Abstract
Background
Positron Emission Tomography-Computed Tomography (PET-CT) has been changing diagnostic and therapeutic strategies for patients with cancers, and several PET-CT-related prognostic factors have been reported. We have focused on metabolic tumor volumes (MTVs) over the whole body and in specific organs using 18F-PET-CT imaging, and have compared clinical data to know the prognosis of patients with diffuse large B cell lymphoma (DLBCL).
Patients and methods
From January 2006 to December 2016, patients who were newly diagnosed for de novo DLBCL and who received 18F-FDG PET-CT scans for disease staging at Ehime University Hospital were reviewed.
Results
A total of forty out of 108 patients with DLBCL were analyzed. The median and the average follow-up were 3.9 years and 3.6 years. Both MTV50 and MTV60 whole-body searching indicated effective prognostic values for patients with DLBCL statistically (P = 0.027). However, analysis of MTVs in the spleen and in bone marrow did not provide any prognostic value. Receiver operating characteristic (ROC) analysis indicated that the cutoff level 25.8 in MTV60 is the most effective prognostic value (P = 0.022) which predicts patient survival after treatment with R-CHOP chemotherapy.
Conclusion
MTV60 using whole-body scanning appears to be an effective indicator in DLBCL and indicates the patient prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron Emission Tomography-Computed Tomography (PET-CT) is the most advanced medical imaging technique available recently, and has dramatically changed not only cancer diagnosis but also treatment strategies for patients with cancers. Recent clinical studies have reported that various types of data derived from PET-CT analysis may influence the prognosis for cancer patients directly or indirectly.
Several studies have reported that the standardized uptake values (SUVs) of F-18 fluorodeoxyglucose (SUVmean and/or SUVmax) are related to tumor proliferation and growth, suggesting that SUVs may affect disease activity (aggressiveness) and prognosis [1]. In addition, the relation between patient prognosis and tumor metabolism based on metabolic tumor volume (MTV; a volumetric measurement of tumor tissues with increased F-18 fluorodeoxyglucose uptake) and total lesion glycolysis (TLG; TLG = SUVmean × MTV) has been reported recently. A number of publications have referred to the stronger relationship of MTVs than SUVs to the prognosis of patients with malignancies, especially with lymphoid malignancies [2,3,4,5,6,7,8,9,10,11], suggesting that the MTVs may be one effective tool to use to predict patient prognosis with lymphoid malignancies [12]. However, the method, the searching area, the cutoff level, and the relation of the SUVs to MTV analysis have not been wholly understood. Here, we have extracted several parameters from metabolic imaging and apply them to PET-CT, and have analyzed clinical data to determine the patient’s disease prognosis focusing on diffuse large B cell lymphoma (DLBCL).
Materials and methods
Patient with DLBCL and the excluding criteria
Between January 2006 and December 2016, patients who were newly diagnosed for de novo DLBCL and who received 18F-FDG PET-CT scans for disease staging at Ehime University Hospital were reviewed. We performed gene rearrangement analysis of the immunoglobulin heavy chain (IgH) region using Southern blot or karyotypic analysis along with flow cytometric analysis for CD20 expression on cell surfaces to confirm that the tumours were of B lymphocyte origin. Most of the samples were of lymph nodes, but selected specimens of extra-nodal (EN) soft tissue were also included. Some patients were excluded taking into consideration past reports indicating they were unsuitable cases for the current analysis, as follows: (1) patients ≥ 80 years of age; (2) patients with stage I disease; (3) patients treated with over 25% reduction doses of chemotherapy and with less than six cycles of treatment; (4) primary DLBCLs of CNS; (5) primary testicular, mammary, and uterine DLBCLs; (6) rheumatoid arthritis- or methotrexate-associated DLBCLs; (7) DLBCLs with concomitant or antecedent follicular lymphoma expressing t(14;18); (8) DLBCLs associated with HIV infection; (9) high-grade B-cell lymphoma; and (10) intravascular large B-cell lymphoma. We selected our patients very strictly to remove the “noise” from the patient selection in the current study. Because diffuse large B cell lymphoma (DLBCL) has several clinical categories with different treatment strategies. We defined de novo DLBCL in the current paper under the excluding criteria from (4) to (10). Also, we removed patients who were ≥ 80 years of age, patients with stage I disease, and patients treated with reduced doses of chemotherapy, because these patients were treated with different strategies from standard R-CHOP chemotherapy [13, 14]. Clinical examination or interview by phone or mail was performed to ascertain patient survival, physical condition and DLBCL status of all surviving patients.
