Abstract
Background
Treatment of the presacral cavity that forms after contained anastomotic leakage of a low pelvic anastomosis is challenging and often results in a permanent stoma. Endosponge™ therapy is a minimally invasive method of treating the presacral cavity which potentially avoids a permanent stoma. We report our initial experience of using Endosponge™ therapy.
Methods
All patients who underwent Endosponge™ treatment for low pelvic anastomotic leakage in our hospital over a 45-month period were identified and data collected from clinical, operative and endoscopic notes.
Results
Eight patients (seven males, one female) underwent Endosponge™ therapy for extraperitoneal pelvic anastomotic leak during the study period; all had had defunctioning ileostomies placed at their original surgery. Six out of eight patients had complete closure or a reduction in the size of the abscess cavity. Five patients have had their ileostomies reversed with good or reasonable bowel function after a median follow-up of 41 months and four of these patients had Endosponge™ therapy instituted within 6 weeks of initial surgery. One patient had Endosponge™ therapy abandoned and conversion to a permanent end colostomy after accidental intraperitoneal placement of the sponge.
Conclusions
Early use of Endosponge™ therapy appears to offer a minimally invasive and effective way of closing the presacral cavity after a pelvic anastomotic leak, reducing the risk of permanent stoma and resulting in acceptable bowel function. Endosponge™-specific complications can occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the improvements in perioperative care and surgical techniques, anastomotic leakage following rectal resection occurs in up to 24 % of cases [1–3]. Patients with low pelvic anastomoses and those who receive neo-adjuvant radiotherapy or chemotherapy have higher leak rates [2–5], which leads to significant additional morbidity and mortality and can result in a permanent stoma in up to 25 % of cases [4, 6, 7]. Anastomotic leak after cancer resection is also associated with increased rates of local recurrence and decreased long-term survival [8].
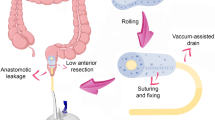
Anastomotic leakage after procedures involving extraperitoneal low pelvic anastomosis such as total mesorectal excision (TME) for low rectal cancer may result in either peritonitis from free intra-peritoneal leakage or a presacral or para-anastomotic abscess cavity. Free intra-peritoneal leakage requires surgical intervention to control the source of sepsis. A contained presacral cavity can occur even in patients who have been defunctioned at the initial operation [9] and may become evident either clinically or radiologically. This abscess can give rise to a non-healing presacral cavity and sinus due to insufficient drainage through the anastomotic defect.
Treating this presacral abscess cavity and subsequent sinus can be challenging. Utilising percutaneous or transanastomotic abscess drainage and antibiotic therapy, with or without abdominal washout, leads to a persistent presacral sinus in 50 % of cases, with healing taking an average of 1 year [10]. Alternatively, numerous surgical options are available, including resection of the failed anastomosis with or without a repeat anastomosis, laparotomy and transabdominal drainage with proximal defunctioning or instillation of agents such as fibrin glue to try and occlude the cavity [11], but none combine reliability with low morbidity.
The choice of treatment is influenced by the condition of the patient and degree of sepsis. It must be kept in mind that reoperation in this patient group will be technically difficult due to adhesions and fibrosis caused by pelvic sepsis. A novel alternative to these strategies is to use endoscopic transanal vacuum-assisted rectal drainage (ETVARD) sponge therapy to reduce the size of the abscess cavity and limit pelvic sepsis. We report our initial experience, the first from the United Kingdom, of using a proprietary vacuum sponge system (Endosponge™, B. Braun Medical, Sheffield, UK) to treat anastomotic leakage in patients with extraperitoneal low pelvic anastomoses.
Materials and methods
All patients who underwent Endosponge™ treatment for anastomotic leakage in our hospital between September 2007 and May 2011 were identified from theatre and endoscopy records and direct inquiry of the hospital’s Colorectal Surgeons. Apart from standard demographic data, we collected details of the patients’ initial surgery, diagnosis of anastomotic leak and subsequent treatment including Endosponge™ therapy and outcomes. Endosponge™ was used according to the manufacturer’s instructions, and the sponge was changed under general anaesthetic with a flexible endoscope.
