PDF with some modifications from the previous report [7] is shown in Supplementary Fig. 1. Cells from the mouse macrophage cell line RAW 246.7 (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM with 10% FBS and 100 U/mL penicillin and 100 mg/mL streptomycin in a humidified 5% CO2 incubator at 37 °C. The cells were seeded onto six-well cell culture plates at a density of 5.0 × 105 cells/mL. After overnight incubation, they were incubated with 0.1 μg/mL lipopolysaccharide (LPS) (Sigma-Aldrich Japan) for 24 h. The cell supernatant was recovered and centrifuged at 3000 × g for 10 min and the collected supernatant was used as stimulus for HUVECs and HUASMCs. The cells were harvested for RT-PCR after 12 h incubation or for western blotting and ELISA after 48 h. We used cells that had undergone 5–8 passages.
Real-Time PCR
Total RNA was extracted from cells using MAXWELL®16 LEV simply RNA tissue kit (Promega, Madison, WI, USA) and MAXWELL®16 instrument (Promega) according to the manufacturer’s instructions. Complementary DNA was purified from 1 µg of total RNA with the PrimeScript RT Reagent kit (Takara Bio Inc). RT-PCR was performed with SYBR Premix Ex Taq™ (Takara Bio Inc) and a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Relative expression levels of human VEGF-A: forward 5′-TCACAGGTACAGGGATGAGGACAC-3′, reverse 5′-CAAAGCACAGCAATGTCCTGAAG-3′; human PDGFB: forward 50-ACTAGAGGCCAGGGCAGCAA-3′, reverse 5′-TGCAGTACACACCCTGCACAAC-3′; human PDGF-Rβ: forward 5′-TCCAGGTGTCATCCATCAAC-3′, reverse 50-ACTTTCTTTGCGGGGGTATG-3′ in cells were determined by the ΔΔCT method using human ribosomal protein 18 s (5′-AAACGGCTACCACATCCAAG-3′ and 5′-CCTCCAATGGATCCTCGTTA-3′) as an internal reference.
Western blotting
Cell lysate was extracted with lysis buffer (mammalian protein extraction reagent, M-PER™; Thermo Scientific, Waltham, MA, USA with a protease inhibitor cocktail; Nacalai Tesque) and centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatant was analyzed. Protein samples (10 µg) were isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 5–20% polyacrylamide gradient gels (PAGEL; Atto, Tokyo, Japan) and applied onto a polyvinylidene difluoride membrane using Trans-Blot Turbo System (BioRad, AbD Serotec, Oxford, UK). Primary and secondary antibodies were diluted in antibody solution (signal enhancer HIKARI, Nacalai Tesque). After incubation with blocking solution (Blocking One, Nacalai Tesque) for 30 min, the membranes were incubated overnight at 4 °C with the primary antibodies. We used the following antibodies: rabbit VEGF-A antibody (ab46154, 1:1000; Abcam, Cambridge, MA, USA), rabbit PDGF-Rβ antibody (ab32570, 1:1000; Abcam), rabbit phospho-p44/42 MAPK (Erk1/2) antibody (9101, 1:1000; Cell Signaling Technology, Danvers, MA, USA), and rabbit p44/42 MAPK (Erk1/2) antibody (9102, 1:1000; Cell Signaling Technology). After being washed in Tris Buffered Saline with Tween 20 (TBS-T) three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies: anti-rabbit IgG antibody (NA934, GE Healthcare, Bucks, UK) for one hour. The bands were detected using an enhanced chemiluminescent kit (11644-40, Chemi-Lumi One Ultra; Nacalai Tesque), captured using a chemiluminescence imaging system (AE-9300 Ez capture MG; Atto). The density of each band was analyzed by ImageJ software (National Institutes of Health).
Enzyme-linked immunosorbent assay (ELISA)
Cell supernatant was recovered and centrifuged at 3000 × g for 10 min at 4 °C, and the collected supernatant was analyzed. To measure the concentration of VEGF-A and PDGF-BB in the supernatant, we used the human VEGF-A ELISA kit (ab119566, Abcam) and PDGF-BB Quantikine ELISA kit (DBB00, R&D Systems, Minneapolis, MN, USA). To measure the concentration of MGO in RAW 264.7 cells, we used a mouse MGO ELISA kit (MBS756134; MyBioSource, San Diego, CA, USA).
