Abstract
Background
The renal resistive index (RI) is a Doppler-derived measure that reportedly correlates with renal histological changes and renal disease severity and outcome. The aim of this study was to investigate the factors related to the RI elevation in chronic kidney disease (CKD).
Methods
Using Doppler ultrasonography, RIs were determined in 30 patients with CKD, after which they were correlated with interstitial fibrosis, arteriosclerosis, arteriolosclerosis and peritubular capillary (PTC) density. PTC-positive areas were determined based on CD34 immunostaining. Interstitial fibrosis was detected with Masson trichrome staining. All histological markers were assessed using quantitative and semi-quantitative analyses and evaluated statistically using Pearson correlation tests, unpaired t tests and stepwise multiple regression analysis.
Results
RI correlated positively with age (r = 0.603, p = 0.0004), systolic blood pressure (r = 0.775, p < 0.0001), diastolic blood pressure (r = 0.575, p = 0.001), interstitial fibrosis (r = 0.381, p = 0.038) and arteriosclerosis (r = 0.520, p = 0.003), and negatively with creatinine clearance (r = −0.471, p = 0.009) and CD34+ (PTC) areas (r = −0.437, p = 0.016). Patients with hypertension or diabetes mellitus showed higher RIs (p < 0.05) than those without the ailments. Multivariate analysis showed PTC and arteriosclerosis to be independent variables correlating with RI (r 2 = 0.321, p < 0.05).
Conclusions
To our knowledge, this is the first report of using RI measurements to evaluate peritubular capillary loss. Our findings indicate that increases in RI are associated with both arteriosclerosis and loss of PTCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The renal resistive index (RI) is a Doppler-derived measure that has been frequently used to evaluate acute rejection of renal transplants [1, 2]. Among the other factors that can affect RI is aging, which contributes to RI elevation [3]. In addition, recent evidence suggests that in patients with hypertension, RI values correlate with organ damage severity [4, 5], and in patients with diabetic nephropathy, it correlates with disease stage [6, 7]. Several studies have also shown that RI is predictive of renal disease outcome [2, 8, 9]. Because measuring RI is convenient and noninvasive, evaluation of the relationship between RI and histopathology is an important means of estimating disease features, especially for the patients who cannot tolerate renal biopsy.
There have been several studies evaluating the correlation between RI and renal histopathology [9–15]. In some of those studies, glomerulosclerosis appeared to contribute to increases in RI [13, 14], though one review reported that diseases limited to the glomeruli were not associated with significant RI elevation [15]. Strong correlations between RI and the severity of vascular lesions, such as with vasculitis or arterio/arteriolosclerosis, were identified in several studies [9, 13–15]. And correlations have also been observed between RI and tubulointerstitial lesions (e.g., acute tubular injury, tubulointerstitial nephritis and interstitial fibrosis) [10, 11, 13, 15]. Considering that glomerulosclerosis, arterio/arteriolosclerosis and vasculitis all involve renal arterial system disturbances, it seems reasonable that these lesions would be associated with elevated RIs. However, it is unclear why RI would correlate with tubulointerstitial lesion severity, as that area does not receive direct blood supply from small to medium-sized arteries.
It is widely accepted that the severity of tubulointerstitial injury correlates with the degree of renal impairment [16]. Peritubular capillaries (PTCs), which comprise the arterial portal system derived from the efferent arteriole, supply adjacent tubules in the cortex and renal medulla. Several human kidney studies have shown that PTC loss is associated with chronic tubulointerstitial injury and renal impairment [17, 18]. In the present study, we evaluated renal histopathology and RI values, focusing on PTCs.
Materials and methods
Subjects
Between 2002 and 2006, 170 patients underwent renal biopsy in our department because of renal function impairment or urinalysis abnormalities. Upon admission for the biopsy, patient profiles were collected. In 30 of the biopsy patients, RI was measured using Doppler ultrasonography on the same day as the renal biopsy. The clinical and histological findings for the patients are summarized in Table 1. The mean ± SD values for the body mass index, systolic blood pressure, diastolic blood pressure and pulse rate were 21.8 ± 2.8 kg/m2, 121.6 ± 2.8 mmHg, 67.6 ± 10.5 mmHg and 68.2 ± 9.3 beats/min, respectively. To treat their hypertension, 7 patients were taking a calcium channel blocker and 10 patients were taking an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) at the time of their renal ultrasonography (Table 1). Creatinine clearance was calculated from 24-h urine samples after correction for the mean body surface area of a Japanese adult (1.73 m2). This study was approved by the ethical committee of the University of Fukui Hospital (reference number 20–31, September 3, 2008), and all patients provided informed consent.
