Abstract
Background
Treatment of recurrent malignant pleural mesothelioma (MPM) remains challenging. Our study examined the efficacy, tolerability, and safety of nivolumab with ipilimumab treatment for recurrent MPM after primary curative-intent surgery.
Methods
Treatment comprised 360 mg nivolumab every 3 weeks and 1 mg/kg of ipilimumab every 6 weeks, both administered intravenously. Both were discontinued for progressive disease or serious adverse events (AEs). Additional post-treatment data were evaluated, including objective response rate (ORR), disease control rate (DCR), post-treatment survival, progression-free survival (PFS), and AEs. Tumor response was assessed using the modified Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Survival analysis was estimated using a Kaplan–Meier plot. Feasibility analysis was performed using the National Cancer Institute Common Terminology Criteria for AEs version 5.0.
Results
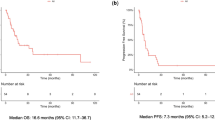
Forty-one patients received nivolumab with ipilimumab for recurrent MPM after primary curative-intent surgery (median follow-up, 10.4 months; median treatment, 5.1 months). Overall, 18 patients exhibited partial response, 13 exhibited stable disease, and 10 had documented progressive disease. ORR and DCR were 43.9 and 75.6%, respectively. The 12-month post-treatment survival rate and PFS rate were 74.2 and 40.0%, respectively (median survival, not calculated; median PFS, 7.3 months). Further, 47 AEs were reported in 29 patients (70.7%), including grade 3–4 AEs in 14 patients (34.1%). Grade 4 hepatobiliary disorders were observed in 2 patients and grade 4 neutropenia was observed in 1.
Conclusion
Nivolumab with ipilimumab treatment in patients with recurrent MPM after primary surgical treatment may be clinically efficacious, although serious AEs may be frequently observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor with most patients suffering from recurrent disease soon after curative-intent surgery [1], which may include either pleurectomy/decortication (P/D) or extra-pleural pneumonectomy (EPP). Both P/D and EPP are highly invasive surgeries and lead to a poor performance status (PS) in surgical patients, whose PS further deteriorates once they are diagnosed with recurrent MPM [2]. PS and treatment may also be compromised due to the delayed diagnosis of recurrent disease, secondary to the lack of symptoms in most patients which leads to significantly more advanced disease being present at the time of diagnosis. [3].
Until 2020, platinum agents with pemetrexed were the only approved first-line treatment regimens for MPM and long-term survival outcomes remained poor [4]. Some cases of poor PS in patients with recurrent MPM could not tolerate the platinum agents with pemetrexed [2]. To overcome these limitations, new and effective therapeutic options are needed. Recently, an immune-checkpoint inhibitor (ICI) for MPM has received considerable attention. In Japan, clinical studies have reported on the efficacy and safety of the ICI, nivolumab, as a second-line primary treatment or for treatment of recurrent disease after primary surgery for MPM [3, 5]. In addition, the open-label, randomized phase 3 study, CheckMate 743, confirmed that nivolumab, a fully human anti-programmed cell death-1 (PD-1) antibody, and ipilimumab, a fully human anti-cytotoxic T lymphocyte 4 (CTLA-4) antibody, demonstrated therapeutic activity in MPM which led to the regulatory approval of nivolumab plus ipilimumab for the treatment of un-resectable MPM [6, 7]. This combination is expected to improve the treatment results in patients with MPM.
To our knowledge, no prior clinical studies have investigated nivolumab with ipilimumab in patients with recurrent MPM after primary curative-intent surgery. This study aimed to evaluate the efficacy, feasibility, and safety of nivolumab with ipilimumab in patients with recurrent MPM.
Materials and methods
Study method and ethics
This is a retrospective cohort study conducted at a single institution. This study followed the standards of the Declaration of Helsinki and current ethical guidelines. All eligible patients were included in the program after providing written informed consent. The institutional review board at the Hyogo College of Medicine (number 4094) approved the study on May 31, 2022. We retrospectively reviewed the medical records, including the radiological data, of all enrolled patients.