Cell tube formation assay
To obtain representative cell proliferation images, we visualized tube formation using HUVECs alone or co-cultured HUVECs and HUASMCs. A pre-thawed Matrigel matrix (Corning Inc., Corning, NY, USA) was added to a pre-chilled 96-well plate. HUVECs and HUASMCs were labeled with a cytomembrane green fluorescent probe and orange fluorescent probe, respectively (CellTracker fluorescence probes; Thermo Fisher Scientific). Fluorescence labeled cells were seeded onto the solidified Matrigel matrix at a ratio of 10:1 (HUVECs, 1.0 × 104 cells/well; HUASMCs, 1.0 × 103 cells/well) for 6 h. Endothelial tube formation was then observed using an all-in-one fluorescence microscope (BZ-9000; Keyence, Osaka, Japan).
Cell count of cultured HUVECs with supernatant of HUASMCs
The HUVECs were seeded onto 96-well cell culture plates at a density of 5.0 × 105 cells/mL. They were incubated with control medium or GDP-containing medium or culture supernatant from 24-h GDPs (MGO, 0.6 µM; 3-DG, 130 µM; and 5-HMF, 14.7 µM), PDGF-BB (10 ng/mL), and combination of them-stimulated HUASMCs. After 48 h of incubation, the nuclei of HUVECs were labeled using Hoechst 33,342 solution (Fujifilm Wako Chemicals, Osaka, Japan). Absorbance (excitation, 355 nm; emission, 460 nm) of the cultures was measured by a multimode plate reader (EnSight Plate Reader; PerkinElmer, Waltham, MA, USA).
Statistical analysis
Parametric variables with a normal distribution were expressed as mean ± SD. Parametric variables between two groups were compared using the Mann–Whitney U test and differences among groups were compared by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. All statistical analyses were performed with EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria), and a modified version of R commander designed to add statistical functions frequently used in biostatistics [8]. A two-tailed value of P < 0.05 was considered statistically significant. We drew graphs using GraphPad Prism version 6.0 for Windows (GraphPad Software).
Results
PDGF-BB is downregulated with MGO, 3-DG, and 5-HMF in ECs
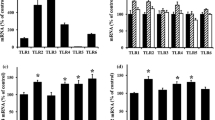
We first investigated whether GDPs increase VEGF-A expression in HUVECs. RT-PCR analysis showed that MGO, 3-DG, and 5-HMF (hereafter referred to as GDPs) did not affect VEGF-A mRNA expression in HUVECs (Fig. 1a). Next, we investigated if GDPs affect the expression of PDGFB mRNA. Even low concentrations of GDPs, such as those observed in peritoneal dialysis fluid, suppressed the expression of PDGF-BB in HUVECs (Fig. 1b). The decrease in PDGF-BB protein is consistent with the decrease in PDGFB mRNA. The expression of VEGF-A, determined by western blotting, was not altered by GDPs (Fig. 1c–e). The GDPs had no effect on VEGF-A secretion in HUVECs (Fig. 1f). Whereas PDGF-BB secreted from HUVECs was suppressed by GDPs (Fig. 1g).