Histological evaluation
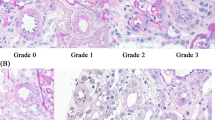
All renal biopsy specimens were examined under a light microscope after staining with periodic acid–Schiff, periodic acid–methenamine silver and Masson trichrome, as well as immunostaining. Only specimens that could not be diagnosed using light microscopy were viewed using electron microscopy. Three pathologists blinded to the patient RI values examined the slides separately. Arteriolosclerosis was defined as the presence of hyalinization in the efferent or afferent arteries. Arteriosclerosis was defined as thickening of the medial wall and intimal fibrosis in small arteries. Both arteriosclerosis and arteriolosclerosis were graded as follows: grade 0 =absence of a lesion, grade 1 =presence of a lesion. PTC immunostaining was performed using the standard diaminobenzidine technique with a mouse monoclonal anti-human CD34 antibody, which is known to be a sensitive marker of human endothelial cells (Endothelial cell markers CD34 clone QBEnd/10, Novocastra, Newcastle-upon-Tyne, UK). Representative images of CD34 staining are shown in Fig. 1. CD34+ areas in the tubulointerstitial area were analyzed using a digital image analysis program (Image pro-plus, Media cybernetics, Gleichen, Germany). Only the cortical areas were evaluated; glomeruli and arteries were excluded from quantification. Tissue analysis was conducted using a standard compound histological microscope at 100× magnification. After randomly selecting 10 tubulointerstitial areas, an attached scanner calculated the percentage of the total area that showed CD34 positivity, and the average value of the 10 fields was calculated for each patient. Interstitial fibrosis was evaluated based on Masson trichrome staining. Each patient specimen was pictorially captured at 40× using picture-editing software (Adobe Photoshop Elements 10, Adobe Systems, San Jose, CA, USA). After excluding the glomeruli, Masson trichrome blue portions of the renal cortex were extracted. Using Image J software (US National Institutes of Health, Bethesda, Maryland, USA), the fibrotic area was calculated as a percentage of the entire cortical area that was blue.
Ultrasonography
Renal Doppler ultrasonography was performed prior to the biopsy by a licensed physician. Images were obtained using a Doppler apparatus (Logiq 500 MD, GE Medical Systems, Japan) equipped with a 3.8-MHz transducer while the patient was in a supine position. The Doppler signal was monitored at the segmental artery for at least two (and in most cases, three) different renal vessels along the medullary pyramid border in each kidney. RI was then calculated using the following equation:
The RI value for each patient was calculated as the mean value for the examined vessels. The patients fasted for at least 4 h prior to the biopsy to exclude digestion effects on the renal blood flow. Anti-hypertensive drugs were administered on the day of the examination because high blood pressure is associated with bleeding risk when renal biopsies are performed on the same day as Doppler ultrasonography.
Statistical analysis
The relationships between RI values and clinical (age, creatinine clearance, body mass index, systolic blood pressure, diastolic blood pressure, pulse rate) and histological (arteriolosclerosis, arteriosclerosis, PTCs, interstitial fibrosis) parameters were evaluated using two-tailed Pearson correlation tests. Because the presence of hypertension or diabetes mellitus in these patients can increase RI, unpaired t tests were performed to compare RIs from patients with and without hypertension, and with and without diabetes mellitus. Because oral administration of an ACEi or ARB may lower RIs, unpaired t tests were performed to compare RIs from patients taking an ACEi or ARB with those not taking one of those drugs. In addition, stepwise multiple regression analysis was used to further evaluate the correlation between increased RI and the parameters shown to correlate with RI with the Pearson correlation test. Values of p < 0.05 were considered statistically significant. All statistical analyses were performed using Stat View, version 5.0 (SAS Institute Inc., NC, USA).
Results
Correlation between RI and clinical and histological parameters
RI correlated positively with age, systolic blood pressure and diastolic blood pressure, and negatively with creatinine clearance (Table 2). We also assessed the effect of hypertension, diabetes mellitus and administration of an ACEi or ARB, since these conditions may affect RI. We found that patients with hypertension or diabetes had higher RIs than patients without those illnesses (p < 0.05) (Table 3). However, there was no significant effect of taking an ACEi or ARB (Table 3).
Bige et al. [9] reported that patients with a baseline RI ≥ 0.65 had a poorer renal outcome than those with a baseline RI < 0.64. Nine patients included in this study had a RI ≥ 0.65 and 21 had a RI < 0.64. The arteriolosclerosis rates in those two groups were 67 and 38 %, respectively, while the arteriosclerosis rates were 67 and 14 %, respectively.
When we assessed the relationships between RI and histological parameters, we found that RI correlated positively with interstitial fibrosis and arteriosclerosis, and negatively with PTC (CD34+ area) (Table 2). We also evaluated the correlation of PTC with other histological markers (e.g., interstitial fibrosis and arteriosclerosis) but found that PTC did not correlate with any other histological markers. Finally, we performed a multiple regression analysis by selecting RI as the dependent variable and choosing from among a set of independent variables, including arteriosclerosis, interstitial fibrosis and PTC. From this analysis, PTC and arteriosclerosis emerged as independent variables associated with RI elevation (r 2 = 0.321, p < 0.05) (Table 4).