Patient data and treatment
We reviewed the medical charts of patients who were registered in the prospective MPM database of our hospital surgery program between January 2004 and October 2022. All patients underwent multimodality treatment which may have included neoadjuvant chemotherapy (NAC) followed by curative-intent surgery (CIS) for MPM or CIS followed by adjuvant chemotherapy or CIS with NAC or adjuvant chemotherapy and localized irradiation. NAC comprised pemetrexed (500 mg/m2) followed by either cisplatin (75 mg/m2) or carboplatin (area under the curve, 5), each administered intravenously on day 1 of a 21-day cycle for a total of 3 cycles. P/D has been the primary curative-intent surgical procedure for MPM since September 2012. Conversion from P/D to EPP was performed when diffuse tumor invasion to the pulmonary parenchyma was identified. Adjuvant chemotherapy (AC) comprised three cycles of pemetrexed and cisplatin or carboplatin administered after P/D. Adjuvant high-dose hemithoracic irradiation using intensity-modulated radiotherapy was performed exclusively after EPP. The protocol used in multimodality treatment for MPM was derived from previous reports [2, 3, 8,9,10].
All patients underwent a physical examination and blood tests for tumor markers and computed tomography (CT) imaging every 3–6 months at our outpatient treatment facility. Recurrent disease was diagnosed and patients were triaged by a cancer board comprised a multidisciplinary team. Beginning in June 2021, nivolumab with ipilimumab was the treatment of choice for patients with an initial recurrence of MPM after P/D with either NAC or AC. The treatment of recurrent MPM consisted of 360 mg of nivolumab administered intravenously every three weeks and 1 mg/kg of ipilimumab administered intravenously every six weeks continuously unless their disease progressed or if they experienced high grade adverse events. In this research, all patients treated with nivolumab with ipilimumab for the recurrent MPM were enrolled.
Outcome evaluation and statistical analyses
Patients were followed at least quarterly until their own mortality or until their last clinic visit. Treatment response was assessed using radiological imaging as per a modified version of the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) based on CT or [18F] fluorodeoxy-D-glucose positron emission tomography (FDG-PET)/CT [11]. Patients were evaluated every 6–12 weeks with radiological imaging throughout nivolumab plus ipilimumab treatment.
The primary endpoint was the treatment response as measured by mRECIST. All radiological outcomes were designated as progressive disease (PD), stable disease (SD), partial response (PR), or complete response (CR). The objective response rate (ORR) was defined as the percentage of patients with complete or partial remission of disease, expressed as the sum of CR and PR. The disease control rate (DCR) is the total percentage of patients with any documented response, designated as the sum of patients with CR, PR, and SD.
Overall survival (OS) was calculated from the time of administration of the first nivolumab with ipilimumab dose for recurrent MPM to the most recent follow-up date or their date of death. Progression-free survival (PFS) was calculated from the time of administration of the first nivolumab with ipilimumab dose for recurrent MPM to the date of the most recent follow-up or the date PD was detected. OS and PFS were estimated using a Kaplan–Meier curve. Analyses were based on data updated on October, 2022 using JMP 14 (SAS Institute Inc., Cary, NC, USA).
Adverse events (AEs) were graded as per the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE v5.0) guidelines [12] to evaluate the safety and tolerability of the combination therapy.
