Abstract
Background
Renal bilateral fluid filled-cyst in polycystic kidney disease (PKD) is associated with abnormal epithelial cell proliferation and transepithelial fluid secretion which leads to end-stage renal disease (ESRD). A chalcone derivative, isoliquiritigenin (ISLQ), has been shown to have various pharmacological properties. Since several studies have shown that ISLQ could inhibit CFTR channel activity, it is interesting to see whether it can inhibit renal cyst enlargement. The present study was aimed to determine an inhibitory effect and the mechanism of chalcone derivatives on MDCK cyst progression and Pkd1 mutant cells.
Methods
MDCK cyst growth and cyst formation experiments, MTT assay, Ussing chamber experiment, BrdU cell proliferation assay and western blot analysis were performed in this study.
Results
Among four compounds of chalcone derivatives tested, CHAL-005 (100 µM) was found to inhibit MDCK cyst growth in a dose-dependent manner without cytotoxicity. It inhibited short-circuit current of chloride secretion as well as CFTR protein expression in MDCK cells. CHAL-005 significantly suppressed cell proliferation. In addition, CHAL-005 strongly reduced phosphorylation ERK1/2 and phosphorylation S6 kinase in MDCK and Pkd1 mutant cells. Interestingly, CHAL-005 activated phosphorylation of AMP kinase protein expression in MDCK and Pkd1 mutant cells.
Conclusion
CHAL-005 slowed MDCK cyst progression by inhibiting CFTR expression and reducing ERK1/2 and mTOR/S6K signaling pathways as well as activating AMPK expression. Therefore, a chalcone derivative could represent as a promising drug candidate for polycystic kidney disease intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cyst progression in polycystic kidney disease (PKD), a common renal inherited disorder, caused by mutation of either PKD1 or PKD2 gene encoding polycystin-1 and polycystin-2 protein, respectively [1]. The characteristics of PKD is the proliferation and accumulation of fluid-filled cysts along the nephron [2]. The numerous fluid-filled cysts destroyed normal renal parenchymal cells leading to the loss of renal function and ending up with end-stage renal disease [3]. Currently, Tolvaptan (V2R antagonist) was approved for treating PKD [4]. However, the side effects of this drug urgent need to find the new candidate for retarding cystogenesis in PKD.
It is known that cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel activity is involved in fluid secretion in PKD pathophysiology [5]. Of note, several compounds which inhibited CFTR channel activity and expression could slow renal cyst enlargement both in an in vitro and in vivo model of PKD [6,7,8]. In addition, it was found that several complex pathways including the upregulation of Raf/MEK/ERK pathway [9, 10] and mTOR/S6K signaling pathway [11] were involved in cyst-lining epithelial cell growth. In contrast, the activation of AMP kinase, a cellular energy sensor, was found to strongly retard renal cystogenesis in a mouse model of PKD through the inhibition of both CFTR channel and mTOR/S6K signaling [12]. Therefore, the combination of target therapies might be more effective for the inhibition of renal cyst enlargement in PKD intervention.
Chalcone derivatives have found to possess several pharmacological properties such as anti-inflammation [13], antiproliferation [14], and anticancer [15]. In addition, there was a report that chalcone derivatives have an anti-diarrheal effect by inhibition of CFTR though the activation of AMPK activity [16]. Chalcone isoliquiritigenin (ISLQ) was found to inhibit ERK1/2 and mTOR signaling in adenoid cystic carcinoma cell [17]. Moreover, the chalcone ISLQ derivative has been shown to inhibit CFTR activity in human colonic epithelial (T84) and MDCK cells [18]. However, the mechanism of chalcone derivatives to slow an in vitro cyst progression on fluid secretion and cell proliferation pathway was unclear. Therefore, this present study was aimed to determine the inhibitory effect and the possible mechanisms of chalcone derivatives on fluid secretion and cell proliferation using an in vitro model of PKD.
Materials and methods
Reagents and compounds
Chalcone derivatives were synthesized and their identities were checked by spectroscopic data [16]. Collagen type I (PureCol) was purchased from Advanced BioMatrix (Fremont, CA, USA). DMEM/Ham F-12, penicillin, streptomycin, and FBS were purchased from Invitrogen (Carlsbad, CA, USA). Interferon γ, blasticidin, GlyH101, and ECL-solution were obtained from Calbiochem (San Diego, CA, USA). Primary antibodies including anti-CFTR, anti-p-ERK1/2 (Thr202/Tyr204), anti-t-ERK1/2, anti-pS6K (Thr421/Ser424), anti-t-S6K, anti-p-AMPK (Thr172), anti-AMPKα, anti-β-actin, were purchased from Cell Signaling (Beverly, MA, USA). Protease inhibitor was obtained from Roche (Indianapolis, IN, USA).