Results
Eight patients (seven males, one female) underwent Endosponge™ therapy for extraperitoneal pelvic anastomotic leak during the 45-month study period. The median age was 66.5 years (range 45–79 years). Patient characteristics are summarised in Table 1.
Seven patients had leaks after low anterior resection (LAR) for rectal cancer, and all had a defunctioning ileostomy sited at initial surgery. Six of them had undergone pre-operative short course radiotherapy and the seventh had previously received radical radiotherapy for carcinoma of the bladder. One patient leaked after restorative proctocolectomy (RP) for ulcerative colitis, which had also been defunctioned.
The median period of time from initial surgery to the detection of anastomotic leakage was 29 days (range 10–115 days) and was detected by contrast computed tomography (CT) scan in four cases, water soluble contrast enema in three cases and examination under anaesthesia (EUA) in one case.
Each patient had only one Endosponge™ placed per application, except a single occasion of double sponge placement, and all were inserted under general anaesthetic. The median number of sponge applications was 4 (range 1–7), over a median treatment period of 26 days (range 7–49 days). One patient complained of discomfort after Endosponge™ placement, but the device remained in situ. The remaining sponges were well tolerated.
Six out of eight patients had complete closure or a reduction in the size of the abscess cavity (Fig. 1). Five patients have had their ileostomies reversed and have what they describe as good or reasonable function over a median follow-up period of 41 months (range 10–45 months). The patient who leaked following RP evacuates his pouch six times daily and can remain continent throughout an 8-h shift at work. Of the three patients who continue with proximal diversion, one developed a colovesical fistula which has been managed conservatively with the defunctioning stoma left permanently in situ, one patient proceeded to abdominoperineal excision of the rectum (APER) due to persistent perianal sepsis, and the third was converted to a permanent end colostomy after resection of the insufficient rectal anastomosis (Hartmann’s operation) after inadvertent placement of the Endosponge™ through the roof of the abscess cavity into the peritoneal cavity (Fig. 2a, b).
Four out of five patients (80 %) who had Endosponge™ therapy instituted within 6 weeks of initial surgery have achieved restoration of bowel continuity with good results; only one of the three (33 %) who had treatment started after the 6 week watershed has achieved bowel continuity. However, the numbers are too small to allow meaningful statistical comparison.
Discussion
Anastomotic leakage is a serious problem in patients undergoing colorectal resections, and whilst there is no difference in anastomotic leak rates in patients with or without a defunctioning stoma [12], it is known that the presence of a stoma does reduce the rate of surgical intervention needed after anastomotic leakage [12, 13], potentially allowing the leak to be managed with minimally invasive techniques.
Endoscopic transanal vacuum-assisted rectal drainage with or without the use of a commercially available system has been described as a minimally invasive method of treating low pelvic extraperitoneal anastomotic leakage [14–21] (Table 2) and includes both rectal cancer and ileoanal pouch surgery. Reports are predominantly from Germany, where the technique was pioneered, and the Netherlands, and no reports of the technique from the United Kingdom have been published.
Outcomes
The two largest series of ETVARD both come from Germany. Weidenhagen et al. [15] reported closure of the presacral cavity in 28/29 cases over a mean treatment period of 34 days and von Bernstorff’s group achieved success in 23/26 cases over a mean period of 21 days [19]; the latter department had previously reported a 94 % success rate in 17 cases of ETVARD use accompanied by fibrin instillation into the presacral cavity, but it is unclear if these are the same patients [16].
The advantages of Endosponge™ therapy before 6 weeks after initial surgery are apparent in our small series and replicate the findings from von Koperen’s study [18], where the healing rates before and after the 6-week point were 75 and 38 %, respectively. When compared to historical data, vacuum therapy closes the para-anastomotic defect in a shorter time than other conservative measures, but direct comparisons are scarce. Mees et al. [17] compared Endosponge™ therapy with daily cavity lavage and demonstrated a slightly shorter overall hospital stay after Endosponge™ treatment, but the in-patient stay from the institution of treatment to discharge was actually slightly longer in those treated by Endosponge™.
