Abstract
Background
High-normal albuminuria is an important risk factor for incident chronic kidney disease in diabetic populations, in contrast to an uncertain association in nondiabetic populations. This study aimed to reveal the relationship between high-normal albuminuria and incident chronic kidney disease in a Japanese nondiabetic population.
Methods
A 10-year follow-up retrospective cohort study was performed involving 1378 Japanese men (mean age 44 ± 5.3 years) without chronic kidney disease and diabetes mellitus. Chronic kidney disease was diagnosed as either estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio ≥ 30 mg/g.
Results
At baseline, age, estimated glomerular filtration rate, and the presence of hematuria, hypertension, and dyslipidemia were independently associated with the albumin-to-creatinine ratio. Among the 1378 participants, 185 (13.4%) fulfilled diagnostic criteria for chronic kidney disease over the 10-year follow-up period. Median annual estimated glomerular filtration rate decline showed a deterioration with increasing quartiles of baseline albumin-to-creatinine ratio (P = 0.004). Participants who had a baseline albumin-to-creatinine ratio in the highest quartile (5.9–28.9 mg/g) were more likely to develop micro- or macroalbuminuria (odds ratio: 16.23, 95% confidence interval 6.56–54.03), chronic kidney disease (odds ratio: 2.48, 95% confidence interval 1.64–3.82), and hypertension (odds ratio 2.06, 95% confidence interval 1.30–3.31), but not diabetes mellitus compared with those who had an albumin-to-creatinine ratio in the lowest quartile (1.3–3.6 mg/g) after adjustment for potential confounders.
Conclusions
High-normal albuminuria was associated with incident chronic kidney disease in this Japanese nondiabetic male population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) affects more than 10% of the population globally and is a world health concern [1]. The financial impact of CKD is also great, with particularly high costs depending on renal replacement therapy and cardiovascular complications [2, 3]. Recently, the kidney disease: improving global outcomes updated their classifications for determining CKD based on glomerular filtration rate (GFR) and urine albumin-to-creatinine ratio (ACR), with a definition of GFR < 60 mL/min/1.73 m2 and of ACR ≥ 30 mg/g [4]. Notably, the risks for important outcomes including all-cause mortality, cardiovascular diseases, and kidney failure have been visually captured in a “heat map” [4], providing recommendations on how GFR and ACR should be estimated together. In addition to GFR and primary diseases, the importance of ACR is widely recognized in clinical practice.
ACR from a spot urine sample reflects the daily amount of urinary albumin excretion [4]. The guidelines propose three categories: normal albuminuria (< 30 mg/g); microalbuminuria (30–300 mg/g); and macroalbuminuria (> 300 mg/g) [4]. In the clinical setting, microalbuminuria is widely used as a urinary marker for the early detection of diabetic nephropathy in patients with type 2 diabetes mellitus (DM) [5], and its clinical usefulness has been established [6]. In the general population, microalbuminuria is reported to be one predictor of a decline in GFR (< 60 mL/min/1.73 m2) [7, 8]. These factors suggest that a very small amount of albuminuria may be a precursor or sign of possible renal dysfunction. Although normal albuminuria is classified as low-risk, a previous study reported that high-normal albuminuria is an important risk factor for incident CKD in patients with DM [9]. High-normal albuminuria is reported to predict not only cardiovascular events but also all-cause mortality [10,11,12]. However, there is currently little information on the association between normal-range albuminuria and future CKD in the nondiabetic population (non-DM).
In this study, we investigated whether increased albuminuria is associated with incident CKD in a non-DM population with an ACR < 30 mg/g. This retrospective cohort study evaluated the correlation between normal albuminuria and the incidence of CKD in 1378 general Japanese men without DM over a 10-year follow-up period.
Materials and methods
Participants
We extracted data of 1709 men from the general health checkup database of the Nippon Telegraph and Telephone West Corp., Chugoku Health Administration Center (Hiroshima, Japan) between April 1999 and March 2004 who had values for serum creatinine and albuminuria that had been measured twice at an interval of 10 years. Four hundred and six people were excluded, because they met exclusion criteria at the first examination. Exclusion criteria were: (1) CKD defined as GFR < 60 mL/min/1.73 m2 or ACR ≥ 30 mg/g; (2) DM defined as hemoglobin A1c (HbA1c) ≥ 6.5%, 2-h plasma glucose ≥ 200 mg/dL with a 75-g oral glucose tolerance test, fasting plasma glucose ≥ 126 mg/dL, or medical history of DM [13]; and (3) antihypertensive drugs that may affect excretion of albumin. The remaining 1378 people were enrolled in this study.