Cell cultures and treatments
Madin-Darby canine kidney (MDCK) type I cells [19] were kindly give from Prof. David N. Sheppard, University of Bristol, Bristol, UK. Mouse renal cystic epithelial cells Pkd1+/− (PH2, heterozygous) and Pkd1−/− (PN24, homozygous) cells [10] were kindly give from Prof. Stefan Somlo, Yale University School of Medicine, Connecticut, USA. Cells were cultured in DMEM/F-12 Ham medium supplemented with 10% FBS, 5 µg/ml insulin, 5 µg/ml transferrin, 5 µg/ml selenium X, 100 U/ml penicillin, and 100 µg/ml streptomycin, and 5 µg/ml interferon γ for Pkd1 mutant cells. These cells were grown at 37 °C and 33 °C, respectively, in a humidified atmosphere with 5% CO2, 95% O2. Cells were trypsinized with 0.25% trypsin and centrifuged at 600 g before seeding.
MDCK cyst experiment
Type I MDCK cells were added in an individual well of a 24-wells plate and suspended in 0.4 ml of 3.0 mg/ml ice-cold collagen supplemented with 10% 10 × minimum essential medium (MEM), 27 mM NaHCO3, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin (pH 7.4 with NaOH) as described previously [7]. For MDCK cyst growth experiment, MDCK cyst photographs were captured at 10 × magnifications using an inverted microscope (Nikon, TE 2000-S) at day 6. Then, the MDCK media containing chalcone derivatives (100 µM) and forskolin (10 µM) were incubated with cysts. The MDCK media containing forskolin and test compounds were changed every 2 days for 6 days onwards. Photographs of individual cyst were taken before adding the test compounds. Cyst diameter (µM) was measured using the Image J software. For MDCK cyst formation experiment, the MDCK media containing forskolin (10 µM) with either CHAL-005 (100 µM) or GlyH-101 (50 μM) were incubated with cysts for 6 days onwards. MDCK cyst photographs were captured at 10 × magnifications using an inverted microscope (Nikon, TE 2000-S) at day 6. Cyst diameter > 50 µM were counted as cyst colonies and cyst diameter < 50 μM were count as non-cyst colonies.
Cell viability assay
The 20,000 cells of MDCK were seeded and grown for 24 h in 96-wells plate. Four chalcone derivative compounds at doses of 100 μM were added and incubated for 24 h. Media containing test compounds were removed, and adherent cells were exposed to serum free media containing 10% MTT solutions (5 mg/ml) for 4 h in humidified atmosphere of 5% CO2, 95% O2 at 37 ℃. Then, the serum-free media containing MTT were removed and 100 μl of DMSO was added. The absorbance of the solution was measured at 530 nm and percentage of cell viability was expressed as 100% of control.
Cell proliferation assay
Cell proliferation assay was measured using BrdU cell proliferation kit (Calbiochem, San diego, CA, USA). MDCK cell (8000 cells) were seeded in 96-wells plate in DMEM/Ham’s F-12 media supplemented with 10% FBS and ITS-supplement and grown for 24 h. Then, cells were incubated with serum free media in the presence or absence of CHAL-005 at doses of 1–100 µM for 24 h. BrdU reagent was added at 18 h later and incubated for 6 h. Blasticidin (20 µg/ml) treatment was used as a positive control. The absorbance was measured at 490 nm by automated microplate reader and BrdU cell proliferation was calculated as 100% of control.
Ussing chamber experiment
MDCK cells (5 × 105 cells/well) were seeded on Snapwell inserts. MDCK media were changed every 2 days. On day 8, media from the apical side of MDCK cell monolayer were removed to form an air–liquid interface to enhance CFTR expression in MDCK epithelia. On day 10, only MDCK polarized epithelia monolayers with resistance > 2000 Ohm.cm2 were used for subsequent Ussing chamber experiments. Apical chloride current measurements were performed as previously described [7].