Outcomes according to indication
As reports of ETVARD are scarce, it is difficult to draw any firm conclusions about possible differences in success rates according to indication. The Dutch multicentre Endosponge™ study described a 46 % success rate in cavity closure after rectal cancer surgery leaks compared to 100 % after RP leaks but the benign surgery group only included three patients [18]. von Koperen et al. [22] have also reported two cases of successful ileoanal pouch salvage following anastomotic leak after RP, although no details of subsequent pouch function are included. Of more potential interest is the impact of neoadjuvant radiotherapy on healing rates following anastomotic leakage in rectal cancer patients. All seven cancer patients in our series had received pelvic irradiation; von Bernstorff et al. [19] reported statistically significant increases in time to diagnosis of leak, time to institution of Endosponge™ therapy, size of presacral cavity and time to healing in the 14 patients receiving neoadjuvant radiotherapy compared to the 12 who did not. In addition, all 3 patients who failed ETVARD had received radiotherapy.
Restoration of continuity and function
Successful obliteration of the abscess cavity does not necessarily equate to restoration of intestinal continuity, represented by reversal of the defunctioning stoma, and rates of intestinal continuity (20–89.6 %) are generally lower than the quoted figures for abscess cavity closure (56–100 %) (Table 2). In part, this is due to reports excluding failed cases of ETVARD [15, 20]. Our rate of restoration of intestinal continuity (62.5 %) is comparable. No studies have invested in objective functional assessment following anastomotic rescue using ETVARD; von Berstorff et al. [19] report that their patients have regular bowel movements and were grossly asymptomatic. Riss et al. [20] reported 20 patients out of 23 with leaks (including 3 from rectal stumps) who successfully healed with Endosponge™ therapy, but then encountered five recurrent abscesses during a median follow-up of 17 months. Three have been converted to Hartmann’s procedures, one is discussing permanent colostomy and the final case was successfully managed by percutaneous drainage.
Complications and costs
Endosponge™ treatment appears to be a safe and well-tolerated therapy [15, 17] with few patients experiencing discomfort necessitating cessation of treatment [18, 19]. A single case report describes the formation of a small bowel fistula into the presacral abscess cavity associated with ETVARD therapy following rectal cancer surgery after neoadjuvant radiotherapy [23]. Nagell and Holte counselled against using ETVARD when small bowel was visible in the roof of the cavity 4 years previously [14]. Our series includes the first description of inadvertent intraperitoneal Endosponge™ placement, which in our case was felt to preclude further vacuum therapy because of the risk of fistulation and led to the decision to convert to an end colostomy. The Endosponge™ kit cost between £140 and £237 (including VAT) per sponge over the duration of the study. All sponge placements in our hospital were performed in an operating theatre under general anaesthesia, largely for logistic reasons, which would have added to the overall costs, but outpatient placement without hospital stay, as described by Weidenhagen et al. [15] would minimise such costs.
Conclusions
Our initial experience suggests that Endosponge™ represents a minimally invasive and well-tolerated method of facilitating restoration of intestinal continuity with good function after extraperitoneal pelvic anastomotic leakage, especially if used within 6 weeks of initial surgery. Based on the literature and our experience, we recommend early institution of vacuum therapy whenever possible and its continuation until the cavity is too small to accept further sponges. Examination under anaesthetic at this point allows an objective assessment of the residual cavity, and if it is closed sufficiently so that the risk of further pelvic sepsis is low, then the stoma should be reversed as soon as is practical. If complete cavity closure has not been achieved but further sponge placement is impractical, then we would observe the patient for approximately 2 months to monitor for any recurrence of pelvic sepsis before planning stoma reversal. We also report a new complication of intraperitoneal placement of the Endosponge™ that necessitated formation of a permanent end colostomy rather than salvage of the original anastomosis.
Further prospective randomised trials are necessary to further clarify the role of Endosponge™ therapy in anastomotic leakage, its effects on reducing morbidity and length of stay coupled with objective assessment of eventual bowel function.