Expression of angiogenic factors in endothelial cells exposed to glucose degradation products. a VEGF-A mRNA expression determined by real-time PCR in HUVECs. n = 3 each. b PDGFB mRNA expression determined by real-time PCR in HUVECs. n = 3 each. c Western blotting of VEGF-A in HUVECs exposed to MGO. Upper panel, VEGF-A; lower panel, β actin as an internal control. Arrow indicates VEGF-A protein. Relative levels of VEGF-A protein normalized to β actin, n = 6 each. d Western blotting of VEGF-A in HUVECs exposed to 3-DG. Upper panel, VEGF-A; lower panel, β actin as an internal control. Quantification of VEGF-A normalized to β actin, n = 6 each. e Western blotting of VEGF-A in HUVECs treated with 5-HMF. Upper panel, VEGF-A; lower panel, β actin as an internal control. Relative levels of VEGF-A normalized to β actin, n = 6. f Secretion of VEGF-A from HUVECs treated with GDPs was measured by enzyme-linked immunosorbent assay, n = 6 each. g Secretion of PDGF-BB from HUVECs treated with GDPs was measured by enzyme-linked immunosorbent assay, n = 6 each. cont control, 5-HMF 5-hydroxymethylfurfural, MGO methylglyoxal, NS not significant, PDGFB, platelet-derived growth factor B, 3-DG 3-deoxyglucosone, VEGF-A vascular endothelial growth factor-A. *P < 0.05
In VSMCs, VEGF-A is upregulated, and PDGF-Rβ is downregulated, by GDPs
To investigate the effect of GDPs on pericytes, we examined HUASMCs that have similar properties to pericytes. The VEGF-A mRNA was significantly upregulated by MGO and 5-HMF (Fig. 2a). However, PDGF-Rβ mRNA, the receptor of PDGF-BB, was suppressed by MGO and 5-HMF but not by 3-DG in HUASMCs (Fig. 2b). Western blot analysis showed that MGO increased the expression of VEGF-A protein and tended to suppress protein levels of PDGF-Rβ in HUASMCs (Fig. 2c). There was no significant difference in the expression of both VEGF-A and PDGF-Rβ in the controls and 3-DG groups in HUASMCs (Fig. 2d). Whereas 5-HMF increased VEGF-A expression and decreased PDGF-Rβ expression compared to controls in HUASMCs (Fig. 2e). VEGF-A secreted from HUASMCs was significantly increased by MGO. In addition, 5-HMF also tend to promote VEGF-A secretion (Fig. 2f) (A significant difference was found in the comparison between the two groups, control and 5-HMF using the Mann–Whitney U test). Next, we assessed whether VEGF-A affects the expression of PDGF-Rβ in HUASMCs. HUASMCs were incubated with VEGF-165A (R&D Systems), a splicing variant of VEGF-A, at the concentrations used in a previous in vitro study [9] for 24 h. The expression of PDGF-Rβ, determined by RT-PCR and western blotting, was not suppressed by VEGF-165A stimulation (Supplementary Fig. 2a, b). These findings indicate that MGO and 5-HMF downregulate PDGF-Rβ independently of increasing VEGF-A in HUASMCs. Furthermore, we investigated whether cell signaling under the PDGF-BB stimulus is suppressed in HUASMCs incubated with MGO and 5-HMF, which are PDGF-Rβ suppressors. HUASMCs were incubated with PDGF-BB at 10 ng/mL (PeproTech, Inc., Cranbury, NJ, USA) in accordance with a previous study [10] in the presence of MGO and 5-HMF. ERK phosphorylation was significantly upregulated by PDGF-BB in HUASMCs, as previously reported. However, PDGF-BB-mediated ERK phosphorylation was suppressed in the presence of 5-HMF, which inhibited the expression of PDGF-Rβ in HUASMCs. MGO with the PDGF-BB stimulus also tended to inhibit PDGF-BB-mediated ERK phosphorylation in HUASMCs (Fig. 2g). These results indicate that MGO and 5-HMF suppress PDGF-BB-mediated downstream cell signaling through downregulation of PDGF-Rβ in HUASMCs.