Western blot analysis
Western blot analysis was performed as described previously [7, 20]. Briefly, MDCK, Pkd1+/−, Pkd1−/− cells were lysed with ice-cold RIPA buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton-X 100, 1 mM NaF, 1 mM Na3VO4, and 1 mM PMSF) containing protease inhibitor cocktail. Samples were centrifuged at 10000 g and supernatant proteins (40 µg) were separated in 8–10% SDS-PAGE gel. Then, samples were transferred to a nitrocellulose membrane. After blocking non-specific binding by 5% non-fat dry milk at room temperature for 1 h, membranes were incubated with primary antibodies of interested proteins overnight at 4 °C. The membranes were washed by TBS-Tween 20 solution 3 times followed by incubation with secondary antibody for 1 h. After that the membranes were washed by TBS-Tween 20 solution 3 times, the band intensity of interested proteins was developed by chemiluminescence (ECL solution).
Statistical analysis
The data of all experiment were expressed as mean ± SEM. The statistical significance of data between control and the treatment groups was determined by one way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test, and repeated measure ANOVA, where appropriate. A value of P < 0.05 was considered statistically significant.
Results
Chalcone derivatives slowed MDCK cyst enlargement
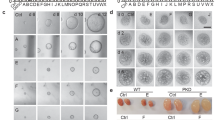
Previously, it was found that four chalcone derivatives (CHAL-005, CHAL-006, CHAL-007, CHAL-011) could inhibit chloride secretion mediated by forskolin in permeabilized MDCK cell monolayers (data not shown). Therefore, these four compounds were examined for their inhibitory effect on MDCK cyst enlargement. To rule out the toxic effect, first, the effect of chalcone derivative compounds on MDCK cell viability was examined prior to the evaluation of their effects on MDCK cyst growth and cyst formation. Using MTT assay, it was found that all chalcone derivatives tested had no effect on MDCK cell viability (Fig. 1b). To further determine the inhibitory effect of the chalcone derivative compounds on MDCK cyst progression, MDCK cells were suspended in the collagen gel media with or without chalcone derivative compounds at the concentration of 100 μM in the presence of forskolin for 6 days (between day 6 and day 12). The result showed that among four compounds examined, only CHAL-005 could significantly inhibit MDCK cyst growth compared to that of control and GlyH-101 (a CFTR inhibitor) (Fig. 1c). Then, the dose–response of an inhibitory effect of CHAL-005 on MDCK cyst growth was performed. The result showed that CHAL-005 could also slow MDCK cyst growth in a dose-dependent manner (10–100 μM) (Fig. 1d, f). To test whether an inhibitory effect of CHAL-005 on inhibiting cyst colony, MDCK cyst formation experiment was performed. It was found that CHAL-005 inhibited cyst formation by 79% compared with the control group and GlyH-101 (Fig. 1e). These results suggested that CHAL-005 significantly inhibits MDCK cyst progression in a dose-dependent manner without cytotoxicity. Therefore, only CHAL-005, whose structure was showed in Fig. 1a, was selected for further study on mechanism of actions in slowing cyst progression in MDCK cells (in vitro) and Pkd1 mutant cells.
Effect of CHAL-005 on MDCK cyst progression. a The structure of CHAL-005 compound. b MDCK cell viability was assayed by MTT assay. The graph represented cell viability after incubated with DMSO (control) or CHAL-005, CHAL-006, CHAL-007, CHAL-011 (100 µM) for 24 h (mean of percent control ± SE, n = 4, NS not significant). c An inhibitory effect of the chalcone derivative compounds on MDCK cyst growth was shown. The graph represented MDCK cyst diameter at day 12 after treatment with DMSO (control), CHAL-005, CHAL-006, CHAL-007, CHAL-011 (100 µM), GlyH-101 (50 μM) for 6 days onward (cyst diameter ± SE, n > 50 cysts, four independent experiments, ***P < 0.001, NS not significant). d Dose–response of CHAL-005 on MDCK cyst growth was shown. The graph represented MDCK cyst growth at day 12 after treatment with CHAL-005 at doses of 0, 1, 10, 50, 100 µM for 6 days onward (cyst diameter ± SE, n > 54–66 cysts, four independent experiments, *P < 0.05, ***P < 0.001, NS not significant). e An inhibitory effect of CHAL-005 on MDCK cyst formation was shown. The graph represented percent of MDCK cyst colonies at day 6 after treatment with DMSO (control), CHAL-005 (100 µM), and GlyH-101 (50 μM) for 6 days (mean ± SE, four independent experiments, n = 4 well/condition, ***P < 0.001). f Representative of light micrographs of MDCK cyst growth in collagen gels was shown. Light micrograph was captured at day 6–12 after cell seeding of MDCK cell presenting with 10 µM of forskolin (control), 1, 10, 50, and 100 µM of CHAL-005 were added for 6 days onward after cell seeding in gels. Scale bar = 100 µM and × 10 magnification
CHAL-005 inhibited apical chloride secretion and CFTR expression in MDCK cell
The effect of chalcone derivative (CHAL-005) on CFTR-mediated apical chloride secretion was determined by short-circuit current measurement in basolaterally permeabilized MDCK cell monolayers. Of note, the CHAL-005 at doses of 50–150 μM significantly reduced an apical chloride current stimulated by 20 μM of forskolin in a dose-dependent manner (Fig. 2). To determine the effect of chalcone derivatives on CFTR expression, western blot analysis was performed. Incubation MDCK cell monolayers with CHAL-005 at the concentrations between 50 and100 µM for 24 h significantly suppressed CFTR expression in these cells (Fig. 3). Taken together, these findings suggested that CHAL-005 inhibits MDCK cyst progression, in part, by inhibiting CFTR chloride channel activity and expression.