Anthropometry, blood pressure, and smoking status
Medical information was obtained via a standardized questionnaire, which included demographic background and medical history. Height and weight were measured, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure (BP) was measured in the sitting position using a mercury sphygmomanometer after a 5-min rest. Hypertension was defined as systolic BP ≥ 140 mmHg and diastolic BP ≥ 90 mmHg [14]. Current smoking was defined as having more than one cigarette a day.
Laboratory analysis
Urine albumin was measured using the latex flocculation immunoturbidimetry assay (Eiken Chemical, Tokyo, Japan). Urine creatinine was measured using an enzymatic method. Urine albumin was divided by urine creatinine to obtain the ACR, and the ACR is expressed in milligrams per gram (mg/g). Hematuria was defined as the presence of ≥ five red blood cells/high-powered field or more than 1 + with the dipstick test.
Blood samples were obtained in the morning after an overnight fast. Serum total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, creatinine, uric acid, and urinary creatinine levels were measured using enzymatic methods (Eiken Chemical). Fasting glucose and HbA1c levels were measured using high-performance-liquid chromatography. After correcting to the value suggested by the Japan Diabetes Society, we estimated the HbA1c level as the National Glycohemoglobin Standardization Program equivalent value using the formula: HbA1c (%) = 1.02 × HbA1c (JDS; %) + 0.25 [15]. We calculated eGFR using the Modification of Diet in Renal Disease equation: eGFR = 194 × Cr− 1.094 × age− 0.287 [16]. Low-density lipoprotein (LDL) cholesterol levels were calculated using Friedewald’s formula [17]. Dyslipidemia was defined as LDL cholesterol ≥ 140 mg/dL, HDL cholesterol < 40 mg/dL, triglycerides ≥ 150 mg/dL, or use of lipid-lowering drugs [18, 19]. Hyperuricemia was defined as urinary acid ≥ 7.0 mg/dL or use of antihyperuricemic drugs [20].
Statistical analysis
Participants were separated by quartiles of ACR (1.3–3.6, 3.7–4.4, 4.5–5.8, and 5.9–28.9 mg/gCr). Variables are expressed as mean ± standard deviation or median and interquartile range (25th–75th percentiles), according to normality of distribution. Kruskal–Wallis and Mantel–Haenszel tests for trend were used to compare the baseline characteristics according to quartiles of ACR. Comparisons between two groups were assessed using Wilcoxon tests, Student’s t tests, and Chi-squared tests. Stepwise multiple regression analysis was performed to find independent predictors of baseline ACR among potential confounders (P < 0.05) in univariate analysis. Final multiple regression analyses were performed. Logistic regression approaches were used to assess determinants of the subsequent incidence of CKD, which are presented as odds ratio (OR) and 95% confidence intervals (95% CI). A P value < 0.05 was considered statistically significant. All analyses were performed using Statistical Package for the Social Sciences software (ver. 21.0; IBM, Armonk, NY, USA).
Results
Median ACR for the 1378 participants was 4.5 mg/g (3.6–5.9 mg/g). Baseline characteristics are shown in Table 1 and are stratified by quartile of ACR. Significant positive trends across ACR quartiles were observed for age (P = 0.002), triglycerides (P = 0.008), eGFR (P < 0.001), BMI (P = 0.001), systolic BP (P < 0.001), diastolic BP (P < 0.001), current smoking (P = 0.007), and hematuria (P < 0.001).
For cross-sectional analysis, variables were selected by stepwise multiple regression analysis among potential confounders (P < 0.05) in univariate analysis. Liner regression analysis revealed that age (P < 0.001), eGFR (P < 0.001), and the presence of hematuria (P < 0.001), hypertension (P < 0.001), and dyslipidemia (P = 0.009) were independently associated with ACR (Table 2).
