Abstract
Background
The association between baseline frailty and health-related quality of life (HRQOL) in patients with prostate cancer (PC) remains unknown.
Methods
We retrospectively evaluated the association of pretreatment frailty with HRQOL in 409 patients with PC from February 2017 to April 2020. Frailty and HRQOL were evaluated using the geriatric 8 (G8) screening tool and QLQ-C30 questionnaire, respectively. The primary objective was comparison of G8 and QOL scores between the localized diseases (M0 group) and metastatic castration-sensitive PC (mCSPC group). Secondary objectives were to study the association of G8 and QOL scores in each group and effect of frailty (G8 ≤ 14) on worse QOL.
Results
The median age of patients was 70 years. There were 369 (surgery: 196, radiotherapy: 156, androgen deprivation therapy alone: 17) patients in the M0 and 40 patients in the mCSPC groups. There was a significant difference between the M0 and mCSPC groups in the G8 score (14.5 vs. 12.5), functioning QOL (94 vs. 87), global QOL (75 vs. 58), and 100–symptom QOL (94 vs. 85) scores. G8 scores were significantly associated with functioning, global, and 100–symptom QOL scores in both M0 and mCSPC groups. The multivariable logistic regression analyses showed that frailty (G8 ≤ 14) was significantly associated with worse global QOL, functioning QOL, and 100–symptom QOL scores.
Conclusion
The baseline frailty and HRQOL were significantly different between the localized and metastatic disease. The baseline frailty was significantly associated with worse HRQOL in patients with PC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the most frequent cancer among the men in Western countries and Japan [1,2,3]. Reflecting on the increasing population of elderly patients with PC, the interest in frailty in those patients has been increasing [4,5,6]. Frailty is a common syndrome in older adults that is theoretically defined as an aging-associated vulnerability and is related to an increased risk for poor health outcomes, such as hospitalization, health care resource utilization, and mortality [7, 8]. Previous studies have demonstrated the utility of frailty on treatment selection (surgery or radiotherapy) [9], prediction of postoperative pain after robotic radical prostatectomy [10], postoperative complications [11, 12], and poor prognosis in patients with PC [13,14,15]. Although the gold standard for frailty assessment is comprehensive geriatric assessment as a multidimensional method, it is time-consuming and requires the expert geriatricians. To address this problem, total of 17 different tools have been studied in 44 different trials to evaluate the best screening test and found the geriatric 8 (G8) was more useful tool than other instruments in terms of sensitivity [16]. However, there are not enough evidences available for the utility of G8 screening tool in patients with PC [15].
Health-related quality-of-life (HRQOL) has been recognized as a non-oncological outcome and was associated with clinical outcomes [17,18,19,20]. Some studies suggested the important role of baseline HRQOL for prognosis in several cancers [21,22,23]. Also, previous studies indicated the association of frailty with HRQOL in the patients with breast cancer [24], lung cancer [25], colorectal cancer [26]. As frailty and HRQOL were suggested to be associated with unfavorable outcomes [21,22,23], we hypothesized that PC patients with frailty might have lower HRQOL compared with patients without frailty. However, no evidence is available regarding the association of frailty with HRQOL in patients with PC. Therefore, we performed a retrospective FRAilty and Quality of life for patients with Prostate Cancer study (FRAQ-PC study) to investigate the association of frailty with HRQOL in patients with PC.
Materials and methods
Ethics statement
This FRAQ-PC study was performed in accordance with the ethical standards established by the Declaration of Helsinki and was approved by the ethics review board (authorization number: 2019–099). The study was registered on the UMIN-CTR (UMIN 000,039,867).
Study population
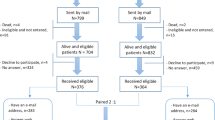
This retrospective study screened 648 patients with prostate cancer who were treated at the Hirosaki University Hospital, Japan, between January 2017 and April 2020. The inclusion criteria called for patients who fulfilled frailty screening and HRQOL questionnaires with untreated localized PC or metastatic castration-sensitive PC (mCSPC).
Patient variables
We evaluated age, Eastern Cooperative Oncology Group performance status (ECOG PS), serum prostate-specific antigen (PSA) at diagnosis, biopsy Gleason score, hypertension (HTN), cerebrocardiovascular disease (CCVD), diabetes mellitus (DM), chronic respiratory disease (CRD), and treatment modality, such as robot-assisted radical prostatectomy (RARP), radiation therapy (RT), androgen deprivation therapy (ADT), standard of care (SOC) for mCSPC, and SOC for mCRPC. The CCVD included cerebral hemorrhage, cerebral infarction and subarachnoid hemorrhage, stroke, heart attack, heart failure, arrhythmia, heart valve problems, coronary artery disease (angina and myocardial infarction). The CRD included chronic obstructive pulmonary disease (COPD) asthma, occupational lung diseases, pulmonary hypertension, and interstitial pneumonia.
