Abstract
Background
The recurrence rate after curative resection for hepatocellular carcinoma (HCC) reaches over 70% after 5 years and early recurrence (within 1 year) is now recognized as having a poor prognosis and has limited treatment options.
Methods
We retrospectively reviewed 184 consecutive patients who underwent curative hepatic resection for HCC. Severe early recurrence was defined as multiple (beyond up-to-7) liver recurrence or distant metastasis after hepatic resection within 1 year. We divided the participants into two groups according to severe early recurrence and analyzed clinicopathological and long-term outcomes.
Results
Among the patients with multiple or distant metastasis (n = 59), 49 patients (83%) had recurrence within 1 year. Overall survival (OS) and recurrence-free survival (RFS) were significantly worse in the severe early recurrence group than in the others group. Logistic regression analysis revealed that severe early recurrence was significantly associated with macroscopic vascular invasion (MVI), tumor burden score (TBS) > 4.70, and ALBI grade 2. In patients with scores of 2 and 3 (the sum of the three factors), OS and RFS rates were significantly poorer than those of patients with scores of 0 or 1. Positive predictive value and negative predictive value for severe early recurrence was 68.4% and 84.2%, respectively. Furthermore, a validation study demonstrated that cases with these factors were at high risk of severe early recurrence and had poor prognosis.
Conclusions
In this retrospective analysis, MVI, TBS, and ALBI could predict severe early recurrence after hepatic resection for HCC, and patients with these risk factors had a poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide. Data from the WHO Global Health Observatory indicated an overall increasing incidence of liver cancer [1, 2]. Hepatic resection is one of the most powerful and curative treatments for HCC, as defined by the Barcelona Clinic Liver Cancer (BCLC) staging system [3]. However, the recurrence rate reaches over 70% at 5 years after hepatic resection [4].
In recent years, systemic therapy for unresectable HCC (u-HCC) has spread widely in Japan, and immunotherapy and tyrosine kinase inhibitors have become available. According to the BCLC staging system, systemic therapy is recommended for patients with multiple intermediate-stage u-HCC classified using the beyond up-to-7 criteria as a transcatheter arterial chemoembolization (TACE)-unsuitable condition, especially for lesions larger than 5 cm, which are less likely to respond to TACE alone and, therefore, may respond better to systemic therapy [5].
Some patients develop early and multiple recurrences or distant metastases after hepatic resection. Uncontrolled intra- or extrahepatic metastasis are the primary causes of the poor prognosis of HCC, and such cases are defined as u-HCC. The Milan criteria have been used as an indication for liver transplantation (LT) in Japan [6]. Furthermore, in recent years, the 5-5-500 rule has been recognized in insurance applications as an extension of LT [7]. Although it is very useful as a criterion for LT, the average age of liver resection patients is over 70 years, and because of the shortage of donors, there are only approximately 400 liver transplants per year in Japan [8]. However, LT is rarely considered as a treatment option in reality. According to the BCLC strategy, prognosis is predicted to be 2.5 years with TACE and 2 years with systemic therapy [3]. Beyond up-to-7 serves as a criterion for treatment selection in TACE or systemic therapy, and as a result, prognostic prediction also changes. Furthermore, early HCC recurrence (within 1 year) is now recognized as a critical determinant for poor prognosis [9,10,11,12,13,14]. We defined HCC patients with recurrence beyond up-to-7 within 1 year as severe early recurrence.
In clinical practice, there is a need for biomarkers that help clinicians to assess the risk of severe early HCC recurrence so that patients with high risk factors can be identified as candidates for preoperative or postoperative systemic therapy. To the best of our knowledge, the risk factors of multiple (beyond up-to-7) liver recurrence or distant metastasis after hepatic resection within 1 year have not yet been studied.
The present retrospective study aimed to investigate the risk factors of severe early recurrence in patients with hepatic resection for HCC.
