Abstract
Background
High serum alkaline phosphatase (ALP) levels are associated with excess all-cause and cardiovascular mortality in patients undergoing hemodialysis (HD). However, the long-term relationship between serum ALP levels and infection-related mortality remains unclear.
Methods
A total of 3502 maintenance HD patients were registered in the Q-Cohort Study, an observational cohort study in Japan. The primary outcome was infection-related mortality during a 10-year follow-up period. The covariate of interest was serum ALP levels at baseline. The association between serum ALP levels and infection-related mortality was calculated using a Cox proportional hazards model and a Fine–Gray subdistribution hazards model with non-infection-related death as a competing risk.
Results
During the follow-up period, 446 patients died of infection. According to their baseline serum ALP levels, the patients were categorized into sex-specific quartiles (Q1–Q4). Compared with patients in the lowest serum ALP quartile (Q1), those in the highest quartile (Q4) had a significantly higher multivariable-adjusted hazard ratio (HR) of 1.70 [95% confidence interval (CI) 1.24–2.32] for infection-related mortality. Furthermore, the HR for every 50 U/L increase in serum ALP levels was 1.24 (95% CI 1.12–1.36) for infection-related mortality. These associations remained consistent in the competing risk model: subdistribution HR, 1.47; 95% CI 1.07–2.03 for Q4 compared with Q1.
Conclusion
Higher serum ALP levels were significantly associated with a higher risk of infection-related mortality in patients undergoing HD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of mortality in hemodialysis (HD) patients is unacceptably high compared with that of the general population [1, 2]. Infection is one of the major leading causes of death in HD patients, accounting for 20.8% of the total [3]. Accumulating evidence has shown several risk factors for infectious disease in this population, such as older age, longer duration on HD, diabetes mellitus, or lower serum albumin [4, 5]. There is an urgent need to identify other modifiable risk factors for infection-related mortality to improve the prognosis of HD patients.
Alkaline phosphatase (ALP) is a membrane-bound enzyme that catalyzes the hydrolysis of organic pyrophosphate and is used as a marker of high bone turnover during the management of chronic kidney disease-mineral bone disorders (CKD-MBD) in daily practice [6,7,8,9]. High serum ALP levels often reflect less effective management of CKD-MBD and have been reported to be associated with a higher risk of mortality. There have been several reports that high serum ALP levels are associated with higher all-cause mortality and a higher prevalence of cardiovascular disease in HD patients [10,11,12,13,14,15,16,17,18]. Besides, there is evidence that high serum ALP levels may represent an immune response to infection [19]. However, little is known about the association between serum ALP levels and infection-related mortality, and previous studies included a relatively brief follow-up period [9, 20, 21].
The present study, therefore, aimed to determine the relationship between serum ALP levels and infection-related mortality using 10-year follow-up data for Japanese HD patients registered in the Q-Cohort Study [22,23,24].
Materials and methods
Study population
The details of the design of the Q-Cohort Study were provided elsewhere [22,23,24]. In the present study, we recruited 3,598 HD outpatients aged ≥ 18 years who attended 39 dialysis facilities between December 2006 and December 2007. The participants were followed until December 2016. We excluded participants for whom baseline data or outcomes were not available (n = 96); therefore, the remaining 3502 patients were enrolled. The study protocol was approved by the Clinical Research Ethics Committee of the Institutional Review Board at Kyushu University (Approval Number 20-31) and all the participating institutions. Written informed consent was obtained from all the participants at the start of the study, which was performed according to the principles of the Declaration of Helsinki. The ethics committees of the participating institutions waived the requirement for written informed consent for the additional follow-up surveys conducted during 2011–2016, because of the retrospective nature of the study. The study was registered in the University Hospital Medical Information Network (UMIN) clinical trial registry (UMIN ID: 000000556).
Outcomes
The primary outcome was infection-related mortality. Infection-related mortality was defined as death from pulmonary infection, sepsis, lower limb infection, cholecystitis/cholangitis, vascular access/catheter-related infection, enteritis, infectious endocarditis, urinary tract infection, cyst infection, cellulitis, or others [25]. The secondary outcome was all-cause mortality. The events were collected from the patients’ medical records. Patient health status was determined annually by local physicians at each HD facility, and by mail or telephone for patients who had started attending other facilities that were not participating in the study.