Treatments for patients with DLBCL
Patients were treated with R-CHOP (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 (maximum 2 mg/body), day 1, and prednisolone 100 mg/body, days 1–5), or R-CHOP-like regimens based on their physician’s choice. Chemotherapy was defined as R-CHOP or R-CHOP-like therapy with ≥ 75% of dose intensity, a dose of an effective drug administered per unit time (mg/m2/week), and ≥ six cycles of treatment at least.
18F-FDG PET-CT imaging
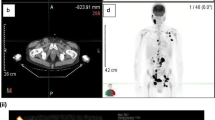
18F-FDG PET-CT imaging was performed by an integrated PET-CT camera (Discovery PET/CT 600; GE Healthcare, Milwaukee, WI, USA) for all patients with DLBCL within 21 days prior to their chemotherapy. All patients fasted for at least 6 h and had a blood glucose level of 80–120 mg/dL before intravenous administration of 18F-FDG (3.7 MBq/kg). The total examination time for PET-CT was approximately 25 min. The PET emission images were corrected for measured attention and reconstructed using an ordered-subset expectation maximization iterative algorithm. Integrated PET and CT images were reviewed on Advantage Workstations Ver.4.5 (GE Healthcare). The display field of view was 60 × 60 cm, which consisted of 192 × 192 matrixes. Voxel size was 3.125 × 3.125 × 3.27 mm3.
18F-FDG PET-CT imaging analysis
PET and CT images were evaluated visually and quantitatively. For each PET-CT dataset, 18F-FDG uptakes among all foci were carefully identified. The maximum standardized uptake value (SUVmax) and the metabolic tumor volume (MTV) were measured on the fused PET-CT images using Workstation. The MTV was estimated in a 3D manner by selecting a volumetric region of interest (VOI), and the size of the VOI was manually regulated on the corresponding coronal and sagittal images to include the entire active tumor in the VOI. As several parameters, isocontour thresholds of 50%, 60%, and 70% of metabolic volume in the MTV (MTVs; MTV50, MTV60, and MTV70) were determined. If there were multiple accumulations in FDG-PET imaging, MTV50, MTV60, and MTV70 were calculated based on the highest values of SUVmax. For example, if the highest SUVmax is 10, the volume of each lesion which indicates 6.0 or more SUV in the accumulation is measured, and the sum of the volumes is aggregated as MTV60. As the SUVmax, we focused on the 18F-FDG uptake in the spleen of the patient with DLBCL and compared this to its uptake in the whole body.
Analysis of overall survival employing 18F-FDG PET-CT imaging
Survival time was from the date of enrollment in any treatment to the date of the last follow-up (at the end of 2018) or death. Overall survival (OS) curves were calculated for each prognostic group based on the median level of SUVmax and MTVs as the cutoff (high; > median range versus low; ≤ median range) according to the Kaplan–Meier method and analyzed using the log-rank univariate test. Fisher’s exact test and Chi-squared test were performed to detect the statistical differences among the groups. P values less than 0.05 were considered significant. A receiver operating characteristic (ROC) curve was used to determine the cut-off value sensitivity and specificity. All statistical analyses were performed using the SAS software package version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics of the study
This study was approved by the Ethics Committee for Clinical Studies at Ehime University Graduate School of Medicine (study IRB#1508024), and carried out in accordance with the ethical standards of the 1995 Declaration of Helsinki (as revised in Brazil 2013). Informed consent was by the opt-out principle; we disclosed information about the study on the associated website (https://www.m.ehime-u.ac.jp/school/clinical.oncology/?page_id=25) and provided an opportunity to decline to participate in this study.