Effect of CHAL-005 on forskolin-mediated chloride secretion in MDCK cells. Under permeabilized condition, MDCK cell monolayers were mount in hemichamber bathing with chloride gradient buffer. a Representative current of apical chloride current after stimulating by forskolin. CHAL-005 at all dose was added into both apical and basolateral hemichamber. The current was record at dose of 20, 50, 100, 150 μM and at the end of the experiment, GlyH-101 (50 μM), a CFTR inhibitor, was added. b Representative of apical short-circuit current tracing after incubation of CHAL-005 at dose of 20–150 μM was shown as a bar graph (six independent experiments, mean of percent control ± SE, *P < 0.05, ***P < 0.001, NS not significant)
Effect of CHAL-005 on CFTR protein expression in MDCK cells. a MDCK cells were incubated with CHAL-005 at all dose for 24 h and were performed by western blot analysis. Representative bands of CFTR and β-actin were shown. b The band intensity was represented as a histogram of indicated proteins in CHAL-005 at dose of 0, 1, 10, 50, 100 μM in MDCK cells. Data were expressed as mean of 100% control ± SE, four independent experiments, **P < 0.01, NS not significant
CHAL-005 inhibited cell proliferation through the suppression of ERK1/2 and mTOR/S6K signaling in MDCK cell and Pkd1 mutant cells
An inhibitory effect of CHAL-005 on cell proliferation was investigated using BrdU cell proliferation assay. The results showed that CHAL-005 (50–100 μM) markedly suppressed cell proliferation in MDCK and Pkd1 mutant cells compared to that of the control (Fig. 4). To determine the mechanism by which chalcone derivative compound (CHAL-005) suppressed cell proliferation, western blot analysis of ERK1/2 and S6 kinase phosphorylation levels was performed. It was found that CHAL-005 at doses between 10 and 100 μM significantly diminished the expression of phosphorylation ERK1/2 and S6K in MDCK cell (Fig. 5a–c). In addition, CHAL-005 at doses 50–100 μM reduced the expression of phosphorylation ERK1/2 and S6K in Pkd1 mutant cells (Fig. 5d–i). Therefore, our findings suggested that CHAL-005 slows MDCK cyst progression, in part, by suppressing cell proliferation through the suppression of ERK1/2 and mTOR/S6K signaling pathways.