Measurements
The details of the assessments of risk factors have been previously published [22,23,24]. Briefly, demographic information [e.g., age, sex, causes of end-stage kidney disease (e.g., diabetic nephropathy), and HD vintage] and clinical data [e.g., hemoglobin, serum levels of ALP, albumin, C-reactive protein (CRP), corrected calcium, phosphate, and parathyroid hormone (PTH), body weight, Kt/V, and systolic blood pressure (BP)] were collected at baseline. Serum levels of ALP were measured using the Japan Society of Clinical Chemistry (JSCC) method and described using the International Federation of Clinical Chemistry (IFCC) method: the following equation was used to convert between the JSCC method and IFCC method: ALP (IFCC method, U/L) = 0.35 × ALP (JSCC method, U/L) [26, 27]. The corrected calcium concentration was calculated using Payne’s formula, as follows: corrected calcium (mg/dL) = observed total calcium (mg/dL) + (4.0—serum albumin concentration (g/dL)), if the serum albumin level was < 4 g/dL [28]. Serum PTH was measured using whole or intact PTH assays: the following equation was used to convert between these: intact PTH (pg/mL) = 1.7 × whole PTH (pg/mL) [29]. The physicians at each HD facility reported on the use of calcium-containing phosphate binders, sevelamer hydrochlorides, vitamin D receptor activators, and antihypertensive drugs, and on the patient history of cardiovascular events, parathyroidectomy, and bone fracture. The history of cardiovascular events included of cerebrovascular disease, coronary artery disease, congestive heart failure, and peripheral vascular disease. Body weight was measured in light clothing, without shoes. Dialysis doses were measured using a single-pool Kt/V for the urea method. BP measured before dialysis was collected from the dialysis records. Laboratory data were recorded for blood samples collected prior to dialysis. The cardiothoracic ratio was calculated from a standard chest radiograph.
Statistical analysis
Baseline data, stratified according to sex-specific quartile of serum ALP levels, are presented as a median and interquartile range, or number and percentage, and their distributions were compared using trend analysis. The Cochran–Armitage and the Jonckheere–Terpstra tests were used to determine the P-values for trends in categorical and continuous variables, respectively. Infection-related and all-cause mortality, stratified according to serum ALP quartile, were described using the Kaplan–Meier methods and compared using the log-rank tests.
Unadjusted, age- and sex-adjusted, and multivariable-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for infection-related and all-cause mortality, stratified according to serum ALP levels, were calculated using a Cox proportional hazards model. The assumption of the proportional hazards analysis was checked graphically using the log cumulative hazard plots for each outcome, stratified according to serum ALP levels, and tested using an analysis of Schoenfeld residuals [30]. The multivariable-adjusted model was adjusted for age; sex; the presence of diabetic nephropathy; the history of cardiovascular events, bone fractures, or parathyroidectomy; HD vintage; body weight; Kt/V; systolic BP; cardiothoracic ratio; blood hemoglobin concentration; serum albumin, CRP, corrected calcium, phosphate, and PTH concentrations; and use of calcium-containing phosphate binders, sevelamer hydrochlorides, vitamin D receptor activators, or antihypertensive agents. These variables were included based on a priori clinical judgment and previous findings. To confirm the robustness of the results obtained by the variable selection, the data were analyzed by the alternative variables selected using the Cox proportional hazard model and stepwise backward elimination with a P-value of < 0.05 for the remaining variables. Age and sex were included as initial candidate variables. To obtain a reliable estimate for each subgroup, serum ALP level was modeled as a continuous variable, and HRs are reported for each 50-U/L increment. The association between serum ALP levels and infection-related mortality was also assessed using a Fine–Gray proportional subdistribution hazards model with non-infection-related death as a competing risk.
Potential interactions were formally tested by including relevant interaction terms. Heterogeneity in the correlations between subgroups was determined by adding a multiplicative interaction term to the relevant Cox model for each 50-U/L increment.
Furthermore, multivariable-adjusted associations between serum ALP levels and infection-related mortality (HRs and 95% CIs) were plotted using restricted cubic spline curves. We used four knots located at the 5th, 35th, 65th, and 95th percentiles of serum ALP levels as previously recommended [31]. Age; HD vintage; body weight; Kt/V; systolic BP; cardiothoracic ratio; blood hemoglobin concentration; and serum albumin, CRP, corrected calcium, phosphate, and PTH concentrations were used as the spline terms. The value of 81.6 U/L, which was the overall median serum ALP level, was chosen as the reference for each spline plot.