Results
Clinical characteristics
One hundred-eight patients with DLBCL in total were treated in the current study period. As mentioned above in the “Materials and Methods” section, we have selected our patients very strictly to remove the “noise” from the patient selection. We removed a total of sixty-eight patients from the current study, (n = 33; patients who were ≥ 80 years of age, n = 20; patients whose disease was in stage I, n = 34; patients treated with over 25% reduction doses of chemotherapy, n = 10; patients whose pathologies had biological heterogenicities from de novo DLBCL, some cases were overlapped). The study included forty patients. Of analyzed patients, 18 (45%) were men and 22 (55%) were women. The median age was 64 years (range, 49–79). Eighteen (18) (45%) patients were in stage II, 6 (15%) in stage III and 16 (40%) in stage IV, respectively. The majority (n = 39) of the patients were treated with R-CHOP. The median and the average follow-up were 3.9 years (47 months) and 3.6 years (43 months), respectively. These clinical characteristics are shown in Table 1. All patients were examined with 18F-FDG PET-CT within 21 days before the initial treatment.
Patient prognosis according to 18F-FDG-PET-CT imaging
Kaplan–Meier analysis was performed to estimate the OS of patients with DLBCL based on several 18F-FDG PET-CT parameters. Figure 1 indicates Kaplan–Meier curves of baseline progression-free survival (PFS) (Fig. 1a) and overall survival (OS) (Fig. 1b) of the currently analyzed patients (n = 40) with DLBCL.
Figure 2 indicates overall survival of patients based on whole-body analysis of SUVmax (Fig. 2a), MTV50 (Fig. 2c), MTV60 (Fig. 2d), and MTV70 (Fig. 2e) to compare prognostic values using the average of patient numbers as the cutoff. Also, Fig. 2 indicates overall survival using analysis of SUVmax (Fig. 2b), MTV50 (Fig. 2f), MTV60 (Fig. 2g), and MTV70 (Fig. 2h) in the spleen of the patients. Regarding the type of analysis, the two parameters of MTV50 and MTV60 in whole-body analysis, not those in spleen analysis, were identified as defining patient prognosis with statistical significance. These data are summarized in Table 2. In addition, we tried MTV analysis using bone marrow accumulation of 18F-FDG to focus on another specific organ; however, the data were unreliable for accurate analysis because of variations (data not shown).
Kaplan–Meier estimates the overall survival (OS) curve in the patients with DLBCL based on several PET-CT parameters, such as the whole-body maximum standardized uptake value of FDG (fluorine-18-2-deoxy-d-glucose) (WB-SUVmax) (a), isocontour thresholds of 50%, 60%, and 70% of metabolic volume in whole-body metabolic tumor volumes (WB-MTVs; WB-MTV50, WB-MTV60, and WB-MTV70) (b–d), maximum standardized uptake value in the spleen (SP-SUVmax) (e) and isocontour thresholds of 50%, 60%, and 70% of metabolic volume in the metabolic tumor volumes in the spleen (SP-MTVs; SP-MTV50, SP-MTV60, and SP-MTV70) (f–h)
Receiver operating characteristic (ROC) analysis to determine cutoff value
To clarify a better prognostic parameter to know the prognosis of patients with DLBCL, as an additional method of data validation, a Receiver Operator Characteristic (ROC) curve was used to determine the real cutoff range among MTV50, MTV60, and MTV70 (Fig. 3a–c). The ROC curve illustrated that MTV60 (cutoff 25.8) had better prognostic value than the conventional cutoff with significant improvement in predictive accuracy of OS (Fig. 3d); a sensitivity of 0.62 and a specificity of 0.63 (log-rank test, p = 0.022), AUC = 0.715.
Discussion
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma and it accounts for approximately 32% of non-Hodgkin lymphomas diagnosed in the world [15]. This disease is recognized to be a chemotherapy-sensitive B-cell malignancy which is curable with R-CHOP or R-CHOP-like regimens [16, 17]. Since several prognostic factors had been reported after clinical observations, prognostic factor-based treatment strategies should be recommended [18]. PET-CT is recently recommended for assessing not only the diagnosis and staging but also the therapeutic response and treatment strategies in the majority of lymphomas [19]. Recent major clinical interests are whether metabolic tools and detection methods concerning 18F-FDG PET-CT should be more strongly involved in determining patient prognosis.