PDGF-Rβ expression in vascular smooth muscle cells was decreased by glucose degradation products. a VEGF-A mRNA expression determined by real-time PCR in HUASMCs incubated with GDPs. n = 3 each. b PDGF-Rβ mRNA expression determined by real-time PCR in HUASMCs exposed to GDPs, n = 3 each. c Western blot analysis of VEGF-A and PDGF-Rβ in HUASMCs incubated with MGO. Upper panel, VEGF-A; middle panel, PDGF-Rβ; lower panel, β actin as an internal control. Arrow indicates VEGF-A protein. Quantification of VEGF-A normalized to β actin shown in c. n = 6 each. Relative levels of PDGF-Rβ protein normalized to β actin, n = 6 each. d Western blotting of VEGF-A and PDGF-Rβ protein in HUASMCs incubated with 3-DG. Upper panel, VEGF-A; middle panel, PDGF-Rβ; lower panel, β actin as an internal control. Quantification of VEGF-A and PDGF-Rβ protein normalized to β actin, n = 6 each. e Western blotting of VEGF-A and PDGF-Rβ protein in HUASMCs incubated with 5-HMF. Upper panel, VEGF-A; middle panel, PDGF-Rβ; lower panel, β actin as an internal control. Relative levels of VEGF-A and PDGF-Rβ normalized to β actin, n = 6. f Secretion of VEGF-A from HUASMCs treated with GDPs was measured by enzyme-linked immunosorbent assay, n = 4 each. g Western blotting of phospho-Erk1/2 and total-Erk1/2 protein in HUASMCs incubated with PDGF-BB and mixture of MGO or 5-HMF. Upper panel, phosphor-Erk1/2; lower panel, total-Erk1/2. Relative levels of phosphor-Erk1/2 normalized to total-Erk1/2, n = 4, each. cont control, 5-HMF 5-hydroxymethylfurfural, MGO methylglyoxal, NS not significant, PDGF-Rβ platelet-derived growth factor receptor beta, 3-DG 3-deoxyglucosone, VEGF-A vascular endothelial growth factor-A. *P < 0.05, **P < 0.01. # P < 0.01 with Mann–Whitney U test
Substances from VSMCs stimulated with GDPs promote cell proliferation in ECs
Next, we investigated the interaction between ECs and VSMCs. First, to obtain representative cell proliferation images, we visualized tube formation using HUVECs either alone or co-cultured HUVECs and HUASMCs. Representative images of co-cultured HUVECs and HUASMCs treated with GDPs are shown in Supplementary Fig. 3a. GDP-containing media tended to promote cell proliferation of HUVECs and disrupt tube formation. However, the co-culture system was not suitable for observing phenotypic changes in the HUVECs alone or for quantifying impaired angiogenesis. Hence, cell proliferation of HUVECs was observed by culturing HUVECs in the medium supernatant of HUASMCs that had been stimulated by the reagent (GDPs, PDGF-BB, or mixture) in advance. HUVECs were incubated for 48 h with culture supernatant from 24-h GDPs (MGO, 0.6 µM; 3-DG, 130 µM; and 5-HMF, 14.7 µM), PDGF-BB (10 ng/mL), and combination of them-stimulated HUASMCs. First, the VEGF-A mRNA from the cell lysate of 24-h GDPs, PDGF-BB, or combination of them-stimulated HUASMCs was assessed. The VEGF-A mRNA was significantly upregulated by GDPs, similar to the aforementioned result (Fig. 2a) in HUASMCs. PDGF-BB and the combination of GDPs and PDGF-BB further increased the VEGF-A mRNA in HUASMCs (Supplementary Fig. 3b). Next, we investigated whether those supernatants of HUASMCs affected the cell proliferation of HUVECs. Control and GDP-containing media alone did not affect the cell growth of HUVECs. In contrast, the supernatant from HUASMCs stimulated by GDPs in advance tended to contribute to cell proliferation of HUVECs. The supernatants from HUASMCs incubated with PDGF-BB and the combination of GDPs and PDGF-BB further enhanced the cell growth of HUVECs (Supplementary Fig. 3c). The finding that GDPs alone did not affect the cell proliferation of HUVECs is consistent with the finding that GDPs did not increase the expression of VEGF-A in HUVECs (Fig. 1a, c–f). In contrast, the culture supernatant from HUASMCs incubated with GDPs in advance might affect the cell proliferation of HUVECs, possibly through enhanced production of VEGF-A in HUASMCs.
Stimulus associated with peritonitis causes angiogenesis in ECs, however, affect impaired VSMC recruitment
To explore the effect of inflammation on the expression changes of VEGF-A, PDGF-BB, and PDGF-Rβ, we used culture supernatant from 24 h LPS-activated RAW 264.7 cells to stimulate HUVEC or HUASMC. The expression of VEGF-A and PDGFB mRNA was increased in HUVECs by macrophage supernatant stimulated by LPS (Fig. 3a, b). In HUASMCs, the levels of VEGF-A mRNA were increased (Fig. 3c). Meanwhile, the expression of PDGF-Rβ mRNA was remarkedly reduced by the culture supernatant of LPS-stimulated RAW 264.7 cells (Fig. 3d). These results were consistent with GDPs-induced changes in the expression of VEGF-A, PDGF-BB, and PDGF-Rβ. Next, we assessed whether LPS-stimulated RAW 264.7 cells may affect the aforementioned mRNA changes through secretion of MGO. MGO secretion was not enhanced in RAW 264.7 cells media regardless of LPS stimulation (0.1 μg/mL) (Supplementary Fig. 4a). This finding indicates that substances from RAW 264.7 cells stimulated with LPS affect mRNA changes involving angiogenesis in HUVECs and HUASMCs in an MGO-independent manner.