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA) and R statistical software, version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P value < 0.05 was considered to represent statistical significance.
Results
Distribution of serum ALP levels, stratified according to sex
Histograms demonstrating the distributions of serum ALP levels according to sex are shown in Fig. 1 and show right-skewed distributions in both male and female patients. Female patients (median value, 88.6 U/L) had higher serum ALP levels than male patients (median value, 77.7 U/L).
Baseline characteristics, stratified according to sex-specific quartile of serum ALP levels
The baseline characteristics of patients, stratified according to the sex-specific quartile of serum ALP levels, are listed in Table 1. Patients with higher ALP levels were older; more likely to have a history of cardiovascular events or bone fracture, and to have been undergoing HD for longer; had higher cardiothoracic ratios. Serum CRP and PTH concentrations also increased with serum ALP. In contrast, blood hemoglobin concentration, serum albumin-corrected calcium, and serum phosphate decreased with increases in serum ALP levels.
Relationship between serum ALP levels and infection-related mortality
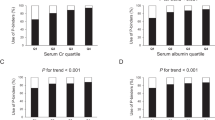
During the follow-up period, 446 (12.7%) patients died of infection. The incident rates of infection-related mortality, stratified according to sex-specific quartile of serum ALP levels, are shown in Fig. 2. The 10-year survival rate decreased with increases in serum ALP (p < 0.001; log-rank test). Patients with the highest serum ALP levels (Q4) showed a 1.70-fold (95% CI 1.24- to 2.32-fold, p < 0.001) higher risk of infection-related mortality than those with the lowest serum ALP levels (Q1), after adjustment for potential confounders (Table 2). As shown in Table 2, 50-U/L increases in serum ALP levels were also continuously associated with a 1.24-fold (95% CI 1.12- to 1.36-fold, p < 0.001) higher risk of infection-related mortality. Another model selected by the stepwise backward elimination was adjusted for age, sex, the presence of diabetic nephropathy, the history of cardiovascular events, HD vintage, body weight, serum albumin, creatinine, CRP, phosphate, and ferritin concentrations, and use of phosphate binders, antihypertensive agents, or iron supplementation, which resulted in almost no changes in the associations (Supplementary Table 1). Similarly, Table 3 shows that patients in Q4 had a significantly higher risk of infection-related mortality than those in Q1 according to the multivariable-adjusted Fine–Gray model with non-infection-related death as a competing risk, where 1284 patients died of non-infectious cause [subdistribution HR (95% CI), 1.47 (1.07–2.03), p < 0.05]. As shown in Fig. 3, the HRs for infection-related mortality increased with increases in serum ALP levels in multivariable-adjusted restricted cubic spline analysis. A significant interaction was identified between the use of calcium-containing phosphate binders and infection-related mortality (p for interaction = 0.002), with a greater contribution of higher serum ALP levels to infection-related mortality among patients using calcium-containing phosphate binders, compared with their counterparts (Fig. 4).
Multivariable-adjusted restricted cubic spline plots of the hazard ratio for infection-related mortality, stratified according to serum ALP levels. Solid lines represent the hazard ratios and dotted lines represent the 95% confidence intervals. The horizontal gray lines correspond to the reference hazard ratio of 1.0. The overall median serum ALP level was 81.6 U/L; therefore, this was chosen as the reference value. The multivariable-adjusted model was adjusted for age; sex; the presence of diabetic nephropathy; the history of cardiovascular events, bone fractures, or parathyroidectomy; HD vintage; body weight; Kt/V; systolic blood pressure; cardiothoracic ratio; blood hemoglobin concentration; serum albumin, CRP, corrected calcium, phosphate, and PTH concentrations; and use of calcium-containing phosphate binders, sevelamer hydrochlorides, vitamin D receptor activators, or antihypertensive agents. ALP alkaline phosphatase, CRP C-reactive protein, HD hemodialysis, PTH parathyroid hormone
Multivariable-adjusted HRs and 95% CIs for infection-related mortality associated with 50-U/L increases in serum ALP levels in patient subgroups. The multivariable-adjusted model was adjusted for age; sex; the presence of diabetic nephropathy; the history of cardiovascular events, bone fractures, or parathyroidectomy; HD vintage; body weight; Kt/V; systolic blood pressure; cardiothoracic ratio; blood hemoglobin concentration; serum albumin, C-reactive protein, corrected calcium, phosphate, and PTH concentrations; and use of calcium-containing phosphate binders, sevelamer hydrochlorides, VDRAs, or antihypertensive agents. ALP alkaline phosphatase, CI confidence interval, HD, hemodialysis, HR hazard ratio, PTH parathyroid hormone, VDRA vitamin D receptor activator
Relationship between serum ALP levels and specific infection-related mortality
Supplementary Table 2 shows details on the specific underlying cause of infection-related death, stratified according to the sex-specific quartile of serum ALP levels. The most common cause of infection-related death was a pulmonary infection, followed by sepsis, lower limb infection, and cholecystitis/cholangitis. The multivariable analyses of the relationship between serum ALP levels and the four major causes of infection-related mortality showed a similar trend to the main analysis, although no statistically significant relationship was found (Supplementary Table 3).