Effect of CHAL-005 on cell proliferation in MDCK cells and Pkd1 mutant cells. MDCK, Pkd1+/−, Pkd1−/− cells proliferation were measured by BrdU incorporation. The graph represented mean of percent MDCK (a), Pkd1+/− (b), Pkd1−/− (c) cells proliferation after incubation with DMSO (control), CHAL-005 at dose of 1–100 µM, and 20 μg/ml of blasticidin (a positive control) for 24 h (mean of percent control ± SE, n = 4, *P < 0.05, **P < 0.01, ***P < 0.001, NS not significant compared to that of control)
Effect of CHAL-005 on phosphorylation of ERK1/2 and phosphorylation of S6K proteins expression in MDCK cells and Pkd1 mutant cells. MDCK (a), Pkd1+/− (d), Pkd1−/− (g) cells lysates were performed by western blot analysis. Representative bands of p-ERK1/2, t-ERK1/2, p-S6K, t-S6K, and β-actin were shown. The band intensity was represented as a histogram of indicated proteins in CHAL-005 treated MDCK (b, c), Pkd1+/− (e, f), Pkd1−/− (h, i) cells (0, 1, 10, 50, 100 μM). Data were expressed as mean of 100% control ± SE, four independent experiments, *P < 0.05, **P < 0.01, NS not significant
CHAL-005 stimulated AMP-activated protein kinase in MDCK and Pkd1 mutant cells
It has been shown that an activation of AMPK strongly retarded renal cystogenesis through the inhibition of CFTR and mTOR/S6K expression in a mouse model of PKD [12]. Since CHAL-005 could reduce CFTR expression as well as mTOR/S6K expression in MDCK cell, its action to slow MDCK cyst enlargement may involve AMPK-dependent mechanism. Thus, the effect of CHAL-005 on AMPK protein expression was determined. Using western blot analysis, the result showed that CHAL-005 at dose of 100 μM stimulated AMPK expression both in MDCK and Pkd1 mutant cells (Fig. 6). These results indicated that CHAL-005 retards MDCK cyst progression through the stimulation of AMP-activated protein kinase.
Effect of CHAL-005 on phosphorylation of AMP-activated protein kinase expression in MDCK and Pkd1 mutant cells. MDCK (a), Pkd1+/− (c), Pkd1−/− (e) cells lysates were performed by western blot analysis. Representative bands of p-AMPK, AMPKα, and β-actin were shown. The band intensity was represented as a histogram of indicated proteins in CHAL-005-treated MDCK (b), Pkd1+/− (d), Pkd1−/− (f) cells (0, 1, 10, 50, 100 μM). Data were expressed as mean of 100% control ± SE, four independent experiments, *P < 0.05, **P < 0.01, NS not significant
Discussion
Chalcone, an α-β unsaturated ketone, has been shown to possess several biological activities including antiproliferative effect [21], anti-inflammatory effect [13], anti-diarrheal effect [16], and antitumor effect [14]. Considering the effect of chalcone derivative, we performed the experiment using MDCK cyst model to determine whether chalcone derivative could retard renal cyst growth and cyst formation in this in vitro model of PKD. An induction of MDCK cyst model to mimic the renal cystogenesis in PKD, MDCK cells were grown and suspended in three-dimensional collagen gels containing forskolin [22]. Chalcone derivatives were incubated with MDCK cyst from day 6 to day 12 onwards. Our results showed that among four chalcone derivatives examined (CHAL-005, CHAL-006, CHAL-007, CHAL-011), only CHAL-005 markedly inhibited MDCK cyst enlargement without cytotoxicity. Interestingly, the effect of CHAL-005 in reducing MDCK cyst growth and cyst formation was much greater than that of GlyH-101 (a CFTR inhibitor). This finding suggested that CHAL-005 may slow MDCK cyst progression not only through the inhibition of fluid secretion but also through the inhibition of cell proliferation pathways as well.
Transepithelial fluid secretion is an important mechanism for cyst enlargement. Several previous reports revealed that CFTR chloride channel plays a major role in drawing fluid into the cyst lumen [5, 19]. Inhibition of CFTR function by CFTR inhibitor (thiazolidinone and glycine hydrazide) could retard renal cystogenesis in MDCK and ADPKD mouse model [6]. In the present study, we demonstrated that CHAL-005 significantly inhibited chloride current-mediated by forskolin in permeabilized MDCK cell monolayers in a dose-dependent manner. Furthermore, our western blot analysis confirmed that CHAL-005 significantly reduced CFTR expression at doses between 50 and 100 μM in MDCK cell. This result correlated well with the previous study showing the ability of a chalcone ISLQ to retard MDCK cyst growth by inhibiting CFTR channel activity [18]. The cell proliferation pathways that trigger the cyst formation are also important for PKD progression [3]. Inhibition of several cell proliferation pathways could retard renal cystogenesis and improved renal function in cell and mouse models of PKD [10, 11, 23]. In this study, we found that CHAL-005 suppressed MDCK and Pkd1 mutant cells proliferation through the reduction of phosphorylation of ERK1/2 expression. Moreover, it also significantly inhibited mTOR/S6 kinase expression at doses between 10 and 100 µM after 24 h incubation both in MDCK and Pkd1 mutant cells. These findings suggested that CHAL-005 inhibited cell proliferation through ERK1/2 and mTOR/S6K expression. In line with this notion, chalcone ISLQ was found to inhibit lung cancer cell proliferation and migration through the suppression of PI3K/AKT/mTOR signaling [21]. In addition, it was reported that ISLQ and chalcone derivative significantly suppressed adenoid cystic carcinoma cell growth via the inhibition of mTOR signaling and ERK1/2 pathways by an upregulation of TSC2 protein [17]. Previous study reported that chalcone ISLQ did not alter cAMP levels in human colonic epithelial (T84) cells [18]. Therefore, the broad effects of CHAL-005 in slowing MDCK cyst progression did not likely to involve an alteration of intracellular cAMP level. Taken together, CHAL-005 slowed MDCK cyst growth in part by inhibiting CFTR expression and by reducing of ERK1/2 and mTOR/S6K signaling pathways.