Results
Patient characteristics
We enrolled 41 patients with recurrent MPM after NAC and P/D, who received nivolumab with ipilimumab between June 2021 and July 2022. The study population included 34 men and 7 women with a median age of 67 years (range 52–83 years). All patients had undergone neoadjuvant chemotherapy followed by P/D at our institution. There were no patients who underwent EPP. Macroscopic complete resection was successfully performed in 38 patients (92.7%). The histological subtypes on final pathology were epithelioid in 36 patients and biphasic in 5 patients. All patients were staged using the pathological stages of the International Mesothelioma Interest Group Staging System (version 8): 9 patients were diagnosed with Stage IA, 11 with Stage IB, 5 with Stage II, 15 with Stage IIIA, and 1 with Stage IIIB disease. Twenty-six patients (63.4%) completed multimodality treatment, including at least3 cycles of AC. The median time to recurrence after surgery was 15.4 months. The sites of the first recurrence were local (ipsilateral hemithorax or mediastinum) in 27 patients and distant (abdomen or contralateral hemithorax, brain, bone, etc.,) in 3 patients. Both local and distant recurrences occurred in 11 patients. Of the 41 patients who received nivolumab with ipilimumab for recurrent MPM after P/D, 16 had an Eastern Cooperative Oncology Group PS of 0, 23 had a score of 1, and 2 had a score of 2 at the time of their first treatment. The demographic features and disease characteristics of the patients are summarized in Table 1.
The median duration of follow-up after the first administration of nivolumab with ipilimumab, was 10.4 months. The median treatment duration was 5.1 months (range 0.7–15 months). Ten of the 41 patients (24.4%) died from either tumor progression (n = 9) or from other diseases (n = 1), 20 (48.8%) stopped nivolumab and ipilimumab treatment, and 11 (26.8%) continued nivolumab with ipilimumab treatment. The reasons for discontinuation (n = 30) included tumor progression (n = 15), AEs (n = 12), patient’s refusal (n = 2), and death due to other disease (n = 1). Of the 27 patients who stopped treatment due to tumor progression or AEs, 10 received pemetrexed with platinum as a 2nd-line chemotherapy, 10 patients received no treatment and were followed up closely and 7 received supportive care alone.
Efficacy evaluation
Radiological evidence of regression was designated as a PR in 18 patients and evidence of no change in tumor size as SD in 13 patients. Ten patients were found to have radiological evidence of increasing tumor size or number of tumors and were designated as PD. The ORR and DCR were 43.9% and 75.6%, respectively (Table 2). The Kaplan–Meier curves for OS and PFS after nivolumab with ipilimumab administration are shown in Supplementary Fig. 1 and Fig. 1. The 6-month and 12-month OS were 87.0 and 74.2%, respectively. The median OS in the study could not be calculated. The 6-month and 12-month PFS rates were 69.8 and 40.0%, respectively. The median PFS was 7.3 months (95% CI, range could not be established). The median duration of response was 7.3 months. At the termination date of the study, 20 of the 41 patients (48.8%) had an ongoing response. Seven patients (17.1%) had an ongoing response for more than one year. Seven of the 12 patients (58.3%) had an ongoing response after discontinuing nivolumab with ipilimumab due to AEs. The details of the treatment exposure and response durations of all patients are presented in Fig. 2.
The swimmer’s plot. The swimmer’s plot (B) shows the treatment duration and response duration, with the bar length corresponding to the duration of follow-up for each patient (n = 41). Results are at data cutoff. Eleven patients (No. 7, 9, 21, 22, 30, 31, 33, 38, 39, 40, and 41) continued nivolumab with ipilimumab treatment. Nine patients (No. 2, 7, 14, 15, 20, 22, 31, 33, and 41) temporarily discontinued nivolumab with ipilimumab treatment due to the development of adverse events, treatment resumed after resolution of the adverse effects. Red triangles show progressive disease. Red diamonds show AEs that led to the temporary discontinuation of nivolumab with ipilimumab. Red crosses show death. Arrows show ongoing treatment of nivolumab with ipilimumab at data cutoff
The DCRs by histological subtype were as follows: 72.2% (26 of 36 patients) and 80.0% (4 of 5 patients) were designated epithelioid and biphasic types, respectively. The DCR by recurrence pattern was as follows: 70.4% (19 of 27 patients) of patients had local recurrences only and 78.6% (11 of 14 patients) of patients had either only distant recurrences or both (local + distant) recurrences.