Relationship between serum ALP levels and all-cause mortality
During the 10-year follow-up period, 1,730 (49.4%) patients died of any causes. The survival rates stratified according to sex-specific quartile of serum ALP levels are shown in Supplementary Fig. 1. The 10-year survival rate decreased with increases in serum ALP levels (p < 0.001; log-rank test). Supplementary Table 4 shows that patients with the highest ALP levels (Q4) had a 1.49-fold (95% CI 1.27- to 1.73-fold, p < 0.001) higher risk of all-cause mortality than those with the lowest ALP levels (Q1), after adjustment for potential confounders.
Discussion
In the present longitudinal follow-up cohort study, we showed that high serum ALP levels were significantly associated with a higher risk of infection-related mortality and that this relationship remained significant after adjustment for confounding factors. Additionally, high serum ALP levels were significantly associated with higher all-cause mortality, as reported previously [10, 11, 13,14,15,16,17]. Patients in the highest quartile of sex-specific ALP levels had a 70% higher risk of infection-related mortality after a multivariable adjustment than those in the lowest quartile of sex-specific ALP levels. Similarly, the risk of all-cause mortality was 49% higher in patients in the highest quartile of sex-specific ALP levels. Our findings indicate that high serum ALP levels are a risk factor for infection-related and all-cause mortality.
This study showed that high serum ALP levels were positively associated with a higher risk of infection-related mortality in patients on HD. To the best of our knowledge, only one study showed that higher serum ALP levels in patients with HD were associated with greater mortality from infection [11]. However, the follow-up period in this previous study was relatively short (mean follow-up: 1.6 years). No other reports have shown an association between serum ALP levels and infection-related mortality in patients receiving maintenance HD. In the present study, patients with high serum ALP levels tended to be older, were more likely to have a history of cardiovascular disease or fracture, and had a long history of dialysis, lower body weight, lower hemoglobin concentrations, lower serum albumin concentrations, and higher serum CRP concentrations. These individuals were considered to be poorly nourished and affected by chronic inflammation, and were considered as having “frailty.” Several reports showed that the severity of frailty was a significant predictor of mortality in patients with HD [32]. These results are consistent with our previous study on the association between the geriatric nutritional risk index and infection-related mortality [33]. A lower geriatric nutritional risk index, which represents a lower body mass index or lower serum albuminemia, was an independent risk factor for infection-related mortality in a patient undergoing HD. However, even when multivariable adjustment was performed using these factors, high serum ALP levels remained a significant risk factor for infection-related mortality. These results indicate that serum ALP levels may be a useful marker of the risk of infection-related mortality, and can be used with established markers of poor nutrition and inflammation.
We also examined the relationship between serum ALP levels and the specific causes of infection-related mortality, including pulmonary infection, sepsis, lower limb infection, and cholecystitis/cholangitis. No significant relationships were found between them, which may be attributed to a lack of power due to the sample size. Further large-scale studies are warranted to determine whether the effect of serum ALP levels differs depending on the site of infection.