Cell metabolism is one of the targets for the treatment of PKD [24]. AMP-activated protein kinase is a regulation of cell sensing energy. In line with this notion, it was fund that PC1 regulated mTOR activity via phosphorylation of ERK [25]. In addition, an upregulation of AMPK by metformin can downregulate ERK1/2 and mTOR activity in Pkd1−/− cell [26]. It has been revealed that an activation of AMPK by metformin strongly slowed MDCK cyst growth and rodent model of ADPKD by inhibiting mTOR/S6K signaling as well as CFTR channel activity and expression [12]. CHAL-005 was also demonstrated to retard MDCK cyst enlargement by inhibiting CFTR activity and expression as well as suppressing mTOR/S6K signaling. Therefore, CHAL-005 might exert its effect to slow MDCK cyst progression via AMPK dependent mechanism. As expected, CHAL-005 (100 μM) was found to significantly increase the level of AMPK both in MDCK and Pkd1 mutant cells. This result was correlated with the previous study reported that a novel chalcone derivative (CHAL-025) inhibits CFTR chloride channel via AMPK activation and has antidiarrheal effect in a mouse closed loop model of cholera toxin induced fluid secretion [16]. Several lines of evidence suggested that ISLQ stimulated AMPK-mediated GSK3β which protected mitochondrial against iron-catalyzed oxidative stress [27]. In addition, ISLQ also had a cardioprotective effect against ischemic injury through the activation of AMPK [28]. Therefore, it is interesting to observe in the present study that chalcone derivative (CHAL-005) also activated AMPK expression which suppressed MDCK cyst progression.
The pharmacological effect of CHAL-005 to slow MDCK cyst progression observed in the present study was found to involve multitarget proteins. CHAL-005 at low concentration (10 μM) was significantly inhibit cell proliferation through the suppression of ERK1/2 and mTOR/S6K expression. While, the high concentration of CHAL-005 (50–100 μM) was found to suppress CFTR expression and activated AMPK expression, respectively. These combination effects of CHAL-005 in inhibiting cyst enlargement may offer the effective treatment since it likely to suppress several pathways of PKD pathogenesis. However, the detailed mechanisms of CHAL-005 on CFTR, ERK1/2, mTOR, and AMPK are further needed to elucidate.
In conclusion, the present study demonstrates novel mechanisms by which CHAL-005 slows MDCK cyst progression. These occur via the stimulation of AMPK and inhibition of CFTR, ERK1/2 and mTOR/S6K signaling. However, further study is required to elucidate the detailed mechanism of chalcone derivative (CHAL-005) on renal cystogenesis in an in vivo model of PKD. Our findings suggested that chalcone derivative (CHAL-005) could represent as a drug candidate for the treatment of polycystic kidney disease.
References
Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13(9):2384–98.
Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta. 2011;1812(10):1291–300.
Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149–68.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Eng J Med. 2017;377(20):1930–42.
Davidow CJ, Maser RL, Rome LA, Calvet JP, Grantham JJ. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 1996;50(1):208–18.
Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19(7):1300–10.
Yuajit C, Homvisasevongsa S, Chatsudthipong L, Soodvilai S, Muanprasat C, Chatsudthipong V. Steviol reduces MDCK Cyst formation and growth by inhibiting CFTR channel activity and promoting proteasome-mediated CFTR degradation. PLoS ONE. 2013;8:e58871.