Methods
Patients characteristics
We retrospectively reviewed data from 184 consecutive patients who underwent initial curative hepatic resection for HCC at the Division of Hepatobiliary and Pancreatic Surgery, Gunma University, between January 2016 and December 2021. This study was approved by the Gunma University Ethics Committee (HS2023-080) and met the guidelines of the Declaration of Helsinki. In addition, a validation study was performed using data from 237 consecutive patients who underwent curative initial hepatic resection for HCC at the Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, the Jikei University School of Medicine, between January 2011 and December 2021. This subsequent study was approved by the Jikei University Ethics Committee (27-177). Informed consent for inclusion in the study was obtained from all the participants. We excluded patients with recurrent HCC and those who had undergone hepatic resection combined with biliary reconstruction or anastomosis of the digestive tract. We also excluded patients with missing clinical data. No patients received systemic therapy prior to hepatectomy.
Severe early recurrence was defined as multiple (beyond up-to-7) liver recurrence or distant metastasis after hepatic resection within 1 year, and we analyzed patients’ clinicopathological and long-term prognoses.
Clinical laboratory data were collected within 1 month before hepatic resection. We defined postoperative complications as Clavien–Dindo grade ≥ III complications (requiring surgical intervention) within 1 month of hepatic resection [15]. We calculated the albumin–bilirubin (ALBI) score using the following formula: ALBI score = (log10 bilirubin × 0.66) + (albumin × − 0.085), with bilirubin concentrations in μM/L and albumin in g/L. We then applied specific cutoffs to generate the following three prognostic groups: ALBI score ≤ − 2.60 (ALBI grade 1); > − 2.60 to ≤ − 1.39 (ALBI grade 2); and > − 1.39 (ALBI grade 3) [16]. We calculated prognostic nutritional indexes (PNIs) using the following formula: 10 × serum albumin concentration (g/dl) + 0.005 × lymphocyte count [17]. The Controlling Nutritional Status (CONUT) scores were calculated based on serum albumin concentrations, peripheral lymphocyte counts, and total cholesterol concentrations [4]. (1) Albumin concentrations ≥ 3.5 g/dL, 3.0–3.49 g/dL, 2.5–2.99 g/dL, and < 2.5 g/dL were scored as 0, 2, 4, and 6 points, respectively; (2) total lymphocyte counts ≥ 1600/mm3, 1200–1599/mm3, 800–1199/mm3, and < 800/mm3 were scored as 0, 1, 2, and 3 points, respectively; and (3) total cholesterol concentrations ≥ 180 mg/dL, 140–179 mg/dL, 100–139 mg/dL, and < 100 mg/dL were scored as 0, 1, 2, and 3 points, respectively. The CONUT score was defined as the sum of (1), (2), and (3). Tumor burden score (TBS) was calculated using the following formula: TBS2 = (maximum tumor diameter)2 + (number of tumors) [2, 18, 19]. This can be shown as one index by combining the two conventionally reported indices of malignancy, namely, tumor diameter and number. Furthermore, TBS has already been established as an index of malignancy in HCC or as a predictor for severe recurrence.
Milan criteria were defined as a single HCC nodule up to 5 cm in diameter or up to three nodules no greater than 3 cm in size without vascular invasion or extrahepatic metastasis [6]. 5-5-500 rule was defined as tumor size ≤ 5 cm in diameter, tumor number ≤ 5, and AFP value ≤ 500 ng/ml [7].
Surgical procedures
Details of the surgical procedures and patient selection criteria have been previously reported [20]. Hepatic dissection was performed using an ultrasonic dissector with a coagulator (CUSA Excel; Integra, USA) under the Pringle maneuver, with systematic ligation of all sizable vessels.