Safety evaluation
Toxicity was determined retrospectively for all patients using the CTCAE v5.0. Table 3 presents the AEs in the 41 patients administered nivolumab with ipilimumab. Forty-seven AEs were reported in 29 patients (70.7%). Grade 3–4 AEs were reported in 14 (34.1%) of the 41 participants. There were no treatment-related deaths. The most common AEs observed were pruritus in 11 patients (26.9%), hypophysitis or adrenal insufficiency in 10 (24.4%), and hepatobiliary disorders in 4 (9.8%). The most commonly reported grade 3–4 AEs were hypophysitis or adrenal insufficiency in 6 (14.6%), colitis in 3 (7.3%) and hyperthyroidism in 3 (7.3%). The DCR of 22 patients with G2-G4 AEs was 72.2%.
AEs that led to the complete discontinuation of nivolumab with ipilimumab (due to either component of the regimen) regardless of grade, were reported in 11 patients. Reasons for complete discontinuation were hepatobiliary disorders in 4 patients, colitis in 2, hypophysitis or adrenal insufficiency in 2, interstitial lung disease in 1 patient, acneiform rash in 1, and hyperthyroidism in 1. On the other hand, 9 patients were temporarily discontinued because of AEs (hypophysitis or adrenal insufficiency in 4 patients, hyperthyroidism in 2, interstitial lung disease in 1, uveitis in 1, and pancreatitis in 1), and the treatment was resumed after the AEs subsided with immune modulating concomitant medication (primarily corticosteroids). Eighteen patients (43.9%) required high-dose steroid treatment. Among them, the most frequent reason for prescribing steroids was for patients with an endocrine disorder (n = 11) and colitis (n = 3). Pulse steroid therapy was prescribed for 3 patients; neutropenia (grade 4, n = 1), acneiform rash (grade 3, n = 1), and nephrotic syndrome (grade 3, n = 1). Three doses of pulse corticosteroid therapy with 500 mg methylprednisolone and subsequent corticosteroid therapy (prednisolone, 1 mg/kg) resulted in recovery from all of these AEs.
Discussion
This is a novel retrospective single-institution cohort study to evaluate the clinical outcome of nivolumab with ipilimumab in patients with recurrent MPM after P/D. The key findings are: first, nivolumab with ipilimumab has promising efficacy in the treatment of recurrent MPM in the post-operative setting; and, second, grade 3–4 AEs were observed in 34.1%, of patients in our study which is higher than in the Checkmate-743 study. For many years, the standard treatment for MPM has been chemotherapy agents. [13]. Since the 1990s, modulation of the immune system in patients with MPM has been the focus of research. The development of checkpoint inhibitors and their successful outcomes in melanoma and lung cancer prompted clinical researchers to test these agents in MPM. Single-agent immuno-oncology studies revealed that significant long-lasting responses could achieved in some patients. Nivolumab was one of the first immunomodulatory found to have promising efficacy in MPM and as a result has received considerable attention. Okada et al. reported the MERIT trial for the second- or third-line treatment of MPM. Thirty-four patients were enrolled and they reported an ORR of 29.4% and DCR of 67.6%. The median OS and PFS were 17.3 and 6.1 months, respectively [5]. Successful developments in the treatment of melanoma and lung cancer have also been studied in hopes of improving OS for recurrent MPM. In the CheckMate-743 study, patients with all histologic subtypes were randomized between the standard of care (cisplatin or carboplatin plus pemetrexed) for six cycles or the combination of ipilimumab plus nivolumab for a maximum of 2 years. There was an ORR of 39.6% and DCR of 76.6% in nivolumab with ipilimumab. Nivolumab with ipilimumab significantly prolonged OS compared to cisplatin or carboplatin with pemetrexed (HR 0.74; 95% CI, 0.60–0.91; P = 0.002), with median duration of response of 11.0 versus 6.7 months, respectively, and estimated rates of patients with ongoing response at 2 years of 32 versus 8%, respectively. Based on these results, in October 2020, the US Food and Drug Administration approved nivolumab with ipilimumab for previously untreated and surgically un-resectable MPM [7].
