Abstract
Background
Hyperuricemia is a known risk factor for end-stage renal disease. Although xanthine oxidase (XO) inhibitors are expected to protect the kidney function, evidence to this end is insufficient at present.
Methods
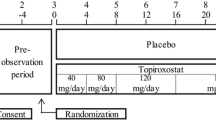
This study was a multi-center, open-labeled, randomized study conducted in Mie Prefecture in Japan. Patients were included if they were between 20 and 80 years old and had a serum uric acid (sUA) level ≥ 7.0 mg/dl with or without gout, estimated glomerular filtration rate (eGFR) of 15–60 ml/min/1.73 m2, and urinary protein creatinine ratio (uPCR) of 0.15–3.5 g/gCr. Patients were randomly assigned to a Topiroxostat or Febuxostat group, and the treatment target for the sUA level was < 6.0 mg/dl. The primary outcome was the change in the uPCR after 24 weeks.
Results
The change in the median uPCR after 24 weeks was not statistically significant after treatment in the Topiroxostat or Febuxostat group (0.05 g/gCr and − 0.04 g/gCr, respectively). However, the sUA levels decreased significantly in both groups (Topiroxostat group: 8.6 ± 1.1 at baseline to 6.0 ± 1.1 mg/dl at 24 weeks, Febuxostat group: 8.4 ± 1.1 mg/dl at baseline to 5.9 ± 1.3 mg/dl at 24 weeks). No significant change in the eGFR after 24 weeks was noted in either the Topiroxostat or Febuxostat group (− 0.04 ± 4.59 ml/min/1.73 m2 and 0.31 ± 4.70 ml/min/1.73 m2, respectively).
Conclusions
In this study, XO inhibitors did not significantly reduce the uPCR in chronic kidney disease stage 3 and 4 patients with hyperuricemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with chronic kidney disease (CKD) have a high risk of cardiovascular events or end-stage renal disease (ESRD), and the treatment of CKD has become more important [1]. However, the current treatments do not show a sufficient efficacy to prevent these events. In a previous observation study, hyperuricemia was a risk factor for kidney dysfunction [2,3,4,5]. However, whether or not the kidney function can be protected by reducing the serum uric acid (sUA) levels remains unclear.
Hyperuricemia evokes xanthine oxidase (XO) activation, generates reactive oxygen species (ROS), and causes endothelial dysfunction [6,7,8]. Topiroxostat and Febuxostat are new XO inhibitors shown to significantly reduce the sUA levels in hyperuricemic patients with or without gout. Indeed, Topiroxostat reduced the sUA levels and urinary albumin excretion in hyperuricemic stage 3 CKD patients with or without gout [9]. Furthermore, Febuxostat reduced the urinary albumin levels more than allopurinol in cardiac surgery patients [10]. However, while XO inhibitors are expected to protect the kidney function, evidence to this end is insufficient at present.
The aim of this study was to investigate the efficacy of XO inhibitors, such as Topiroxostat and Febuxostat, in reducing urinary protein levels in CKD patients with hyperuricemia.
Subjects and methods
Study design
This study was a multi-center, open-labeled, randomized study conducted in Mie Prefecture in Japan. Patients were enrolled between January 2016 and December 2017, from 8 hospitals in Mie Prefecture. Patients were included if they were between 20 and 80 years old and had a sUA level ≥ 7.0 mg/dl with or without gout, estimated glomerular filtration rate (eGFR) of 15–60 ml/min/1.73 m2, and urinary protein–creatinine ratio (uPCR) of 0.15–3.5 g/gCr and provided their written informed consent.
Patients were excluded from the study if they had had a gout attack within the past 4 weeks, had been treated for collagen disease or vasculitis, or were complicated with severe heart disease, liver dysfunction, hematologic disease, malignant disease. The prohibited drugs in this study were anti-hyperuricemic (probenecid, bucolome, benzbromarone, allopurinol), anti-tuberculosis (pyrazinamide, ethambutol), immunosuppressive agents (mizoribine, cyclosporine, mercaptopurine, azathioprine), and theophylline, because of their potential effects on the sUA levels. In principle, renin-angiotensin system (RAS) inhibitors or diuretics were continued without changing the doses during the observation period.