Follow-up strategy and recurrence patterns
Following discharge, all patients were examined monthly for recurrence by ultrasonography and examination of tumor marker concentrations, such as alpha-fetoprotein and des-gamma-carboxy prothrombin (DCP), and by computed tomography every 6 months. When recurrence was suspected, additional investigations such as gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging were performed as indicated. Recurrent HCC was treated by repeat hepatectomy, ablation therapy, and systemic therapy, in accordance with the recommendations of previous studies [21, 22]. The locoregional treatment for recurrent HCC was defined as hepatectomy, ablation therapy, or TACE.
Histological findings
Tumor differentiation, microvascular invasion, intrahepatic metastasis, and histological liver cirrhosis were assessed according to the criteria of the Liver Cancer Study Group of Japan [23]. Portal vein tumor thrombus was categorized as main trunk/contralateral branch (Vp4), first-order branch (Vp3), and second-order branch (Vp2), according to the Japanese staging system. Hepatic vein tumor thrombosis was also categorized as IVC (Vv3), major hepatic vein (Vv2), and peripheral hepatic vein (Vv1). Bile duct tumor thrombus was categorized as main trunk (B4) and first-order branch (B3) according to the Japanese staging system. Macrovascular invasion (MVI) was defined as Vp2–4, Vv2–3, or B2–3. The breakdown of MVI in this study was as follows: Vp2: 8 cases; Vp3: 6 cases; Vp4: 3 cases; Vv2: 3 cases; Vv3: 3 cases; B2: 0 cases; and B3: 12 cases. Some of these included duplicates. Fibrosis stage was scored on a scale of 0–4 using the METAVIR classification as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; and F4, cirrhosis [24].
Statistical analysis
Associations between continuous and categorical variables and the relevant outcome variables were assessed using Student’s t-test and the χ2 test, respectively. We also performed logistic stepwise regression analysis using variables with P-values of < 0.05 in the univariate analyses to predict postoperative complications. We excluded albumin from the logistic stepwise regression analysis because this variable was a confounding factor for ALBI grade.
All analyses were performed using JMP version 14 software (SAS Institute, Cary, NC, USA). P < 0.05 was considered to denote statistical significance.
Results
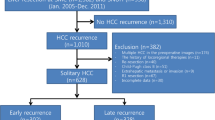
Among 184 patients in this series, 100 patients (54.3%) had recurrence, and the mean observation period was 2.8 years. The recurrence pattern was divided into hepatic solitary (n = 35), hepatic multiple, up-to-7 in recurrence (n = 6), up-to-7 out recurrence (n = 32), and distant metastasis (n = 27). Among the patients with hepatic multiple, up-to-7 out, and distant metastasis (n = 59), 49 patients (83%), namely those with the severe early recurrence pattern, had recurrence within 1 year. Figure 1 details prognosis according to the recurrence pattern. Kaplan–Meier analysis revealed differences in overall survival (OS) (Fig. 1A) and recurrence-free survival (RFS) rates (Fig. 1B) according to the recurrence pattern. OS and RFS rates were both significantly worse in the severe early recurrence group than in the others group. Median survival time (MST) for severe recurrence was 1.98 year and for others was 6.69 year. Median recurrence-free survival time (MRFST) for severe recurrence was 0.49 year and for others was 4.03 year.
Prognosis according to recurrence pattern. The recurrence pattern was divided into hepatic multiple (beyond up-to-7), or distant metastasis within 1 year after hepatectomy (black lines), and others including no recurrence (gray lines). Overall survival (A) and recurrence-free survival (B) were significantly worse in patients with beyond up-to-7 or distant metastasis compared with those with others
To prove the validity of definition of severe recurrence, Fig. 2 details the prognosis according to recurrence after hepatic resection within 1 year beyond Milan criteria and also Fig. 3 details the prognosis according to recurrence after hepatic resection within 1 year beyond 5-5-500 rule. Kaplan–Meier analysis revealed differences in OS (Fig. 2A and 3A) and RFS rates (Fig. 2B and 3B) according to the recurrence pattern. MST for recurrence beyond Milan criteria was with 2.02 year and MST for recurrence beyond 5-5-500 rule was with 1.98 year. MRFST for recurrence beyond Milan criteria was with 0.52 year and MST for recurrence beyond 5-5-500 rule was with 0.49 year.