Among the 1378 participants, 185 (13.4%) fulfilled diagnostic criteria for CKD over the 10-year follow-up period. When participants who developed incident CKD were compared with participants in whom renal function remained normal, baseline age (P < 0.001), ACR (P < 0.001), BMI (P = 0.003), systolic BP (P < 0.001), diastolic BP (P < 0.001), and urinary acid (P = 0.032) were significantly greater, and HDL cholesterol (P = 0.016) and eGFR (P < 0.001) were significantly lower (Table 3).
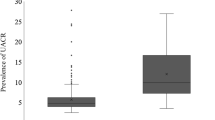
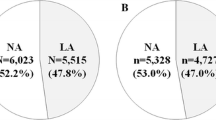
Median subsequent decline in eGFR worsened with increasing quartiles of baseline ACR (P = 0.004 for trend) (Fig. 1). There was 22.6% of participants who developed CKD in the highest quartile of baseline ACR and the rate of incident CKD increased with increasing quartiles of ACR (P = 0.002 for trend) (Fig. 2). In the highest quartile of baseline ACR, 61.6% of participants remained within the same range of ACR over the 10-year period, while 15.8% developed micro- or macroalbuminuria (Fig. 3). The rates of incident micro- or macroalbuminuria were higher with increasing quartiles of ACR (P < 0.001 for trend). In the highest quartile of baseline ACR, 22.6% of participants showed a change to a lower range over the 10-year period (Fig. 3).
With logistic regression analyses, when compared with participants in the lowest ACR quartile, the OR for incident CKD for participants in the highest quartile was 2.48 (95% CI 1.64–3.82; P < 0.001) (Table 4). This difference persisted after adjustment for age, baseline eGFR, BMI, smoking status, and the presence of hypertension, hematuria, hyperuricemia, and dyslipidemia (OR 3.57, 95% CI 2.25–5.76; P < 0.001). When the highest and lowest quartiles of ACR were compared, the OR for incident micro- or macroalbuminuria was 16.23 (95% CI 6.56–54.03; P < 0.001) (Table 4), which also persisted after adjustment for age, baseline eGFR, BMI, smoking status, and the presence of hypertension, hematuria, hyperuricemia, and dyslipidemia (OR 14.03, 95% CI 5.49–47.58; P < 0.001).
Because there was the possibility that some patients at the early stage of glomerular nephritis were included in the population with hematuria, we also analyzed those participants without hematuria at baseline. When compared with participants in the lowest quartile of ACR, the OR for incident CKD and incident micro- or macroalbuminuria for participants in the highest quartile were 2.63 (95% CI 1.71–4.11; P < 0.001) and 16.87 (95% CI 6.79–56.31; P < 0.001), respectively (Table 4). These differences persisted after adjustment for age, baseline eGFR, BMI, smoking status, and the presence of hypertension, hyperuricemia, and dyslipidemia (OR 3.70, 95% CI 2.30–6.06; P < 0.001 and OR 13.26, 95% CI 5.17–45.06; P < 0.001, respectively) (Table 4).
Over the 10-year follow-up period, 75 participants (5.4%) met the diagnostic criteria for DM. Although the highest quartile of ACR was associated with an increased risk for incident DM compared with the lowest quartile of ACR (OR 2.10, 95% CI 1.13–4.08; P = 0.019), there was no significant association between ACR and incident DM after adjustment for age, baseline GFR, hematuria, BMI, smoking status, hyperuricemia, and dyslipidemia (Table 4). Among the 1124 participants without hypertension at baseline, 194 (17.2%) met the diagnostic criteria for hypertension over the 10-year follow-up period. When compared with participants in the lowest quartile of ACR in this population, the OR for incident hypertension for participants in the highest quartile of ACR was 2.18 (95% CI 1.41–3.39; P < 0.001) (Table 5). This difference persisted after adjustment for age, baseline eGFR, hematuria, BMI, smoking status, hyperuricemia, and dyslipidemia (OR 2.06, 95% CI 1.30–3.31; P = 0.002).