Some potential explanations for the relationship between serum ALP levels and infection-related mortality can be proposed. A previous study showed that ALP had a role in lipopolysaccharide (LPS) detoxification in vivo and in vitro [19]. In another study, endogenous intestinal ALP induced by the inflammatory agent resolvin-E1 reduced the activity of nuclear factor-kB, which resulted in a decrease in the release of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin 6, and LPS-binding protein [34]. The LPS to toll-like receptors-4 (LPS-TLR4) pathway is also associated with ALP [35]. The LPS-TLR4 pathway enhances nuclear factor-kB signaling to induce an inflammatory response and secrete inflammatory and anti-inflammatory cytokines such as TNF-α throughout the body. Additionally, ALP interacts with the LPS-TLR4 pathway and decreases LPS through dephosphorylation of LPS [35]. Furthermore, detrimental adenosine triphosphate, which is released during cellular stress caused by inflammation, can be converted by ALP into anti-inflammatory and tissue-protective adenosine [35]. These results collectively suggest that high serum ALP levels reflect subclinical infectious diseases in the patient, resulting in overt infection. However, further research is required to clarify the relationship between serum ALP levels and infectious diseases.
In the stratified analysis, the use of calcium-containing phosphate binders showed an interaction with the risk of infection-related mortality. Although we are unable to propose mechanisms underlying these associations, a possible explanation may be an increase in circulating fibroblast growth factor 23 concentrations. Calcium overload upregulates fibroblast growth factor 23 synthesis and secretion [36, 37]. Fibroblast growth factor 23 reduces the host defense by interference with chemokine signaling and integrin activation in CKD [38]. Therefore, the combination of high serum ALP levels and the use of calcium-containing phosphate binders may facilitate the incidence of infectious diseases.
Which fraction of the serum ALP level, including the liver, bile duct system, bone, thyroid, placenta, small intestine, and kidney, is associated with infection-related mortality is controversial. No studies have reported whether elevated levels of any fraction of ALP are associated with infection-related mortality in the general population or in patients on HD. Patients on HD with liver diseases, such as ascites, hypoalbuminemia, or a history of hepatitis C, tend to have higher serum ALP levels [11]. This finding suggests that liver-type ALP levels are elevated in patients on HD with liver disease [11]. Another study reported that the variability in ALP was due to bone ALP in patients on HD without the liver disease [39]. Additionally, small intestinal ALP was reported to be 15% higher in patients on HD than in the general population [40]. Therefore, postulating which factors contribute to serum ALP levels in patients on HD without measuring fractions of ALP is difficult [41]. Future studies are required to determine which fraction of ALP is associated with the prognosis.
In this study, high serum ALP levels were significantly associated with higher all-cause mortality. A large-scale observational study in the United States also showed that high serum ALP levels in patients on HD increased the risk of all-cause mortality [13]. Additionally, in Japanese patients with HD, high serum ALP levels were associated with higher all-cause and cardiovascular mortality [14]. Notably, ALP inactivates pyrophosphate, which is an endogenous inhibitor of hydroxyapatite formation, resulting in vascular calcification and contributing to cardiovascular morbidity and mortality [42]. Additionally, smooth muscle cells undergoing medial calcification express high ALP levels [43]. These results suggest that high serum ALP levels and vascular calcification may have reciprocal effects. High serum ALP levels are also an independent risk factor for coronary calcification in patients on HD [44]. Taken together, these findings suggest that higher serum ALP levels may predispose toward cardiovascular mortality, thereby increasing the risks of all-cause mortality in patients on HD.
The main strength of our study is that it was a large-scale cohort study with a relatively long follow-up period, and it showed associations between serum ALP, a routinely used biomarker, with infection-related and all-cause mortality in patients undergoing HD. However, this study has several limitations. First, we could not obtain information regarding the liver statuses, such as serum aspartate aminotransferase and alanine aminotransferase activities. Furthermore, we had no data on the prevalence of hepatitis B and C viral infections in patients registered in the Q-Cohort Study. Liver disease may be associated with high serum ALP levels, which are also associated with higher mortality. However, a previous study showed that high serum ALP levels remained associated with higher mortality, even when the data were stratified according to serum aspartate aminotransferase activity [10]. Second, we had no data regarding the history of malignant diseases. Ectopic expression of ALP is associated with a variety of cancers [45]. Among patients on HD, cancer was associated with a ≥ 20% increase in the rate of infection-related hospitalization [4]. The lack of this information may have biased our results to some extent. Third, we did not measure serum bone-specific ALP levels. In daily practice in Japan, serum bone-specific ALP levels are not measured because of the relatively high cost of this measurement. Fourth, baseline data were obtained at a single time point, which may have resulted in the misclassification of the study participants. However, if such misclassification had occurred, the association would have been weakened, biasing the results toward the null hypothesis. Finally, most of the study participants were not taking calcimimetics, such as cinacalcet hydrochlorides, which have revolutionized CKD-MBD treatment strategies, at the start of the study. The Q-Cohort Study enrolled patients between December 2006 and December 2007, which was before calcimimetics had been put on the market in Japan. Additionally, only seven (0.2%) patients in our study were using calcimimetics at the start of the follow-up. A previous study showed that the proportion of patients using cinacalcet hydrochlorides had reached nearly 30% in Japan in 2009 [46]. Therefore, some of the participants in the present study might have been using calcimimetics during the follow-up, which would have affected serum ALP levels by reducing PTH synthesis and secretion [47]. Despite these limitations, we believe that the present study permits a better understanding of the relationship between serum ALP levels and infection-related mortality in patients on maintenance HD.