References
Ptok H, Marusch F, Meyer F, Schubert D, Gastinger I, Lippert H (2007) Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg 94:1548–1554
Jung SH, Yu CS, Choi PW et al (2008) Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum 51:902–908
Lee WS, Yun SH, Roh YN et al (2008) Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg 32:1124–1129
Pakkastie TE, Luukkonen PE, Jarvinen HJ (1994) Anastomotic leakage after anterior resection of the rectum. Eur J Surg 160:293–297
Eriksen MT, Wibe A, Norstein J, Haffner J, Wiig JN (2005) Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis 7:51–57
Chow A, Tilney HS, Paraskeva P, Jeyarajah S, Zacharakis E, Purkayastha S (2009) The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. Int J Colorectal Dis 24:711–723
den Dulk M, Smit M, Peeters KC et al Dutch Colorectal Cancer Group (2007) A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol 8:297–303
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253:890–899
Morton DG, Sebag-Montefiore D (2006) Defunctioning stomas in the treatment of rectal cancer. Br J Surg 93:650–651
van Koperen PJ, van der Zaag ES, Omloo JM, Slors JF, Bemelman WA (2010) The persisting presacral sinus after anastomotic leakage following anterior resection or restorative proctocolectomy. Colorect Dis 13:26–30
Swain BT, Ellis CN (2004) Fibrin glue treatment of low rectal and pouch-anal anastomotic sinuses. Dis Colon Rectum 47:253–255
Gastinger I, Marusch F, Steinert R, Wolff S, Koeckerling F, Lippert H (2005) Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg 92:1137–1142
Tan WS, Tang CL, Shi L, Eu KW (2009) Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg 96:462–472
Nagell CF, Holte K (2006) Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). Int J Colorectal Dis 21:657–660
Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW (2008) Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 22:1818–1825
Glitsch A, von BW, Seltrecht U, Partecke I, Paul H, Heidecke CD (2008) Endoscopic transanal vacuum-assisted rectal drainage (ETVARD): an optimized therapy for major leaks from extraperitoneal rectal anastomoses. Endoscopy 40:192–199
Mees ST, Palmes D, Mennigen R, Senninger N, Haier J, Bruewer M (2008) Endo-vacuum assisted closure treatment for rectal anastomotic insufficiency. Dis Colon Rectum 51:404–410
van Koperen PJ, van Berge Henegouwen MI, Rosman C et al (2009) The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc 23:1379–1383
von Berstorff W, Glitsch A, Schreiber A, Partecke LI, Heidecke CD (2009) ETVARD (endoscopic transanal vacuum-assisted rectal drainage) leads to complete but delayed closure of extraperitoneal rectal anastomotic leakage cavities following neoadjuvant radiochemotherapy. Int J Colorectal Dis 24:819–825
Riss S, Stift A, Kienbacher C et al (2010) Recurrent abscess after primary successful endo-sponge treatment of anastomotic leakage following rectal surgery. World J Gastroenterol 16:4570–4574
Verlaan T, Bartels SA, van Berge Henegouwen MI, Tanis PJ, Fockens P, Bemelman WA (2011) Early, minimally invasive closure of anastomotic leaks: a new concept. Colorectal Dis 13:18–22
Van Koperen PJ, Van Berge Henegouwen MI, Slors JF, Bemelman WA (2008) Endo-sponge treatment of anastomotic leakage after ileo-anal pouch anastomosis: report of two cases. Colorectal Dis 10:943–944
Hoogenboom FJ, Hoff C, Koopal SA (2010) Small intestinal-colorectal anastomotic fistula developing during endo-sponge treatment. Colorectal Dis 12:E337–E338
Acknowledgments
We are grateful to Mr MM Bassuni and Miss M Mottahedeh, Consultant Colorectal Surgeons, for permission to report their patients.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srinivasamurthy, D., Wood, C., Slater, R. et al. An initial experience using transanal vacuum therapy in pelvic anastomotic leakage. Tech Coloproctol 17, 275–281 (2013). https://doi.org/10.1007/s10151-012-0911-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-012-0911-9