Expression changes of angiogenetic factors in endothelial cells or smooth muscle cells with conditioned medium of LPS-stimulated macrophage. a VEGF-A mRNA expression determined by real-time PCR in HUVECs incubated with conditioned medium. n = 3 each. b PDGFB mRNA expression in HUVECs incubated with conditioned medium. n = 3 each. c Quantification of VEGF-A mRNA expression determined RT-PCR in HUASMCs incubated with conditioned medium. n = 6. d Expression of PDGF-Rβ mRNA in HUASMCs incubated with conditioned medium. n = 6 each. cont control, 5-HMF 5-hydroxymethylfurfural, LPS lipopolysaccharide, MGO methylglyoxal, PDGFB platelet-derived growth factor B, PDGF-Rβ platelet-derived growth factor receptor beta, 3-DG 3-deoxyglucosone, VEGF-A vascular endothelial growth factor-A. **P < 0.01
Discussion
The present study assesses how GDPs affect the expression of angiogenic factors. At concentrations found in peritoneal dialysis fluid all GDPs tested (MGO, 3-DG, and 5-HMF) decreased expression of PDGF-BB in ECs. In VSMCs, MGO and 5-HMF increased the expression of VEGF-A, MGO and 5-HMF decreased the expression of PDGF-Rβ (the receptor for PDGF-BB secreted from ECs and VSMCs). Schema of normal angiogenesis is shown in Fig. 4a. Our data indicate that VEGF-A is upregulated by GDPs, and that PDGF-Rβ is downregulated in VSMCs. An immature angiogenesis by GDPs is illustrated in Fig. 4b. In normal angiogenesis, PDGF-BB is secreted from ECs along with VEGF-A. PDGF-BB is recognized by PDGF-Rβ in VSMCs, and VSMCs are recruited to the perivascular area. However, in impaired angiogenesis, GDPs induce overexpression of VEGF-A but the expression of PDGF-BB from ECs, and PDGF-Rβ from VSMCs is decreased, resulting in failure of recruiting VSMCs.
The primary isolated HUVECs are probably the most popular ECs used worldwide because human umbilical veins are relatively more available than other types of blood vessels. HUVECs express many important endothelial markers, e.g. ICAM-1, VCAM-1 and selectins, as well as signaling molecules associated with vascular physiology such as NO [11]. Moreover, HUVECs have been shown to be responsive to physiological and/or pathological stimuli such as high glucose, LPS and shear stress [12,13,14]. Thus, although human umbilical veins are only at certain stages of human life, HUVECs have been considered as a general model for ECs both in normal and diseased conditions [15]. While, VSMCs constitute the internal layer of the blood vessels, the tunica media. Together with ECs, VSMCs are responsible for the regulation of the blood flow and the immunomodulation of inflammatory processes in the vascular wall and the surrounding tissue. HUASMC is also taken from human umbilical cord arteries, easy and low-cost with the expression of VSMCs markers, such as alpha-smooth muscle actin, smooth muscle myosin heavy chain [16]. Moreover, HUVEC and HUASMC are used to investigate vascular sprouting, capillary extension, and stabilization [17]. Pericytes and VSMCs have been identified as vascular mural cells. They belong to the same lineage and form a morphological and functional continuum along blood vessels. Previous ultrastructural studies demonstrated that pericytes and VSMCs share common features [3, 18, 19]. To our knowledge, the culture method of primary peritoneal pericytes in animals has not previously been reported. Thus, we used HUVECs and HUASMCs as model to study capillary formation in peritoneum. The impaired angiogenesis phenomena shown using HUVECs and HUASMCs may also be observed in capillaries.