Conclusion
This study shows that high serum ALP levels in patients on maintenance HD increase the risks of infection-related and all-cause mortality. These results suggest that patients with HD with high serum ALP levels are a high-risk group for infection-related mortality and that prevention and aggressive treatment of infections are imperative. Further research is required to determine the acceptable threshold for serum ALP levels in patients with HD, and whether strategies aimed at reducing serum ALP will improve their prognosis.
References
Daratha KB, Short RA, Corbett CF, Ring ME, Alicic R, Choka R, et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol. 2012;7:409–16.
Masakane I, Taniguchi M, Nakai S, Tsuchida K, Goto S, Wada A, et al. Annual dialysis data report 2015, JSDT renal data registry. Ren Replace Ther. 2018;4:19.
Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, et al. An overview of regular dialysis treatment in Japan (As of 31 December 2013). Ther Apher Dial. 2015;19:540–74.
Dalrymple LS, Mu Y, Nguyen DV, Romano PS, Chertow GM, Grimes B, et al. Risk factors for infection-related hospitalization in-center hemodialysis. Clin J Am Soc Nephrol. 2015;10:2170–80.
Abbasi SH, Aftab RA, Chua SS. Risk factors associated with nosocomial infections among end stage renal disease patients undergoing hemodialysis: a systematic review. PLoS ONE. 2020;15: e0234376.
Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008;73:989–91.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1–59.
National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1-201.
Guideline Working Group, Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial. 2008;12:514–25.
Regidor DL, Kovesdy CP, Mehrotra R, Rambod M, Jing J, McAllister CJ, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–203.
Blayney MJ, Pisoni RL, Bragg-Gresham JL, Bommer J, Piera L, Saito A, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–63.
Drechsler C, Verduijn M, Pilz S, Krediet RT, Dekker FW, Wanner C, et al. Bone alkaline phosphatase and mortality in dialysis patients. Clin J Am Soc Nephrol. 2011;6:1752–9.
Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–80.
Maruyama Y, Taniguchi M, Kazama JJ, Yokoyama K, Hosoya T, Yokoo T, et al. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol Dial Transplant. 2014;29:1532–8.
Lee GH, Benner D, Regidor DL, Kalantar-Zadeh K. Impact of kidney bone disease and its management on survival of patients on dialysis. J Ren Nutr. 2007;17:38–44.
Waziri B, Duarte R, Naicker S. High serum alkaline phosphatase, hypercalcaemia, race, and mortality in South African maintenance haemodialysis patients. Int J Nephrol. 2017;2017:2795432.
Beddhu S, Baird B, Ma X, Cheung AK, Greene T. Serum alkaline phosphatase and mortality in hemodialysis patients. Clin Nephrol. 2010;74:91–6.
Zhu JG, Cheng BC, Lee WC, Li LC, Lee CH, Long G, et al. Serum alkaline phosphatase levels are not associated with increased death risk in prevalent hemodialysis patients: 5-year experience in a single hemodialysis center. Kidney Blood Press Res. 2016;41:498–506.
Koyama I, Matsunaga T, Harada T, Hokari S, Komoda T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin Biochem. 2002;35:455–61.
Ye H, Lin X, Qiu Y, Guo Q, Huang F, Yu X, et al. Higher alkaline phosphatase was associated with the short-term adverse outcomes of peritoneal dialysis-related peritonitis. Curr Chem Lab Med. 2015;53:e113–6.