Yuajit C, Muanprasat C, Gallagher AR, Fedeles SV, Kittayaruksakul S, Homvisasevongsa S, et al. Steviol retards renal cyst growth through reduction of CFTR expression and inhibition of epithelial cell proliferation in a mouse model of polycystic kidney disease. Biochem Pharmacol. 2014;88(3):412–21.
Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63(6):1983–94.
Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet. 2008;17(11):1505–16.
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103(14):5466–71.
Takiar V, Nishio S, Seo-Mayer P, King JD, Li H, Zhang L, et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA. 2011;108(6):2462–7.
Li W, Sun YN, Yan XT, Yang SY, Kim S, Lee YM, et al. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch Pharmacal Res. 2014;37(2):186–92.
Zhang XR, Wang SY, Sun W, Wei C. Isoliquiritigenin inhibits proliferation and metastasis of MKN28 gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. Mol Med Rep. 2018;18(3):3429–36.
Peng F, Du Q, Peng C, Wang N, Tang H, Xie X, et al. A review: the pharmacology of isoliquiritigenin. Phytother Res. 2015;29(7):969–77.
Yibcharoenporn C, Chusuth P, Jakakul C, Rungrotmongkol T, Chavasiri W, Muanprasat C. Discovery of a novel chalcone derivative inhibiting CFTR chloride channel via AMPK activation and its anti-diarrheal application. J Pharmacol Sci. 2019;140(3):273–83.
Sun ZJ, Chen G, Zhang W, Hu X, Huang CF, Wang YF, et al. Mammalian target of rapamycin pathway promotes tumor-induced angiogenesis in adenoid cystic carcinoma: its suppression by isoliquiritigenin through dual activation of c-Jun NH2-terminal kinase and inhibition of extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2010;334(2):500–2.
Muanprasat C, Sirianant L, Soodvilai S, Chokchaisiri R, Suksamrarn A, Chatsudthipong V. Novel action of the chalcone isoliquiritigenin as a cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor: potential therapy for cholera and polycystic kidney disease. J Pharmacol Sci. 2012;118(1):82–91.
Li H, Findlay IA, Sheppard DN. The relationship between cell proliferation, Cl- secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int. 2004;66(5):1926–38.
Yuajit C, Muanprasat C, Homvisasevongsa S, Chatsudthipong V. Steviol stabilizes polycystin 1 expression and promotes lysosomal degradation of CFTR and beta-catenin proteins in renal epithelial cells. Biomed Pharmacother. 2017;94:820–6.
Tian T, Sun J, Wang J, Liu Y, Liu H. Isoliquiritigenin inhibits cell proliferation and migration through the PI3K/AKT signaling pathway in A549 lung cancer cells. Oncol Lett. 2018;16(5):6133–9.
Sullivan LP, Wallace DP, Grantham JJ. Chloride and fluid secretion in polycystic kidney disease. J Am Soc Nephrol. 1998;9(5):903–16.
Li A, Xu Y, Fan S, Meng J, Shen X, Xiao Q, et al. Canonical Wnt inhibitors ameliorate cystogenesis in a mouse ortholog of human ADPKD. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.95874.
Chang MY, Ong ACM. Targeting new cellular disease pathways in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2018;33(8):1310–6.
Distefano G, Boca M, Rowe I, Wodarczyk C, Ma L, Piontek KB, et al. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol. 2009;29(9):2359–71.
Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med. 2013;19(4):488–93.
Choi SH, Kim YW, Kim SG. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol. 2010;79(9):1352–62.
Zhang X, Zhu P, Zhang X, Ma Y, Li W, Chen JM, et al. Natural antioxidant-isoliquiritigenin ameliorates contractile dysfunction of hypoxic cardiomyocytes via AMPK signaling pathway. Mediat Inflamm. 2013;2013:390890.
Acknowledgements
This work was supported by Ubon Ratchathani University (Grant to CY), Thailand Research Fund (TRF) and Office of the Higher Education Commission (MRG6180153 to CY). Financial support from TRF through the grant DBG6180029 (to CM) was also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Veeraphan, P., Chavasiri, W., Muanprasat, C. et al. A chalcone derivative retards renal cyst enlargement by inhibiting fluid secretion and cell proliferation in an in vitro model of polycystic kidney disease. Clin Exp Nephrol 25, 944–952 (2021). https://doi.org/10.1007/s10157-021-02080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-021-02080-1