VEGF-A is a representative molecule for angiogenesis that promotes lumen formation by ECs [20]. ECs in the lumen recruit PDGF-Rβ-expressing pericytes by secreting PDGF-BB, leading to complete angiogenesis. Normal angiogenesis requires pericyte recruitment and covering vascular ECs with pericytes. The interaction between ECs and pericytes is necessary to maintain tissue homeostasis [21]. In the pathogenesis of diabetes mellitus, recruiting of pericyte to the endothelium in angiogenesis is suppressed. This may influence retinal angiogenesis and contribute to fundal hemorrhage [22]. Although increases in VEGF-A alone upregulates vascular permeability [23], angiogenesis without pericytes induces further vascular leakage and upregulated permeability [4].
Glucose is partially decomposed during heat sterilization of PDF to form GDPs. GDPs induce the formation of advanced glycation end-products [24] and impaired cellular and peritoneal membrane function. GDPs induce toxicity on peritoneal mesothelial cells and suppress cell signaling [25]. Novel neutral pH dialysates have been developed. In contrast to the acidic PD solution, this neutral pH solution includes lower levels of GDPs using a two-chamber system [26]. However, 3-deoxyglucosone remains in the drainage of PD solutions [27]. While MGO is present at a very low percentage, 3-DG and 5-HMF are said to account for approximately 90% or more of total GDPs contained in PD effluent [7, 27]. Therefore, reducing 3-DG and 5-HMF is important for lowering the GDP in PD solution. However, there are few reports on the effects of 3-DG and 5-HMF on angiogenesis. Our research theme was to examine the effects of them on angiogenesis in ECs and VSMCs.
A previous study proposed a “two-hit theory” to explain the development of EPS, in which the peritoneum deteriorates by exposure to PD solution (first hit) and is then exposed to additional deteriorating factors (second hit) [28]. The experiment on GDPs in PD solution is related to the first hit. We also assessed the effect of inflammation during PD-related peritonitis, a typical second hit on the expression of angiogenic factors. Intraperitoneal administration of LPS, a key component of the outer membrane of Gram-negative bacteria, initiates rapid and coordinated mobilization and activation of leukocytes, followed by excessive production of inflammatory mediators. An LPS-induced peritonitis model is often used to imitate PD-related peritonitis [29]. In our study, LPS alone showed no effect on angiogenic factors in ECs and VSMCs. However, culture supernatant of LPS-activated RAW 264.7 cells had a significant effect on the expression of angiogenic factors, such as VEGF-A and PDGF-BB, and PDGF-Rβ was decreased in VSMCs. LPS increases the production of VEGF-A in macrophages [30], moreover neo-angiogenesis is related to fibrosis through the TGFβ1-VEGF-A pathway in mesothelial cells and fibroblasts [31]. Our study showed that inflammatory cytokines secreted by macrophages (including TGFβ1) may increase VEGF-A production in ECs and VSMCs. Furthermore, inflammation from peritonitis may result in normal angiogenesis in ECs but impair angiogenesis in VSMCs. Our finding that the supernatant of LPS-stimulated RAW 264.7 cells increased VEGF-A in HUVECs and HUASMCs is similar to the finding of GDP-induced upregulation of VEGF-A in HUASMCs. Furthermore, inhibition of PDGF-Rβ by the supernatant of LPS-stimulated RAW 264.7 cells is similar to the finding of GDP-induced downregulation of PDGF-Rβ in HUASMCs. However, induction of PDGF-BB by the supernatant of LPS-stimulated RAW 264.7 cells is in contrast to the finding that GDPs suppress the expression and secretion of PDGF-BB in HUVECs. Although the reported incidence of PD-related peritonitis ranges widely, a retrospective study of 1677 incident PD patients revealed a first-year peritonitis rate of 42 per 100 patient-years [32]. Thus, the incidence of peritonitis is not high, and as a result, the decline in PDGFB induced by continuous exposure of the peritoneum to GDPs may be more important for impaired angiogenesis. Furthermore, regardless of the increase or decrease in the ligand PDGF-BB in ECs, suppression of its receptor PDGF-Rβ in VSMCs may contribute more to impaired angiogenesis through inhibition of VSMC coverage of ECs. Hence, these results indicate that peritonitis-induced inflammation may cause an imbalance in PDGF-BB and PDGF-Rβ expression, resulting in impaired angiogenesis.