Discussion
This is a retrospective cohort study of data of medical examinations from 1378 Japanese men without DM. We found that ACR correlated with age, hematuria, hypertension, dyslipidemia, and decline in eGFR. Although ACR is a urinary marker for diabetes nephropathy [9], the results indicate that a high-normal ACR is independently associated with not only the development of micro- or macroalbuminuria but also with the incidence of CKD in this non-DM population. These findings suggest that people with high-normal ACR should be considered as potential future CKD patients.
We found that 13.4% of participants in the study were diagnosed with CKD over the 10-year period from medical checkups. The previous studies have reported that age, DM, obesity, hypertension, smoking, and low HDL cholesterol levels are risk factors for incident CKD [21, 22]. Although these factors worsen renal function, the findings of the current study suggest that high-normal ACR independently correlated with an increased risk for incident CKD even after adjustment for these factors (Table 4; Model 2). A possible explanation is that high-normal ACR may reflect the early phase of renal damage. Therefore, evaluation of ACR may be a useful tool to help predict future incident CKD even in patients within the normal range.
In this study, we found that high-normal ACR was a predictive factor for incident hypertension in this population, a finding supported by the previous research [23, 24]. The previous studies report that glomerular endothelial cells act as a barrier against albumin filtration [25], and that endothelial cell dysfunction, such as loss of the glycocalyx, leads to increased albumin excretion [26]. Some studies have also reported an association between urinary albumin excretion and endothelial dysfunction [27,28,29,30]. These findings indicate that the relationship between increased albuminuria and the progression of cardiovascular disease as well as kidney disease is suggestive of systemic endothelial dysfunction. In contrast, high-normal ACR was not associated with incident DM in the current study after adjustment for potential confounders, suggesting that normal-range ACR is not a predictor of future impaired glucose tolerance.
In the current study, even though median baseline eGFR increased with increasing quartiles of ACR, the subsequent decline in the eGFR was greatest in the highest quartile. A previous study reported that albuminuria in the range of 15–30 mg/24 h was independently associated with increased glomerular filtration in a non-DM population [31], which eventually leads to renal damage. Baseline albuminuria may increase in response to glomerular hemodynamic changes and, therefore, predict the incidence of CKD. Protein overload may contribute to exacerbate tubulointerstitial injury [32, 33], but the direct influence of low-grade albuminuria on tubulointerstitial damage is poorly understood. Therefore, further studies are needed to help determine the mechanisms.
From baseline data in the current study, multivariate analysis revealed that ACR independently correlated with baseline age, eGFR, and the presence of hematuria, hypertension, and dyslipidemia. In contrast, a past study reported that ACR levels not only increased but also decreased in a non-DM population over time [34, 35]. At the completion of the 10-year follow-up period in the current study, 22.6% of participants were observed to change to a lower ACR range. These findings suggest that the necessity of therapeutic intervention should be evaluated with other risk factors, such as age, baseline eGFR, hematuria, BMI, smoking status, hypertension, hyperuricemia, and dyslipidemia.
In the cross-sectional analysis, the presence of microscopic hematuria was independently associated with albuminuria. We did not exclude extrarenal causes of microscopic hematuria and some patients at the early stage of glomerular nephritis may be included in the group. However, we also found that high-normal ACR was associated with incident CKD, even in participants without hematuria. The previous studies have reported that persistent asymptomatic microscopic hematuria is a predictive risk marker of end-stage renal disease [36, 37]. In the current study, the presence of microscopic hematuria was not associated with the subsequent incidence of CKD and micro- or macroalbuminuria over the 10-year follow-up period (data not shown).
This study has several limitations. First, data were derived from records of medical checkups at a company that we could follow for 10 years retrospectively. As a result, the proportion of female participants was less than 9.1%, and we could not examine the impact of ACR on the incidence of CKD in females. Second, because the study group was people of Japanese ethnicity, the results need to be replicated in other ethnic groups and should not be generalized without caution. Finally, we only used a single urine specimen to assess the ACR, which has day-to-day variability [38].
In summary, we found that high-normal albuminuria was associated with incident CKD in this non-DM population of Japanese men. We also found that baseline ACR correlated with age, hematuria, hypertension, dyslipidemia, and eGFR. These results suggest that evaluation of ACR and ACR-related factors at medical checkups may help to prevent the future incidence of CKD.
References
Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–69.
Kerr M, Bray B, Medcalf J, et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(Suppl 3):iii73–i80.