Discussion
In the present study, we observed significant correlations between RI and age, the presence of hypertension or diabetes mellitus, and creatinine clearance. Among histological factors, RI correlated with arteriosclerosis. These results are consistent with a number of earlier studies [3–7, 9, 13–15]. In addition, evaluation of the relationship between CD34+ area and RI showed that PTC correlated negatively with RI, while multiple regression analysis showed that both PTC and arteriosclerosis correlated with RI elevation. Ikee et al. also reported a correlation between arteriosclerosis and RI; however, our study is the first to show a correlation between RI and PTC loss, as well as arteriosclerosis.
It was recently suggested that tubulointerstitial injury due to chronic hypoxia serves as a final common pathway to end-stage renal disease [16]. In animal studies, capillary repair following tubular cell injury was shown to be impaired by alterations in the local expression of both angiogenic (vascular endothelial growth factor) and antiangiogenic (thrombospondin 1) factors in the kidney [18]. In addition, PTC density was found to be reduced and renal fibrosis developed following acute renal failure [19]. Similarly, in human kidneys, PTC injury develops in concert with tubulointerstitial injury [17]. We hypothesized that arteriosclerosis may cause downstream hypoxia leading to PTC loss. To test that idea, we examined the correlation between PTC loss and arteriosclerosis or interstitial fibrosis, but PTC loss did not correlate with either of those pathological changes.
Two limitations of our study are the small number of the subjects and the influence of anti-hypertensive drugs, including ACE inhibitors and ARBs, during RI measurements.
In conclusion, the present study shows that increases in RI are associated with both arteriosclerosis and loss of PTCs.
References
Rifkin MD, Needleman L, Pasto ME, Kurtz AB, Foy PM, McGlynn E, et al. Evaluation of renal transplant rejection by duplex Doppler examination: value of the resistive index. AJR Am J Roentgenol. 1987;148:759–62.
Radermacher J, Mengel M, Ellis S, Stuht S, Hiss M, Schwarz A, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115–24.
Terry JD, Rysavy JA, Frick MP. Intrarenal Doppler: characteristics of aging kidneys. J Ultrasound Med. 1992;11:647–51.
Shimizu Y, Itoh T, Hougaku H, Nagai Y, Hashimoto H, Sakaguchi M, et al. Clinical usefulness of duplex ultrasonography for the assessment of renal arteriosclerosis in essential hypertensive patients. Hypertens Res. 2001;24:13–7.
Florczak E, Januszewicz M, Januszewicz A, Prejbisz A, Kaczmarska M, Michałowska I, et al. Relationship between renal resistive index and early target organ damage in patients with never-treated essential hypertension. Blood Press. 2009;18:55–61.
Soldo D, Brkljacic B, Bozikov V, Drinkovic I, Hauser M. Diabetic nephropathy. Comparison of conventional and duplex Doppler ultrasonographic findings. Acta Radiol. 1997;38:296–302.
Ishimura E, Nishizawa Y, Kawagishi T, Okuno Y, Kogawa K, Fukumoto S, et al. Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int. 1997;51:1920–7.
Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease: results of a 4-year follow-up. Clin Exp Nephrol. 2011;15:114–20.
Bigé N, Lévy PP, Callard P, Faintuch JM, Chigot V, Jousselin V, et al. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol. 2012;13:139.
Rigler AA, Vizjak A, Ferluga D, Kandus A, Buturović-Ponikvar J. Ultrasonography parameters and histopathology findings in transplanted kidney. Transplant Proc. 2013;45:1630–4.
Sugiura T, Nakamori A, Wada A, Fukuhara Y. Evaluation of tubulointerstitial injury by Doppler ultrasonography in glomerular diseases. Clin Nephrol. 2004;61:119–26.
Platt JF, Rubin JM, Ellis JH. Lupus nephritis: predictive value of conventional and Doppler US and comparison with serologic and biopsy parameters. Radiology. 1997;203:82–6.
Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H, et al. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis. 2005;46:603–9.
Mostbeck GH, Kain R, Mallek R, Derfler K, Walter R, Havelec L, et al. Duplex Doppler sonography in renal parenchymal disease histopathologic correlation. J Ultrasound Med. 1991;10:189–94.
Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol. 1990;154:1223–7.
Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004;43:9–17.
Choi YJ, Chakraborty S, Nguyen V, Nguyen C, Kim BK, Shim SI, et al. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol. 2000;31:1491–7.
Kang D-H, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–16.
Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–99.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kimura, N., Kimura, H., Takahashi, N. et al. Renal resistive index correlates with peritubular capillary loss and arteriosclerosis in biopsy tissues from patients with chronic kidney disease. Clin Exp Nephrol 19, 1114–1119 (2015). https://doi.org/10.1007/s10157-015-1116-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1116-0