Prognosis according to recurrence pattern. The recurrence pattern was divided into hepatic multiple, beyond Milan criteria, or distant metastasis within 1 year after hepatectomy (black lines), and others including no recurrence (gray lines). Overall survival (A) and recurrence-free survival (B) were significantly worse in patients with beyond Milan criteria or distant metastasis compared with those with others
Prognosis according to recurrence pattern. The recurrence pattern was divided into hepatic multiple, beyond 5-5-500 rule or distant metastasis within 1 year after hepatectomy (black lines), and others including no recurrence (gray lines). Overall survival (A) and recurrence-free survival (B) were significantly worse in patients with beyond 5-5-500 or distant metastasis compared with those with others
Patients’ clinicopathological characteristics according to severe early recurrence or not are shown in Table 1. Severe early recurrence was significantly associated with lower albumin, ALBI grade 2, PNI < 45, neutrophil lymphocyte ratio (NLR) > 3, CONUT > 2, tumor size 5 cm or more, multiple tumors, higher TBS, higher DCP, MVI, anatomical resection, longer surgery time, large blood loss, and blood transfusion.
The results of the logistic regression analysis aimed at identifying independent risk factors for severe early recurrence are presented in Table 2. MVI, TBS > 4.70, and ALBI grade 2 were significantly associated with severe early recurrence. Figure 4 shows the OS (Fig. 4A) and RFS (Fig. 4B) rates according to the sum of the three factors in the multivariate analysis (MVI, TBS > 4.70, and ALBI grade 2). OS and RFS rates of patients with scores of 2 and 3 (n = 38) were significantly poorer than those of patients with scores of 0 or 1. Among patients with scores of 2 and 3, 26 patients (68.4%) had severe early recurrence and 23 patients (15.7%) had severe early recurrence among patients with scores of 0 and 1, and there were significant differences. Figure 5 shows the surgical resection rates and locoregional treatment for recurrent HCC according to the sum of the three factors (MVI, TBS > 4.70, and ALBI grade 2). Among patients with recurrent HCC, the surgical resection rates and locoregional treatment rates were significantly decreased for scores of 2 or 3 compared with scores of 0 or 1.
Overall survival (A) and recurrence-free survival (B) according to the sum of the three factors in the multivariate analysis (MVI, TBS > 4.70, and ALBI grade 2). The sum of 0 was scored 0 (thin gray lines), 2 was scored 2 (gray lines), and 3 was scored 3 (black lines). Overall and recurrence-free survival rates of patients with scores of 2 and 3 (n = 38) were significantly poorer than those of patients with scores 0 or 1. MVI macrovascular invasion, TBS tumor burden score, ALBI albumin–bilirubin
Surgical resection rates and locoregional treatment for recurrent HCC according to the sum of the three factors in the multivariate analysis (MVI, TBS > 4.70, and ALBI grade 2). The surgical resection rates (black lines) and locoregional treatment rates (gray lines) were significantly decreased in scores of 2/3 compared with scores of 0/1. HCC hepatocellular carcinoma, MVI macrovascular invasion, TBS tumor burden score, ALBI albumin–bilirubin
Patients’ clinicopathological characteristics according to the sum of the three factors (MVI, TBS > 4.70, and ALBI grade 2) for severe early recurrence are shown in Table 3. As the sum of the risk factors increased, the tumor size, number, TBS, and tumor markers increased, which means the degree of malignancy increased. The frequency of severe recurrence was also correlated with the sum of the risk factors. In addition, surgery time, blood loss, transfusion rate, and complications increased, and the number of risk factors was proportional to short-term postoperative outcomes.