Patients were randomly assigned to receive Topiroxostat or Febuxostat with dynamic randomization, which was conducted by The Critical Research Support Center at Mie University Hospital according to the minimization method by site (each site), uPCR (< 1.0 or ≥ 1.0 g/gCr), and RAS inhibitors (user or non-user). Topiroxostat was given at 40 mg daily and Febuxostat at 10 mg daily as the starting doses. The treatment periods of XO inhibitors were 24 weeks, and the treatment target of the sUA level was < 6.0 mg/dl. Patients were evaluated at baseline and after 12 and 24 weeks of treatment by a physical examination, assessments of adverse effects and outcomes, and any other tests deemed necessary by the attending clinician. As a historical control group, we retrospectively investigated the 24-week changes in the eGFR and uPCR values of the same participants as were included in this study. The values were obtained from the medical records of the participants before their enrollment.
The primary outcome was the change in the uPCR in the 2 groups between baseline and 24 weeks. The secondary outcomes were the changes in the eGFR, sUA levels, systolic and diastolic blood pressure (SBP and DBP, respectively), and urinary liver-type fatty acid-binding protein (L-FABP) between baseline and 24 weeks.
We named this study the Multicenter Investigation of Xanthine Oxidase Inhibitor Efficacy for CKD patients with Hyperuricemia (MIE-CKD), a prospective randomized study in Mie Prefecture in Japan.
Data management
The Critical Research Support Center at Mie University Hospital (https://www.medic.mie-u.ac.jp/chiken/) performed data management. Clinical data were obtained according to the study protocol and registered in the database of the Critical Research Support Center at Mie University Hospital via the Internet by each institution.
Statistical analyses
In this study, the dose of XO inhibitor was adjusted, so that sUA levels were ≤ 6.0 mg/dL. Based on the 58-week administration study performed during the development of Topiroxostat [11], the estimated necessary dose of Topiroxostat was predicted to be between 80 and 120 mg, with 60% of the patients receiving 80 mg daily and 40% of those receiving 120 mg daily (data not shown). Using the results of Phase III trial evaluating the effect of Topiroxostat on urinary albumin in patients with CKD and hyperuricemia [9], the change in urinary protein levels in the Topiroxostat group was able to be predicted, since the change in the urinary protein levels and in the urinary albumin levels showed high correlation. Based on the change in the urinary albumin creatinine ratio (uACR) at the end of administration of 120 mg Topiroxostat [9], the logarithmic transformations of the mean and the standard deviation of the change in the uACR were Log10 0.665 ≒ − 0.178 and Log10 1.981 ≒ 0.297, respectively. The target sample size for obtaining a significant difference in the uPCR change amount after logarithmic transformation using a paired t test at a significance level of 5% (two-sided test) and power of 80% was determined to be 25 samples. Based on the change in the uACR at the end of administration of 80 mg Topiroxostat [11], the logarithmic transformations of the mean and the standard deviation of the change in uACR were Log10 0.841 ≒ − 0.075 and Log10 1.636 ≒ 0.214, respectively. The target sample size for obtaining a significant difference in the uPCR change amount after logarithmic transformation using a paired t test at a significance level of 5% (two-sided test) and power of 80% was determined to be 66 samples. Based on the weighted average considering the dose distribution at the end of administration in this study, the target sample size was set at 50 samples (66 samples × 60% + 25 samples × 40% ≒ 50 samples). Since Febuxostat analyses were assumed to provide similar results, the target sample size was set at 50 samples for that group as well.
The normal distribution data were summarized as the mean ± standard deviation. To assess differences between the two groups, a t test was performed for clinical and laboratory data. Fisher's exact test was performed for categorical data. The uPCR, obtained as non-normally distributed data, was summarized as the median (25–75th percentile), and Wilcoxon’s rank-sum test was used to compare the two groups. Comparisons between baseline and 12 and 24 weeks for normally distributed data were performed by a paired t test, and uPCR values were compared by Wilcoxon’s signed rank test. Comprisons among three groups (two treated groups and historical controls) for normally distributed data were performed by an analysis of covariance (ANCOVA), and uPCR values were compared by the Kruskal–Wallis test. A P value < 0.05 was accepted as significant. Analyses were performed using the SAS software program, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 101 patients were initially enrolled, and 7 were excluded from the data analysis after enrollment because 5 did not meet inclusion criteria and 2 met the exclusion criteria (Fig. 1). Therefore, 46 patients were assigned to the Topiroxostat group and 48 to the Febuxostat group for the analysis. Eighty-eight patients were included as historical controls.