Travers K, Martin A, Khankhel Z, et al. Burden and management of chronic kidney disease in Japan: systematic review of the literature. Int J Nephrol Renovasc Dis. 2013;6:1–13.
Detlef Schlondorff KDIGO 2012. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Association AD. Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl 1):S4–36.
Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care. 2014;37:867–75.
O’Seaghdha CM, Lyass A, Massaro JM, et al. A risk score for chronic kidney disease in the general population. Am J Med. 2012;125:270–77.
Halbesma N, Jansen DF, Heymans MW, et al. Development and validation of a general population renal risk score. Clin J Am Soc Nephrol. 2011;6:1731–8.
Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–9.
Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–5.
Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81.
Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324.
Association AD. Erratum. Classification and diagnosis of diabetes. Section 2. In Standards of Medical Care in Diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S13–22 (Diabetes Care. 2016;39:1653).
James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
National Cholesterol Education Program (NCEP). Expert panel on detection Ea, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
Okamura T, Tanaka H, Miyamatsu N, et al. The relationship between serum total cholesterol and all-cause or cause-specific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis. 2007;190:216–23.
Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15:189–92.
Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50.
Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71:159–66.
Forman JP, Fisher ND, Schopick EL, et al. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19:1983–8.
Jessani S, Levey AS, Chaturvedi N, et al. High normal levels of albuminuria and risk of hypertension in Indo-Asian population. Nephrol Dial Transplant. 2012;27(Suppl 3):iii58–i64.
Ballermann BJ, Stan RV. Resolved: capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol. 2007;18:2432–8.
Rabelink TJ, de Zeeuw D. The glycocalyx-linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol. 2015;11:667–76.
Clausen P, Jensen JS, Jensen G, et al. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869–74.
Dogra G, Rich L, Stanton K, et al. Endothelium-dependent and independent vasodilation studies at normoglycaemia in type I diabetes mellitus with and without microalbuminuria. Diabetologia. 2001;44:593–601.
Clausen P, Feldt-Rasmussen B, Jensen G, et al. Endothelial haemostatic factors are associated with progression of urinary albumin excretion in clinically healthy subjects: a 4-year prospective study. Clin Sci (Lond). 1999;97:37–43.
Martens RJ, Henry RM, Houben AJ, et al. Capillary rarefaction associates with albuminuria: the Maastricht Study. J Am Soc Nephrol. 2016;27(12):3748–57.
Pinto-Sietsma SJ, Janssen WM, Hillege HL, et al. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol. 2000;11:1882–8.
Nangaku M, Pippin J, Couser WG. C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J Am Soc Nephrol. 2002;13:928–36.
Zoja C, Benigni A, Remuzzi G. Cellular responses to protein overload: key event in renal disease progression. Curr Opin Nephrol Hypertens. 2004;13:31–7.
Brantsma AH, Atthobari J, Bakker SJ, et al. What predicts progression and regression of urinary albumin excretion in the nondiabetic population? J Am Soc Nephrol. 2007;18:637–45.
Suzuki K, Konta T, Takasaki S, et al. High variability of albuminuria in nondiabetic population: the Takahata Study. Clin Exp Nephrol. 2009;13:452–9.
Vivante A, Afek A, Frenkel-Nir Y, et al. Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA. 2011;306:729–36.
Gutiérrez E, González E, Hernández E, et al. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin J Am Soc Nephrol. 2007;2:51–7.
Naresh CN, Hayen A, Weening A, et al. Day-to-day variability in spot urine albumin–creatinine ratio. Am J Kidney Dis. 2013;62:1095–101.
Acknowledgements
The authors thank the participants in this study and all research assistants involved in the data acquisition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exist.
Ethical approval
This study was performed in accordance with the guidelines contained within the Declaration of Helsinki and the protocol was licensed by the hospital ethics committee of Hiroshima University Hospital (Approval no. E-223).
Informed consent
Informed consent was obtained from all individual participants included in this study.
About this article
Cite this article
Ashitani, A., Ueno, T., Nakashima, A. et al. High-normal albuminuria and incident chronic kidney disease in a male nondiabetic population. Clin Exp Nephrol 22, 835–842 (2018). https://doi.org/10.1007/s10157-017-1522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1522-6