SUVmax has been reported as an easy and simple indicator to show the prognosis of patients with lymphoid malignancies among several parameters of 18F-FDG PET [1]. Not only maximum glucose uptake but also tumor volume and areas with increased 18F-FDG uptake have been recognized as indicators of patient prognosis [3]. In contrast, MTV, which is based on relative thresholds, shows the percentage of SUV volumes, which three-dimensional regions of interest are drawn around individual lesions, and indicates the volume of glucose-avid malignancies. MTV may indicate lesional dosimetry, patient prognosis, and response to therapy beyond SUVmax, and its MTV is easy to detect technically. MTV50 (margin threshold of 50% SUVmax) is often universally used as a model of isocontour threshold of tumor metabolic volume from total-body to a specific organ [20, 21]. However, tumor volume delineation based on a fixed percentage of SUVmax might be affected by the variability and “noise” inherent in SUVmax itself and MTV50 may be relatively too low to be able to show the tumor metabolism and prognosis for aggressive metabolic tumors (high SUVmax tumors) such as DLBCL [22]. In this study, we have selected additional parameters (MTV60 and MTV70) to know the best cutoff range of MTV for determining the prognosis of patients with DLBCL.
First in this study, we have focused on MTV imaging in the bone marrow and spleen as specific organs in which tumors may be involved and in which involvement may affect the prognosis of patients with lymphoid malignancies [23, 24]. However, a search of the entire bone marrow employing MTV is technically difficult. This is partly because bone marrow is present in several parts of the body, such as in limbs, the skull, trunk, and pelvis. Also, it is present in bone matter of various sizes and it lacks cohesion which impedes analysis. In addition, it is difficult to distinguish between reactive and pathological accumulation of 18F-FDG. Therefore, we have changed our strategies and have focused on the analysis of the relation between whole-body and spleen MTVs and patient prognosis.
Our analysis indicates that MTV50 and MTV60 in the whole body are both useful to predict the prognosis of patients with DLBCL, and ROC analysis indicates MTV60 cutoff 25.8 is the most effective level, and the range of AUC in MTV60 shows 0.715. In contrast, analysis of metabolic volumes in the spleen is not a useful indicator of patient prognosis, and no association of MTV scanning between the spleen and the whole body was found in the current analysis. Several reasons for this negative result may exist. First, SUVmax in the spleen was at a relatively low level (2.20 vs 22.80) and MTVs in the spleen existed at a relatively high range compared to those in the whole body (69.93 vs 30.78 in MTV50, 45.06 vs 15.71 in MTV60, and 19.38 vs 8.69 in MTV70). These results suggest that the incidence of tumor involvement in the spleen in the current cases may be small and not suitable for statistical analysis. Second, accurate current evaluation of infiltration of tumors into the spleen by focusing on MTVs might be impossible. This is because glucose accumulation in the spleen exists at a relatively high level in cases without tumor involvement. Also, if the spleen has a high-density spot of glucose accumulation, which points to tumor infiltration, MTVs in the spleen appear at a relatively lower level compared to those in the whole body, because the area to be measured appears smaller instead of the SUVmax getting larger. In contrast, if the spleen is biologically enlarged without tumor involvement and the accumulation in the spleen is uniform, MTVs in the spleen will be relatively high in the calculation, especially in the cases with inflammatory and immunological activations [25]. Such “noise” or false positivity may affect the negative relation between MTVs in the spleen and the patient prognosis. The above “noise” may be involved in negative results of MTV analysis involving the spleen in the current analysis [26].
In summation, MTV60 (cutoff 25.8) using whole-body scanning, not other scanning of specific organs, appears to be an effective indicator of aggressive tumor metabolism in DLBCL and indicates the patient prognosis. However, we are aware of the limitations of our analysis, because it was confined to a single institution and to a small number of cases. Further study should be recommended to determine the magnitude of MTVs using PET-CT in researching lymphoid malignancies, especially DLBCL.