The baseline characteristics of the patients are presented in Table 1. There were significant differences between the Topiroxostat and Febuxostat groups in the SBP, hemoglobin, and urinary L-FABP at baseline. The average doses of Topiroxostat and Febuxostat were 62.2 ± 9.2 mg and 19.8 ± 2.8 mg daily at 24 weeks.
Primary outcome
The changes in the uPCR are depicted in Fig. 2. The uPCR variation did not satisfy the lognormal distribution, so a statistical analysis was performed using a nonparametric method. In both groups and all periods, the median change in the uPCR was not statistically significant after treatment. The median uPCR in the Topiroxostat group was 0.56 g/gCr at baseline and 0.58 g/gCr at 24 weeks, and that in the Febuxostat group was 0.69 g/gCr at baseline and 0.50 g/gCr at 24 weeks. The changes in the median uPCR after 24 weeks in the Topiroxostat and Febuxostat groups were 0.05 g/gCr and − 0.04 g/gCr, respectively (P = 0.706 and P = 0.653, respectively; Wilcoxon’s rank-sum test). No statistically significant differences were noted between the 2 groups (P = 0.672; Wilcoxon’s rank-sum test).
Secondary outcomes
The changes in the sUA level are depicted in Fig. 3. The sUA levels in the Topiroxostat and Febuxostat groups were significantly decreased from baseline after treatment. The sUA levels in the Topiroxostat group were 8.6 ± 1.1 mg/dl at baseline and 6.0 ± 1.1 mg/dl at 24 weeks, and those in the Febuxostat group were 8.4 ± 1.1 mg/dl at baseline and 5.9 ± 1.3 mg/dl at 24 weeks. The changes in the sUA level after 24 weeks in the Topiroxostat and Febuxostat groups were − 2.6 ± 1.0 mg/dl and − 2.7 ± 1.1 mg/dl at 24 weeks, respectively.
The changes in the eGFR are depicted in Fig. 4. In both groups and all periods, the eGFR showed no significant change after treatment. The eGFR in the Topiroxostat group was 35.9 ± 12.2 ml/min/1.73 m2 at baseline and 36.1 ± 13.0 ml/min/1.73 m2 at 24 weeks, while that in the Febuxostat group was 35.9 ± 11.4 ml/min/1.73 m2 at baseline and 36.7 ± 12.6 ml/min/1.73 m2 at 24 weeks. The changes in the eGFR after 24 weeks in the Topiroxostat and Febuxostat group were − 0.04 ± 4.59 ml/min/1.73 m2 and 0.31 ± 4.70 ml/min/1.73 m2, respectively.
In the historical control group, the change in the median uPCR was − 0.03 g/gCr, and the change in the eGFR was − 1.2 ± 4.1 ml/min/1.73 m2 during the observation period. Neither the Topiroxostat nor the Febuxostat group showed any significant difference in the changes in the median uPCR or eGFR compared with historical controls.
The SBP, DBP, and urinary L-FABP showed no significant changes in either group in all periods. No severe adverse events caused by XO inhibitors were observed in our study.
Discussion
In this study, we investigated the efficacy of XO inhibitors on reducing the urinary protein level in CKD stage 3 and 4 patients. No significant reduction in the uPCR was noted during the observation period in the Topiroxostat or Febuxostat groups.
Xanthine oxidoreductase (XOR) is a general term for xanthine dehydrogenase (XDH) and XO. Typically, XDH catalyzes the degradation of purine bodies to uric acid (UA) in the liver and bowels without producing ROS. However, hypoxia as well as inflammation induce XDH expression in the liver, bowels, and vascular endothelial cells, and XDH is thus released into the circulation, where it is rapidly converted to XO. XO then oxidizes hypoxanthine to xanthine and subsequently oxidizes xanthine to UA, resulting in the production of ROS [7]. Other studies have found that hyperuricemia induces endothelial dysfunction associated with mitochondrial alterations [8]. In theory, XO inhibitors should help to reduce ROS produced by XO and protect endothelial cells or organs, such as the kidneys, liver, heart, and vessels.