Our prior report on post-recurrence treatment for MPM included 57 patients who developed recurrence after NAC followed by P/D between September 2012 and December 2017. Forty-three of these patients (75.4%) developed recurrent disease and were treated with ≥ 3 cycles of chemotherapy, surgery, or radiofrequency ablation. The survival time was significantly longer in patients who received treatment for their recurrence, than in those who received best supportive care (median survival with recurrent disease: 24.1 vs 4.2 months) [2], which supports the provision of treatment for recurrent MPM. Of the 39 patients who received initial chemotherapy for recurrence, 20 (51.3%) received a platinum agent plus pemetrexed. The 12-month post-recurrence survival rate was 59.5% (median, 14.4 months) [2]. After that, nivolumab for the second-line treatment of MPM was approved in Japan in August 2018, while we reported that nivolumab in 35 patients with post-operative recurrence of MPM. Of the 35 patients, ORR was 20.0%, and the DCR was 77.1%. The 12-month post-treatment survival rates and PFS rates were 54.1 and 17.1%, respectively (median survival, 13.1 months; median PFS, 4.4 months) [3]. Hence, the CIS for MPM is highly invasive and frequently leads to a poor PS, particularly in those patients who subsequently develop recurrent MPM. Treatment of recurrent MPM remains challenging due to a limitation of treatment options and few studies providing evidence of benefit. The current study presents a novel treatment option and evidence of benefit with an ORR of 43.9%, DCR of 75.6% and the 12-month post-treatment survival and PFS were 74.2 and 40.0%, respectively (median survival, not calculated; median PFS, 7.3 months), with this proposed combination therapy in patients with recurrent MPM after P/D, with either AC or NAC. Several of our patients were documented to have a response to treatment that exceeded the length of our study. These findings were similar to the results of Checkmate-743 [7, 8] and provide evidence for the efficacy of nivolumab plus ipilimumab for patients with recurrent MPM after P/D.
One area of concern in the prospective widespread use of nivolumab in combination with ipilimumab is the reported frequency of grade 3–4 AEs which could lead to discontinuation of the treatment. Checkmate-743 reported that grade 3–4 AEs were documented in 30.3%, whereas we documented grade-3–4 AEs in 34.1% of the patients in our study [7]. AEs affecting the endocrine systems were among the most common and were found to be affecting 26.8% (11 patients.) Endocrine AEs included effects on the pituitary, adrenal and thyroid endocrine systems, which contrasts with our previously reported data on nivolumab therapy as a single agent for recurrent MPM after P/D which found a low frequency of endocrine AEs and grade 3 AEs (11.4%) [3, 5]. This indicates that ipilimumab, a monoclonal antibody against CTLA-4, is likely to be the factor associated with the development of several endocrine AEs in the combination therapy protocol. The mechanism underlying ipilimumab-induced endocrine AEs implicates immune, inflammatory, and genetic pathways, the specifics of which remain to be elucidated [14]. Our study provides evidence that the inflammation and reduced PS following surgery may directly affect the pathogenesis of endocrine AEs. The management of endocrine AEs primarily involves replacement therapy of deficient hormones possibly with concurrent high-dose steroids, particularly with pituitary, and adrenal involvement. Discontinuation of ICI may also be either temporarily or permanently required in severe cases. [14]. We found that most endocrine AEs resolved with either high-dose steroids or supportive treatment making it possible to subsequently resume ICI.
Combination therapy with nivolumab and ipilimumab is an important treatment option for recurrent MPM after P/D. Checkmate-743 and this study have both demonstrated efficacy and long-term responsiveness to this combination protocol and while our patients experienced a high frequency of AEs, most all of the AEs were manageable with treatment protocol-specific guidelines. This protocol provides a novel treatment option for recurrent MPM at a time when few options are available [15].
Our study has several limitations. This is a single-center, single arm, retrospective cohort study which necessarily introduces bias which should not be ignored. We also concluded the study before all survival data could be collected. The follow-up period on all patients after the initiation of nivolumab with ipilimumab administration was relatively short. Thus, OS and PFS data were immature. We evaluated the initial effects and the feasibility of nivolumab with ipilimumab administration; however, the prognostic factors, including programmed death ligand 1, were not investigated. Further research is warranted to further examine the efficacy of this protocol with more patients for a longer duration and involving more institutions.