In conclusion, we revealed that GDPs or an inflammatory response can induce an immature, impaired angiogenesis through the imbalance of angiogenetic factors in ECs and VSMCs.
Abbreviations
- EPS:
-
Encapsulated peritoneal sclerosis
- GDPs:
-
Glucose degradation products
- PDGF-BB:
-
Platelet-derived growth factor BB
- PDGF-Rβ:
-
Platelet-derived growth factor receptor beta
- VEGF-A:
-
Vascular endothelial growth factor-A
References
Hamada C, Honda K, Kawanishi K, et al. Morphological characteristics in peritoneum in patients with neutral peritoneal dialysis solution. J Artif Ogans. 2015;18(3):243–50.
Shi J, Yu M, Sheng M. Angiogenesis and inflammation in peritoneal dialysis: the role of adipocytes. Kidney Blood Press Res. 2017;42(2):209–19.
van Dijk CG, Nieuweboer FE, Pei JY, et al. The complex mural cell: pericyte function in health and disease. Int J Cardiol. 2015;190:75–89.
Gianni-Barrera R, Bartolomeo M, Vollmar B, et al. Split for the cure: VEGF, PDGF-BB and intussusception in therapeutic angiogenesis. Biochem Soc Trans. 2014;42(6):1637–42.
Schmitt CP, Aufricht C. Is there such a thing as biocompatible peritoneal dialysis fluid? Pediatr Nephrol. 2017;32(10):1835–43.
Nakano T, Kumiko T, Mizumasa T, et al. The glucose degradation product methylglyoxal induces immature angiogenesis in patients undergoing peritoneal dialysis. Biochem Biophys Res Commun. 2020;525(3):767–72.
Yamamoto T, Izumotani T, Okuno S, et al. Glucose degradation products in neutralized peritoneal dialysis solution. J Jpn Soc Dial Ther. 2004;37(12):2069–77.
Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Pfister C, Pfrommer H, Tatagiba MS, et al. Vascular endothelial growth factor signals through platelet-derived growth factor receptor β in meningiomas in vitro. Br J Cancer. 2012;107(10):1702–13.
Muratoglu SC, Mikhailenko I, Newton C, et al. Low density lipoprotein receptor-related protein 1 (LRP1) forms a signaling complex with platelet-derived growth factor receptor-beta in endosomes and regulates activation of the MAPK pathway. J Biol Chem. 2010;285(19):14308–17.
Caniuguir A, Krause BJ, Hernandez C, et al. Markers of early endothelial dysfunction in intrauterine growth restriction-derived human umbilical vein endothelial cells revealed by 2D-DIGE and mass spectrometry analyses. Placenta. 2016;41:14–26.
Patel H, Chen J, Das KC, et al. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol. 2013;12:142.
Walshe TE, dela Paz NG, D’Amore PA. The role of shear-induced transforming growth factor-beta signaling in the endothelium. Arterioscler Thromb Vasc Biol. 2013;33(11):2608–17.
Jang J, Jung Y, Kim Y, et al. LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974. Sci Rep. 2017;7:41612.
Cao Y, Gong Y, Liu L, et al. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J Appl Toxicol. 2017;37(12):1359–69.
Mazza G, Roßmanith E, Lang-Olip I, et al. Marker profile for the evaluation of human umbilical artery smooth muscle cell quality obtained by different isolation and culture methods. Cytotechnology. 2016;68(4):701–11.
Janke D, Jankowski J, Rüth M, et al. The “artificial artery” as in vitro perfusion model. PLoS ONE. 2013;8(3): e57227.
Chiaverina G, di Blasio L, Monica V, et al. Dynamic interplay between pericytes and endothelial cells during sprouting angiogenesis. Cells. 2019;8(9):1109.
Attwell D, Mishra A, Hall CN, et al. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–5.
Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2(7): a006502.
Caporali A, Martello A, Miscianinov V, et al. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacol Ther. 2017;171:56–64.
Park DY, Lee J, Kim J, et al. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017;8:15296.
Xiao X, Prasadan K, Guo P, et al. Pancreatic duct cells as a source of vegf in mice. Diabetologia. 2014;57(5):991–1000.