Although the sUA levels were significantly decreased by XO inhibitors in both groups, we found no evidence of kidney protection. We propose several reasons for this finding. First, small doses of intervention drugs might have kept the uPCR from being reduced to a significant degree. At 24 weeks, only two patients (4.4%) in the Topiroxostat group and one patient (2.3%) in the Febuxostat group were treated with the maximum dose (Topiroxostat 160 mg, Febuxostat 60 mg), respectively. Hosoya et al. reported that Topiroxostat 160 mg effectively reduced the uACR in hyperuricemic CKD patients with or without gout [9]. Similarly, the ETUDE study reported that Topiroxostat 160 mg reduced the uACR in diabetic nephropathy patients with hyperuricemia [12], and while the UPWARD study reported that Topiroxostat 160 mg did not reduce the uACR, it still prevented a decline in the eGFR in diabetic nephropathy patients with hyperuricemia [13]. Of note, Febuxostat (40 mg daily) slowed the eGFR decline in CKD patients compared to placebo [14]. In another report, Febuxostat (maximum 60 mg daily) exerted superior antioxidant and anti-inflammatory effects to allopurinol (maximum 300 mg daily) in cardiac surgery patients with CKD [10]. Furthermore, in basic research, XO inhibitor dose-dependently decreased the urinary albumin excretion and inhibited plasma XO activity [15]. Second, it might not be appropriate to assume that the sUA levels reflect kidney protection. Sunagawa et al. reported that the sUA level was not correlated with the plasma XO activity [16]. In addition, in the present study, the inhibition of the XO activity may not have been sufficiently achieved to improve uPCR, although the sUA levels did clinically decrease. Third, it might be difficult for CKD patients with overt proteinuria to obtain a kidney protection effect with XO inhibitors. Indeed, a sub-analysis of the FEATHER study showed that Febuxostat improved the reduction in the eGFR in CKD patients with negative proteinuria, but had no effect on those with positive proteinuria [17]. On the other hand, several meta-analyses of clinical trials centering on allopurinol have shown that XO inhibitor treatment provides effective renal protection [18,19,20,21]. Well-designed, placebo-controlled RCTs of newly XO inhibitors are needed to resolve these problems.
Limitations
Several limitations associated with the present study warrant mention, including the small number of patients and short term of observation. We calculated the sample size based on the previous reports that evaluated the uACR. It might, therefore, be difficult to establish any differences by comparing uACR and uPCR values. We must confirm the effect of XO inhibitors on CKD patients in larger populations with a longer observation period. The urinary PCR after 24 weeks was highly variable. The observation period of our study was 24 weeks, so proteinuria might have shown seasonal fluctuations [22]. Three cases (1 in the Topiroxostat group; 2 in the Febuxostat group) in which the urine protein level increased by ≥ 3 g/gCr were all diabetic patients who were not taking RAS inhibitors. An analysis by excluding these 3 “outliners” showed a significant reduction in proteinuria in the Febuxostat group (log change in PCR: − 0.084 ± 0.277, P = 0.049). The influence of cases with extreme urinary protein fluctuations would counteract the urinary protein reduction effects of XO inhibitors; however, excluding such “outliers” was thought to be a violation of the protocol established before the start of the study.
Conclusion
In the present study, newly developed XO inhibitors had no marked effects on reducing the uPCR in CKD stage 3 and 4 patients with hyperuricemia.
References
Kimura K. Preface: evidence-based clinical practice guidelines for CKD: an abridged English version. Clin Exp Nephrol. 2014;18:339–40.
Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691–7.
Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PLoS ONE. 2014;9:e100801.
Odden MC, Amadu AR, Smit E, Lo L, Peralta CA. Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988–1994 and 1999–2002. Am J Kidney Dis. 2014;64:550–7.
Kawashima M, Wada K, Ohta H, Terawaki H, Aizawa Y. Association between asymptomatic hyperuricemia and new-onset chronic kidney disease in Japanese male workers: a long-term retrospective cohort study. BMC Nephrol. 2011;12:31.
Maxwell AJ, Bruinsma KA. Uric acid is closely linked to vascular nitric oxide activity. Evidence for mechanism of association with cardiovascular disease. J Am Coll Cardiol. 2001;38:1850–8.
Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biol. 2013;1:353–8.
Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121:e71–e7878.
Hosoya T, Ohno I, Nomura S, Hisatome I, Uchida S, Fujimori S, et al. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin Exp Nephrol. 2014;18:876–84.
Sezai A, Soma M, Nakata K, Osaka S, Ishii Y, Yaoita H, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU-FLASH trial for CKD). J Cardiol. 2015;66:298–303.
Hosoya T, Ishikawa T, Ogawa Y, Sakamoto R, Ohashi T. Multicenter, open-label study of long-term topiroxostat (FYX-051) administration in Japanese Hyperuricemic patients with or without gout. Clin Drug Investig. 2018;38:1135–43.