References
Schöder H, Noy A, Gönen M et al (2005) Intensity of 18 fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol 23:4643–4651
Kanoun S, Rossi C, Berriolo-Riedinger A et al (2014) Baseline metabolic tumor volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 41:1735–1743
Sasanelli M, Meignan M, Haioun C et al (2014) Pretherapy metabolic tumor volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 41:2017–2022
Adams HJ, de Klerk JM, Fijnheer R et al (2015) Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol 94:532–539
Trotman J, Luminari S, Boussetta S et al (2014) Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol 1:PE17-E27. https://doi.org/10.1016/S2352-3026(14)70008-0
Ceriani L, Martelli M, Zinzani PL et al (2015) Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood 126:950–956
Cottereau AS, Becker S, Broussais F et al (2016) Prognostic value of baseline total metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with peripheral T-cell lymphoma (PTCL). Ann Oncol 27:719–724
Cottereau AS, Lanic H, Mareschal S et al (2016) Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res 22:3801–3809
Mikhaeel NG, Smith D, Dunn JT et al (2016) Combination of baseline metabolic tumor volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging 43:1209–1219
Schöder H, Zelenetz AD, Hamlin P et al (2016) Prospective study of 3′-deoxy-3′-18F-fluorothymidine PET for early interim response assessment in advanced-stage B-cell lymphoma. J Nucl Med 57:728–734
Meignan M, Cottereau AS, Versari A et al (2016) Baseline metabolic tumor volume predicts outcome in high–tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol 34:3618–3626
Guo B, Tan X, Ke Q et al (2019) I Prognostic value of baseline metabolic tumor volume and total lesion glycolysis in patients with lymphoma: a meta-analysis. PLoS ONE 14:e0210224. https://doi.org/10.1371/journal.pone.0210224
Otsuka M, Yakushijin Y, Hamada M et al (2004) Role of CD21 antigen in diffuse large B-cell lymphoma and its clinical significance. Br J Haematol 127:416–424
Hasebe S, Tanaka K, Miyake Y et al (2018) Analysis of clinical factors and mortality in diffuse large B-cell lymphoma patients over or under 80 years of age. Int J Gerontol 12:100–104
Armitage JO, Weisenburger DD (1998) New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 16:2780–2795
Sehn LH, Berry B, Chhanabhai M et al (2007) The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857–1861
Sehn LH, Donaldson J, Chhanabhai M et al (2005) Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23:5027–5033
Lossos IS, Morgensztern D (2006) Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol 24:995–1007
Voltin CA, Mettler J, Grosse J et al (2020) FDG-PET Imaging for Hodgkin and diffuse large B-cell lymphoma-an updated overview. Cancers (BASEL) 12. pii:E601. https://doi.org/10.3390/cancers12030601
Bang JI, Lim Y, Paeng JC et al (2017) Comparison of quantitative methods on FDG PET/CT for treatment response evaluation of metastatic colorectal cancer. Nucl Med Mol Imaging 51:147–153
Gao A, Wang S, Fu Z et al (2015) 18F-FDG avid volumes on pre-radiotherapy FDG PET as boost target delineation in non-small cell lung cancer. Int J Clin Med 8:7561–7568
Soret M, Bacharach SL, Buvat I (2007) Partial-volume effect in PET tumor imaging. J Nucl Med 48:932–945
St-Pierre F, Broski SM, LaPlant BR et al (2019) Detection of extranodal and spleen involvement by FDG-PET imaging predicts adverse survival in untreated follicular lymphoma. Am J Hematol 94:786–793
Song MK, Yang DH, Lee GW et al (2016) High total metabolic tumor volume in PET/CT predicts worse prognosis in diffuse large B cell lymphoma patients with bone marrow involvement in rituximab era. Leuk Res 42:1–6
Dercle L, Seban RD, Lazarovici J et al (2018) 18F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with Hodgkin lymphoma treated by anti–programmed death 1 immune checkpoint inhibitor. J Nucl Med 59:15–24
Mayerhoefer ME, Raderer M, Jaeger U et al (2018) Ultra-early response assessment in lymphoma treatment: [18F] FDG PET/MR captures changes in glucose metabolism and cell density within the first 72 hours of treatment. Eur J Nucl Med Mol Imaging 45:931–940
Acknowledgements
We appreciate the cooperation of the staff members of the chemotherapy room in Ehime University Hospital.
Author information
Authors and Affiliations
Contributions
SY and YY carried out the design of the study, collected the clinical data, and drafted the manuscript. MM, YS, TF, and KT participated in the design of the study and oversaw the clinical data. SH and KT carried out the statistical analysis of the study. YY conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read the draft, revised it critically, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yamanaka, S., Miyagawa, M., Sugawara, Y. et al. The prognostic significance of whole-body and spleen MTV (metabolic tumor volume) scanning for patients with diffuse large B cell lymphoma. Int J Clin Oncol 26, 225–232 (2021). https://doi.org/10.1007/s10147-020-01807-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01807-6