Hwang SD, Kim SH, Kim YO, Jin DC, Song HC, Choi EJ, et al. Serum alkaline phosphatase levels predict infection-related mortality and hospitalization in peritoneal dialysis patients. PLoS ONE. 2016;11: e0157361.
Eriguchi R, Taniguchi M, Ninomiya T, Hirakata H, Fujimi S, Tsuruya K, et al. Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: the Q-Cohort study. J Nephrol. 2015;28:217–25.
Yotsueda R, Taniguchi M, Tanaka S, Eriguchi M, Fujisaki K, Torisu K, et al. Cardiothoracic ratio and all-cause mortality and cardiovascular disease events in hemodialysis patients: the Q-Cohort Study. Am J Kidney Dis. 2017;70:84–92.
Tanaka S, Ninomiya T, Hiyamuta H, Taniguchi M, Tokumoto M, Masutani K, et al. Apparent treatment-resistant hypertension and cardiovascular risk in hemodialysis patients: ten-year outcomes of the Q-Cohort Study. Sci Rep. 2019;9:1–8.
Hiyamuta H, Yamada S, Taniguchi M, Nakano T, Tsuruya K, Kitazono T. Causes of death in patients undergoing maintenance hemodialysis in Japan: 10-year outcomes of the Q-Cohort Study. Clin Exp Nephrol. 2021;25:1121–30.
Yamadate S, Nakayama T. The future topics of discussion on JSCC recommended methods. Rinsho Byori. 2016;64:544–9.
International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), Schumann G, Aoki R, Ferrero CA, Ehlers G, Férard G, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Clin Chem Lab Med. 2006;44:1146–55.
Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4:643–6.
Kazama JJ. Japanese society of dialysis therapy treatment guidelines for secondary hyperparathyroidism. Ther Apher Dial. 2007;11(Suppl 1):S44–7.
Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41.
Harrell FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. New York: Springer; 2013.
Fu W, Zhang A, Ma L, Jia L, Chhetri JK, Chan P. Severity of frailty as a significant predictor of mortality for hemodialysis patients: a prospective study in China. Int J Med Sci. 2021;18:3309–17.
Matsukuma Y, Tanaka S, Taniguchi M, Nakano T, Masutani K, Hirakata H, et al. Association of geriatric nutritional risk index with infection-related mortality in patients undergoing hemodialysis: the Q-Cohort Study. Clin Nutr. 2019;38:279–87.
Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci USA. 2010;107:14298–303.
Peters E, Heemskerk S, Masereeuw R, Pickkers P. Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am J Kidney Dis. 2014;63:1038–48.
Gravesen E, Mace ML, Hofman-Bang J, Olgaard K, Lewin E. Circulating FGF23 levels in response to acute changes in plasma Ca(2+). Calcif Tissue Int. 2014;95:46–53.
Shikida Y, Mizobuchi M, Inoue T, Hamada T, Ogata H, Koiwa F, et al. Effect of continuous intravenous calcium loading on fibroblast growth factor 23 in normal and uremic rats. Calcif Tissue Int. 2018;103:455–64.
Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstädt HJ, Meersch M, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126:962–74.
Haarhaus M, Monier-Faugere MC, Magnusson P, Malluche HH. Bone alkaline phosphatase isoforms in hemodialysis patients with low versus non-low bone turnover: a diagnostic test study. Am J Kidney Dis. 2015;66:99–105.
Skillen AW, Pierides AM. Serum alkaline phosphatase isoenzyme patterns in patients with chronic renal failure. Clin Chim Acta. 1977;80:339–46.
Haarhaus M, Gilham D, Kulikowski E, Magnusson P, Kalantar-Zadeh K. Pharmacologic epigenetic modulators of alkaline phosphatase in chronic kidney disease. Curr Opin Nephrol Hypertens. 2020;29:4–15.
Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–30.
Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–76.
Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–14.
Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem. 2014;29:269–78.
Akizawa T, Kido R, Fukagawa M, Onishi Y, Yamaguchi T, Hasegawa T, et al. Decreases in PTH in Japanese hemodialysis patients with secondary hyperparathyroidism: associations with changing practice patterns. Clin J Am Soc Nephrol. 2011;6:2280–8.
Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–25.