Mittelmaier S, Pischetsrieder M. Multistep ultrahigh performance liquidchromatography/tandem mass spectrometry analysis for untargetedquantification of glycating activity and identification of most relevantglycation products. Anal Chem. 2011;83(24):9660–8.
Distler L, Georgieva A, Kenkel I, et al. Structure- andconcentration-specific assessment of the physiological reactivity of alpha-dicarbonyl glucose degradation products in peritoneal dialysis fluids. Chem Res Toxicol. 2014;27(8):1421–30.
Park S-H, Do J-Y, Kim YH, et al. Effects of neutral pH and low-glucose degradation product- containing peritoneal dialysis fluid on systemic markers of in-flammation and endothelial dysfunction: a randomized controlled 1-year follow-up study. Nephrol Dial Transplant. 2012;27(3):1191–9.
Thornalley PJ, Dobler D, Babaei-Jadidi R, et al. Concentrations of glucose degradation products, glyoxal, methylglyoxal and 3-deoxyglucosone, in current generation peritoneal dialysis fluids determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J Am Soc Nephrol. 2005;16:268A.
Honda K, Nitta K, Horita S, et al. Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial. 2003;19:169–75.
Ni J, McLoughlin RM, Brodovitch A, et al. Nitric oxide synthase isoforms play distinct roles during acute peritonitis. Nephrol Dial Transplant. 2010;25(1):86–96.
Koide N, Odkhuu E, Naiki Y, et al. Augmentation of LPS-induced vascular endothelial cell growth factor production in macrophages by transforming growth factor-β1. Innate Immun. 2014;20(8):816–25.
Kariya T, Nishimura H, Mizuno M, et al. TGF-β1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am J Physiol Renal Physiol. 2018;314(2):F167–80.
Pulliam J, Li NC, Maddux F, et al. First-year outcomes of incident peritoneal dialysis patients in the United States. Am J Kidney Dis. 2014;64(5):761–9.
Acknowledgements
We thank the Research Support Center and Kyushu University Graduate School of Medical Sciences for technical support. We also thank Tamara Leahy, Ph.D., from Edanz Group (https://www.jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was supported by the 19th Baxter PD Fund Award, and partly by a JSPS KAKENHI Grant-in-Aid for Scientific Research C (No. 23590400) grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author(s) declare no conflict of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10157_2022_2272_MOESM1_ESM.pptx
Supplementary file1: Fig. 1 Schema of glucose degradation products in peritoneal dialysis fluid. Fig. 2 (a) PDGF-Rβ mRNA expression determined by real-time PCR in HUASMCs incubated with VEGF-165A, n=3 each. (b) Western blot analysis of PDGF-Rβ in HUASMCs incubated with VEGF-165A. Upper panel, PDGF-Rβ; lower panel, β actin as an internal control. n=5 each. Abbreviations: NS, not significant; PDGF-Rβ, platelet-derived growth factor receptor beta; VEGF-165A, vascular endothelial growth factor-165A. Fig. 3 (a) Representative image of co-cultured HUVECs and HUASMCs. White scale bars represent 300 μm. (b) VEGF-A mRNA expression determined by real-time PCR in HUASMCs incubated with GDPs, PDGF-BB, or mixture of them, n=3 each. (c) Cell proliferation in endothelial cells by labeling the nuclei and measuring with a multiwell plate reader. Abbreviations: GDPs, Glucose degradation products; NS, not significant; PDGF-BB, platelet-derived growth factor BB; PDGF-Rβ, platelet-derived growth factor receptor beta; VEGF-A, vascular endothelial growth factor-A. *P<0.05, **P<0.01. Fig. 4 (a) Secretion of methylglyoxal from RAW 264.7 was measured by enzyme-linked immunosorbent assay, n=3 each. Abbreviations: LPS, lipopolysaccharide; NS, not significant.
About this article
Cite this article
Aihara, S., Nakano, T., Torisu, K. et al. Glucose degradation products in peritoneal dialysis solution impair angiogenesis by dysregulating angiogenetic factors in endothelial and vascular smooth muscle cells. Clin Exp Nephrol 26, 1160–1169 (2022). https://doi.org/10.1007/s10157-022-02272-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02272-3