Conclusions
Nivolumab with ipilimumab treatment in patients with recurrent MPM after P/D was found to have promising efficacy but was also complicated by serious AEs. Further multi-institutional studies to evaluate long-term survival, prognostic factors and development of AEs with this protocol deserve consideration.
Data availability
Raw data were generated at Hyogo College of Medicine. Derived data supporting the findings of this study are available from the corresponding author AN on request.
References
Baldini EH, Richards WG, Gill RR et al (2015) Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 149:1374–1381
Nakamura A, Takuwa T, Hashimoto M et al (2020) Clinical outcomes with recurrence after pleurectomy/decortication for malignant pleural mesothelioma. Ann Thorac Surg 109:1537–1543
Nakamura A, Kondo N, Nakamichi T et al (2020) Initial evaluation of nivolumab in patients with post-operative recurrence of malignant pleural mesothelioma. Jpn J Clin Oncol 50:920–925
Zalcman G, Mazieres J, Margery J et al (2016) Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma avastin cisplatin pemetrexed study (MAPS): a randomised, controlled, open-label, phase 3 trial, et al. Lancet 387:1405–1414
Okada M, KijimaAoe T et al (2019) Clinical efficacy and safety of nivolumab: results of a multicenter open-label, single-arm, Japanese phase ii study in malignant pleural mesothelioma (MERIT). Clin Cancer Res 25:5485–5492
Baas P, Scherpereel A, Nowak AK et al (2021) First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicenter, randomised, open-label, phase 3 trial. Lancet 397:375–386
Peters S, Scherpereel A, Cornelissen R et al (2022) First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol 33:488–499
Nakamura A, Hashimoto M, Kodama H et al (2021) Clinicopathological features of radiological early malignant pleural mesothelioma with no apparent tumor or pleural thickening. Int J Clin Oncol 26:95–103
Nakamura A, Kondo N, Nakamichi T et al (2021) Complications and predictive factors for air leak > 10 days with neoadjuvant chemotherapy followed by pleurectomy/decortication for malignant pleural mesothelioma. Ann Surg Oncol 28:3057–3065
Nakamura A, Hashimoto M, Matsumoto S et al (2021) Outcomes of conversion to extrapleural pneumonectomy from pleurectomy/decortication for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 33:873–881
Byrne MJ, Nowak AK (2004) Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 15:257–260
National Cancer Institute. Maryland: Common Terminology Criteria for Adverse Events, version 5.0 (CTCAEv5.0). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed Oct 15 2022
Baas P (2022) Nivolumab plus ipilimumab should be the standard of care for first-line unresectable epithelioid mesothelioma. J Thorac Oncol 17:30–33
Tsoli M, Kaltsas G, Angelousi A et al (2020) Managing ipilimumab-induced hypophysitis: challenges and current therapeutic strategies. Cancer Manag Res 12:9551–9561
Travert C, Tomasini P, Greillier L (2022) Nivolumab plus ipilimumab in malignant pleural mesothelioma. Expert Rev Anticancer Ther 22:815–822
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Takashi Kijima reports receiving speakers bureau honoraria and research funding from Ono Pharmaceutical. Seiki Hasegawa reports endowed department (The Department of Thoracic Oncology, Hyogo Medical University.) from Ono pharmaceutical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2023_2292_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1 Overall survival. Kaplan–Meier curves of overall survival from the time of first nivolumab with ipilimumab. The 12-month survival rate was 74.2% (TIF 5415 KB)
About this article
Cite this article
Nakamura, A., Hashimoto, M., Kondo, N. et al. Efficacy and safety of nivolumab with ipilimumab for recurrent malignant pleural mesothelioma after primary surgical intervention. Int J Clin Oncol 28, 409–415 (2023). https://doi.org/10.1007/s10147-023-02292-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02292-3