Acknowledgements
We would like to express our appreciation to the participants in the Q-Cohort Study and the members of the Society for the Study of Kidney Disease. The following personnel (institutions) participated in the study: Takashi Ando (Hakozaki Park Internal Medicine Clinic), Takashi Ariyoshi (Ariyoshi Clinic), Koichiro Goto (Goto Clinic), Fumitada Hattori (Nagao Hospital), Harumichi Higashi (St Mary’s Hospital), Tadashi Hirano (Hakujyuji Hospital), Kei Hori (Munakata Medical Association Hospital), Takashi Inenaga (Ekisaikai Moji Hospital), Hidetoshi Kanai (Kokura Memorial Hospital), Shigemi Kiyama (Kiyama Naika), Tetsuo Komota (Komota Clinic), Hiromasa Kuma (Kuma Clinic), Toshiro Maeda (Kozenkai-Maeda Hospital), Junichi Makino (Makino Clinic), Dai Matsuo (Hirao Clinic), Chiaki Miishima (Miishima Clinic), Koji Mitsuiki (Japanese Red Cross Fukuoka Hospital), Kenichi Motomura (Motomura Naika Clinic), Sadatoshi Nakamura, Hidetoshi Nakamura (Kokura Daiichi Hospital), Koichi Nakashima (Ohashi Internal Circulatory Clinic), Nobumitsu Okita (Shiroishi Kyoritsu Hospital), Shinichiro Osato (Osato Jin Clinic), Sakura Sakamoto (Fujiyamato Spa Hospital), Keiko Shigematsu (Shigematsu Clinic), Kazumasa Shimamatsu (Shimamatsu Naika Iin), Yoshito Shogakiuchi (Shin-Ai Clinic), Hiroaki Takamura (Hara Hospital), Kazuhito Takeda (Iizuka Hospital), Asuka Terai (Chidoribashi Hospital), Hideyoshi Tanaka (Mojiko-Jin Clinic), Suguru Tomooka (Hakozaki Park Internal Medicine Clinic), Jiro Toyonaga (Fukuoka Renal Clinic), Hiroshi Tsuruta (Steel Memorial Yawata Hospital), Ryutaro Yamaguchi (Shiseikai Hospital), Taihei Yanagida (Saiseikai Yahata General Hospital), Tetsuro Yanase (Yanase Internal Medicine Clinic), Tetsuhiko Yoshida (Hamanomachi Hospital), Takahiro Yoshimitsu (Gofukumachi Kidney Clinic, Harasanshin Hospital), and Koji Yoshitomi (Yoshitomi Medical Clinic). We also thank Mark Cleasby, Ph.D, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This study was supported by the Kidney Foundation (H19 JKFB 07-13, H20 JKFB 08-8, H23 JKFB 11-11) and the Japan Dialysis Outcome Research Foundation (H19-076-02, H20-003), without restriction on publication.
Author information
Authors and Affiliations
Contributions
Hiromasa Kitamura contributed to the study design, statistical analysis, interpretation of data, and drafting of the manuscript. Ryusuke Yotsueda contributed to the study design, statistical analysis, interpretation of data, and drafting of the manuscript. Hiroto Hiyamuta contributed to the study design, data cleaning, interpretation of data, and drafting of the manuscript. Masatomo Taniguchi contributed to the acquisition of data, and critical revision of the manuscript. Shigeru Tanaka contributed to the study design, the acquisition of data, interpretation of data, and drafting of the manuscript. Shunsuke Yamada contributed to the interpretation of data and drafting of the manuscript. Kazuhiko Tsuruya contributed to the data cleaning and critical revision of the manuscript. Toshiaki Nakano contributed to the funding, acquisition of data, study design, and drafting of the manuscript. Takanari Kitazono contributed to the critical revision of the manuscript and supervision of the study. All authors provided critical reviews of the draft and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Clinical Research Ethics Committee of the Institutional Review Board at Kyushu University (Approval Number: 20-31) and with the 1964 Helsinki declaration.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kitamura, H., Yotsueda, R., Hiyamuta, H. et al. Serum alkaline phosphatase and infection-related mortality in hemodialysis patients: ten-year outcomes of the Q-cohort study. Clin Exp Nephrol 26, 1119–1129 (2022). https://doi.org/10.1007/s10157-022-02255-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02255-4