Figure 6 shows the OS (Fig. 6A) and RFS (Fig. 6B) rates according to the sum of the three factors in the multivariate analysis (MVI, TBS > 4.70, and ALBI grade 2) in a validation study using data from the Jikei University School of Medicine. OS and RFS rates of patients with scores of 2 and 3 (n = 76) were significantly poorer than those of patients with scores of 0 or 1. Among patients with scores of 2 and 3, 11 patients (73.3%) had severe early recurrence and 4 patients (26.7%) had severe early recurrence among patients with scores of 0 and 1, and there were significant differences of positive predict value.
Overall survival (A) and recurrence-free survival (B) according to the sum of the three factors for severe recurrence (MVI, TBS > 4.70, and ALBI grade 2) in the validation study. The sum of 0 was scored 0 (thin gray lines), 2 was scored 2 (gray lines), and 3 was scored 3 (black lines). Overall and recurrence-free survival rates of patients with scores of 2 and 3 (n = 76) were significantly poorer than those of patients with scores of 0 or 1. MVI macrovascular invasion, TBS tumor burden score, ALBI albumin–bilirubin
Discussion
In this study, patients with recurrence beyond up-to-7 within 1 year were defined as severe early recurrence. OS and RFS rates were significantly worse in the severe early recurrence group. Multivariate analysis identified significant associations between severe early recurrence and MVI, TBS > 4.70, and ALBI grade 2. Patients with two or more of the three risk factors were prone to recurrence, failed to receive locoregional therapy for recurrent HCC, and had poor OS. The validation study also demonstrated that cases with these factors were at high risk of severe recurrence and had a poor prognosis.
In this study, patients with recurrence beyond up-to-7 within 1 year were defined as severe early recurrence, but the prognosis was compared using other criteria such as Milan criteria or 5-5-500 rule. Beyond up-to 7 and 5-5-500 rule were almost equivalent, and Milan criteria showed a slightly better prognosis. However, more patients will be eligible for the beyond up-to-7 criteria.
Most previous studies identified the presence of vascular invasion, intrahepatic metastases, large tumor size, multiple tumors, high AFP level, and positive surgical margin as risk factors of early recurrence [9,10,11,12,13,14]. In particular, the prognosis of patients with Vp4 or Vv3 was extremely poor, and the surgical indications for Vp4 or Vv3 patients required further investigation [25]. Lee et al. reported that ALBI grade 2 was also a prognostic factor for early recurrence after hepatic resection for HCC [11]. Both tumor factors and liver function are important for early recurrence.
Some nutritional and immunological statuses were shown to affect surgical prognosis in HCC, and we analyzed simple scoring systems such as NLR, CONUT, and PNI in this study [11, 26, 27]. Although previous reports showed that NLR, CONUT, and PNI were predictive of worse OS and RFS, these scores were significantly associated with severe early recurrence in the univariate analysis, and immune-nutrition status and inflammatory score were related to poor prognosis, but did not show significant differences for severe early recurrence in multivariate analysis.
In the era of multidisciplinary treatment, BCLC is clinically useful because it shows target cases, treatment methods, and prognosis prediction [3], and it is also a useful guideline for recurrent HCC after hepatic resection. Repeat hepatic resection for recurrent HCC is also the treatment of choice for patients in whom recurrence has developed after a disease-free interval of 1 year or more and in whom the recurrent tumor has no portal invasion [28], but the indication of repeat hepatic resection is limited in patients with severe recurrence. In addition to surgery, there are treatment methods such as TACE and systemic therapy. TACE provides survival benefits with an expected overall median survival of 40 months or a 5-year survival of 35% [29, 30], and has been recommended as the first-line treatment for BCLC-B HCC, but the prognosis of patients meeting beyond up-to-7 criteria is unsatisfactory [31]. Systemic therapy is recommended for patients with multiple intermediate-stage u-HCC classified using the beyond the up-to-7 criteria as a TACE-unsuitable condition. In September 2020, atezolizumab plus bevacizumab treatment (Atez/Bev) was approved as a new treatment to be administered with an immune checkpoint inhibitor and anti-VEGFR for u-HCC. An updated analysis showed that the median survival of the Atez/Bev arm was 19.2 months and PFS was 6.8 months [32]. Although the prognosis of u-HCC has been improved by systemic therapy, we would like to avoid such a severe recurrence in the early postoperative period because surgical resection can induce impaired liver function and treatment after surgery is limited. Although HCC has a high recurrence rate, it is controversial whether upfront hepatic resection is appropriate because of the high possibility of severe early recurrence; however, neoadjuvant treatment with embolization has shown negative results [33, 34]. In addition, the STORM trial, which randomized patients to sorafenib versus placebo after resection or ablation, showed no benefit in RFS [35]. There is no clear evidence for the efficacy of any of the adjuvant or neoadjuvant protocols.