Mizukoshi T, Kato S, Ando M, Sobajima H, Ohashi N, Naruse T, et al. Renoprotective effects of topiroxostat for hyperuricaemic patients with overt diabetic nephropathy study (ETUDE study): a prospective, randomized, multicentre clinical trial. Nephrology (Carlton). 2018;23:1023–30.
Wada T, Hosoya T, Honda D, Sakamoto R, Narita K, Sasaki T, et al. Uric acid-lowering and renoprotective effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in patients with diabetic nephropathy and hyperuricemia: a randomized, double-blind, placebo-controlled, parallel-group study (UPWARD study). Clin Exp Nephrol. 2018;22:860–70.
Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized placebo-controlled trial. Am J Kidney Dis. 2015;66:945–50.
Nakamura T, Murase T, Nampei M, Morimoto N, Ashizawa N, Iwanaga T, et al. Effects of topiroxostat and febuxostat on urinary albumin excretion and plasma xanthine oxidoreductase activity in db/db mice. Eur J Pharmacol. 2016;780:224–31.
Sunagawa S, Shirakura T, Hokama N, Kozuka C, Yonamine M, Namba T, et al. Activity of xanthine oxidase in plasma correlates with indices of insulin resistance and liver dysfunction in patients with type 2 diabetes mellitus and metabolic syndrome: a pilot exploratory study. J Diabetes Investig. 2019;10:94–103.
Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72:798–810.
Qu LH, Jiang H, Chen JH. Effect of uric acid-lowering therapy on blood pressure: systematic review and meta-analysis. Ann Med. 2017;49:142–56.
Sampson AL, Singer RF, Walters GD. Uric acid lowering therapies for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst Rev. 2017;10:Cd009460.
Liu X, Zhai T, Ma R, Luo C, Wang H, Liu L. Effects of uric acid-lowering therapy on the progression of chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2018;40:289–97.
Pisano A, Cernaro V, Gembillo G, D'Arrigo G, Buemi M, Bolignano D. Xanthine Oxidase inhibitors for improving renal function in chronic kidney disease patients: an updated systematic review and meta-analysis. Int J Mol Sci. 2017;18.
Wada Y, Hamamoto Y, Ikeda H, Honjo S, Kawasaki Y, Mori K, et al. Seasonal variations of urinary albumin creatinine ratio in Japanese subjects with Type 2 diabetes and early nephropathy. Diabet Med. 2012;29:506–8.
Acknowledgements
We are grateful to Dr. Tomomi Yamada and Dr. Kazuya Murata for assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The MIE-CKD study was sponsored by Sanwa Kagaku Kenkyusho CO., LTD., which had no role in the data collection or analysis, decision to publish, or preparation of the manuscript. S.N. received lecture fees of more than 500,000 yen from Otsuka Pharma Inc. in 2016. M.I received lecture fees of more than 500,000 yen from Bayer Yakuhin, Ltd., Daiichi Sankyo Co. Ltd. and Mitsubishi Tanabe Pharma Corporation in 2016. The Department of Cardiology and Nephrology, Mie University Graduate School of Medicine, was supported in part by unrestricted research grants of more than 1,000,000 yen from Daiichi Sankyo Co. Ltd., Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., MSD K.K., Astellas Pharma Inc., Takeda Pharmaceutical Company Limited and Pfizer Japan Inc. in 2016. The Division of Hemodialysis Center, Mie University Hospital, was supported in part by unrestricted research grants of more than 1,000,000 yen from Sanwa Kagaku Kenkyusho Co., Ltd., in 2016. No relevant conflicts of interest related to the article were disclosed by the authors.
Ethical approval
The protocol was initially approved by the Medical Research Ethics Review Committee of Mie University Hospital (reference number 2778) and subsequently by the institutional review boards at all other participating hospitals in accordance with the Declaration of Helsinki. This study was registered in the UMIN Clinical Trials Registry (registration ID number: UMIN000016172).
Informed consent
Written informed consent was obtained from all patients before enrolment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Matsuo, H., Ishikawa, E., Machida, H. et al. Efficacy of xanthine oxidase inhibitor for chronic kidney disease patients with hyperuricemia. Clin Exp Nephrol 24, 307–313 (2020). https://doi.org/10.1007/s10157-019-01829-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01829-z