Liver transplantation (LT) has been established as an acceptable therapy for small or few tumors associated with cirrhosis. However, the limited availability of donor organs hampers LT, especially deceased donor LT, in individual patients, especially because Child–Pugh class C patients without significant risk factors should be evaluated for living donor LT; moreover, this procedure is covered by the government insurance in Japan. Although the Milan criteria have been used for a long time [6], the use of extended criteria for LT, such as up-to-7 and UCSF, has been an active area of investigation [36, 37] but has not been indicated for patients with beyond up-to-7. TBS was recently reported to stratify the long-term outcomes of patients with HCC. In addition, TBS can accurately predict recurrence beyond the MC, which means that salvage transplantation is not recommended [18, 19]. In this study, recurrence beyond up-to-7 was a severe recurrence pattern that exceeded the MC, and TBS was a useful tool associated with severe recurrence.
Yoh et al. defined the resectability classification of HCC [38]. Borderline resectable (BR) HCC was defined as resectable HCC with MVI and/or ICG-Krem ≥ 0.03– < 0.05. The 5-year survival rate of BR-HCC was 35.6%, exhibiting poorer OS compared with resectable HCC. To evaluate the risk of postoperative liver failure and tumor recurrence, liver function and tumor aggressiveness should be considered. It is desirable that the BR-HCC concept is examined at multiple centers. Currently, upfront surgery is preferred for resectable HCC, but the definition of BR-HCC and the improvement of prognosis through clinical trials are required.
In this retrospective analysis, we found that MVI, TBS > 4.70, and ALBI grade 2 can predict severe early recurrence after hepatic resection for HCC, and patients with these risk factors of HCC had a poor prognosis. These patients with severe early HCC recurrence should be defined as borderline resectable HCC.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Cin 71(3):209–249
Huang J, Lok V, Ngai CH et al (2021) Disease burden, risk factors, and recent trends of liver cancer: a global country-level analysis. Liver Cancer 10(4):330–345
Reig M, Forner A, Rimola J et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 76(3):681–693
Harimoto N, Yoshizumi T, Inokuchi S et al (2018) Prognostic significance of preoperative Controlling Nutritional Status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol 25(11):3316–3323
Kudo M, Matsui O, Izumi N et al (2014) JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 3(3–4):458–468
Mazzaferro V, Regalia E, Doci R et al (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334(11):693–699
Shimamura T, Akamatsu N, Fujiyoshi M et al (2019) Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: the 5-5-500 rule - a retrospective study. Transplant Int 32:356–368
Umeshita K, Eguchi S, Egawa H et al (2019) Liver transplantation in Japan: registry by the Japanese Liver Transplantation Society. Hepatol Res 49(9):964–980
Kamiyama T, Nakanishi K, Yokoo H et al (2012) Analysis of the risk factors for early death due to disease recurrence or progression within 1 year after hepatectomy in patients with hepatocellular carcinoma. World J Surg Oncol. 10:107
Hayashi M, Shimizu T, Hirokawa F et al (2011) Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma. Am Surg 77(5):572–578
Lee YH, Koh YS, Hur YH et al (2018) Effectiveness of the albumin-bilirubin score as a prognostic factor for early recurrence after curative hepatic resection for hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg 22(4):335–343
Chan AWH, Zhong J, Berhane S et al (2018) Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 69(6):1284–1293
Shah SA, Greig PD, Gallinger S et al (2006) Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 202:275–283
Park JH, Koh KC, Choi MS et al (2006) Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg 192:29–33
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Johnson PJ, Berhane S, Kagebayashi C et al (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33(6):550–558
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients (in Japanese). Nippon Geka Gakkai Zasshi 85:1001–1005
Tsilimigras DI, Mehta R, Guglielmi A et al (2020) Recurrence beyond the Milan criteria after curative-intent resection of hepatocellular carcinoma: a novel tumor-burden based prediction model. J Surg Oncol 122(5):955–963
Yen YH, Liu YW et al (2023) Alpha-fetoprotein combined with radiographic tumor burden score to predict overall survival after liver resection in hepatocellular carcinoma. Cancers (Basel) 15(4):1203
Araki K, Harimoto N, Kubo N et al (2020) Functional liver volumetry using Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) predicts post-hepatectomy liver failure in resection of more than one segment. HPB 22(2):318–327
Taketomi A, Kitagawa D, Itoh S et al (2007) Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute’s experience with 625 patients. J Am Coll Surg 204(4):580–587
Harimoto N, Shirabe K, Ikegami T et al (2015) Postoperative complications are predictive of poor prognosis in hepatocellular carcinoma. J Surg Res 199(2):470–477
Liver cancer study group of Japan. General rules for the clinical and pathological study of primary liver cancer, Second English edition, pp34–35, Kanehara& Co., Tokyo, 2003
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24(2):289–293
Kokudo T, Hasegawa K, Matsuyama Y et al (2016) Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 65:938–943
Mano Y, Shirabe K, Yamashita Y et al (2013) Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 258(2):301–305
Saito Y, Imura S, Morine Y et al (2021) Preoperative prognostic nutritional index predicts short- and long-term outcomes after liver resection in patients with hepatocellular carcinoma. Oncol Lett 21(2):153
Minagawa M, Makuuchi M, Takayama T et al (2003) Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg 238(5):703–710
Burrel M, Reig M, Forner A et al (2012) Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 56:1330–1335
Takayasu K, Arii S, Kudo M et al (2012) Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol 56:886–892
Scaffaro LA, Stella SF, Alvares-Da-Silva MR et al (2015) Survival rates according to barcelona clinic liver cancer sub-staging system after transarterial embolization for intermediate hepatocellular carcinoma. World J Hepatol 7(3):628–632
Galle PR, Finn RS, Qin S et al (2021) Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol 22(7):991–1001
Samuel M, Chow PKH, Shih-Yen EC, et al. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst. Rev. 2009(1)CD001199.
Lee JH, Lee Y, Lee M et al (2015) A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br J Cancer 113(12):1666–1676
Bruix J, Takayama T, Mazzaferro V et al (2015) Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 16(13):1344–1354
Mazzaferro V, Llovet JM, Miceli R et al (2009) Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 10(1):35–43
Yao FY, Xiao L, Bass NM et al (2007) Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 7(11):2587–2596
Yoh T, Ishii T, Nishio T et al (2023) A Conceptual Classification of Resectability for Hepatocellular Carcinoma. World J Surg 47(3):740–748
Acknowledgements
We thank H. Nikki March, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. No funding was received specifically for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Harimoto, N., Tsukagoshi, M., Seki, T. et al. Predictors for early recurrence beyond up-to-7 or distant metastasis after hepatocellular carcinoma resection: proposal for borderline resectable HCC. Int J Clin Oncol 29, 195–204 (2024). https://doi.org/10.1007/s10147-023-02434-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02434-7