Assessment of frailty
We assessed frailty using the G8 screening tool (ranges from 0 to 17) with a frailty cutoff of ≤ 14 [9, 10, 15]. The G8 assessment was administered during the initial outpatient clinic visit as a part of clinical practice.
Evaluation of QOL
We used the European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire C30 (QLQ-C30) for QOL evaluation [27, 28], and patients answered this questionnaire at the time of the initial outpatient clinic visit. The questionnaire consisted of five functioning QOL scales (physical, social, role, cognitive, and emotional functioning), a scale for global QOL, and nine-symptom QOL scales (fatigue, pain, nausea/vomiting, dyspnea, appetite loss, sleep disturbance, constipation, diarrhea, and financial difficulties). All scales were converted to linear QOL scores ranging from 0 to 100 in accordance with the scoring manual (Scoring QLQ-C30 version 3.0) [23] and summarized in three components: the functioning QOL (physical, role, cognitive, and emotional scores), global QOL, and symptomatic QOL (fatigue, pain, sleep disturbance, nausea/vomiting, appetite loss, constipation, diarrhea, and dyspnea scores). A high score for functioning and global QOL scales represents a good QOL. Since a high score for a symptom scale equates to a high number of symptoms or problems, we used a 100-symptomatic QOL scale to come up with a higher scale that represents a good QOL. Optimal cutoff score of QOL for G8 ≤ 14 was defined at the time of data analysis by receiver operating characteristic (ROC) curve.
Outcomes
The primary objective was to compare the G8 and QOL scores between the M0 and mCSPC groups. Our secondary objective was to study the association of G8 and QOL scores in each group and effect of frailty (G8 ≤ 14) on worse QOL under a multivariable logistic regression analysis. Exploratory objectives were to compare the G8 and QOL scores among the treatments in the M0 group.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 7.00, Bell Curve for Excel, and R 3.6.1. Categorical variables were compared using Fisher’s exact test or the χ2 test. Quantitative variables were expressed as means ± standard deviation. The significance of between-group differences was determined using Student’s t test for normally distributed data or the Mann–Whitney U test for non-normally distributed data. The Kruskal–Wallis test was used to analyze differences among the three groups. The correlation between two indices was analyzed using Spearman’s correlation coefficient (RS). A P value < 0.05 was considered statistically significant. Odds ratio (OR) with 95% confidence intervals (95% CI) was calculated using the multivariable logistic regression model for lower HRQOLs after controlling for potential confounders, including age, frailty (G8 ≤ 14), number of comorbidities (HTN, CCVD, DM, plus CRD; range, 0–4), and metastatic disease.
Results
Patient selection and characteristics
Among the 648 patients subjected in this study, we excluded 224 patients who did not provide complete response to the G8 or QLQ-C30 questionnaires and 15 patients with castration-resistant PC. This left us with a total of 409 patients for the analysis. The main reason for exclusion was the lack of some questionnaires of QLQ-C30. The median age, PSA, and Gleason score were 70 years, 9 ng/mL, and 7, respectively (Table 1). The median G8 score, global QOL, functioning QOL, and symptom QOL scores were 14.5, 75, 94, and 93, respectively. Of 409 patients, 369 and 40 were in the M0 and mCSPC groups, respectively. The number of patients who were treated with RARP, RT, and ADT alone in the M0 group was 196, 156, and 17, respectively. There was a significant difference in background between patients with M0 and mCSPC groups in the PSA, Gleason score (Table 1).
Primary outcomes
A significant difference was observed between patients with M0 and mCSPC groups in the G8 score (Fig. 1a, 14.5 vs. 12.5: P = 0.002), functioning QOL (Fig. 1b, 94 vs. 87, P = 0.002), global QOL (Fig. 1c, 75 vs. 58, P < 0.001), and 100-symptom QOL scores (Fig. 1d, 94 vs. 85, P = 0.001).
Secondary Outcomes
The G8 scores were significantly associated with the functioning QOL (RS = 0.32, P < 0.001), global QOL (RS = 0.25, P < 0.001), and 100-symptom QOL (RS = 0.25, P < 0.001) in the M0 group (Fig. 1a), although the magnitudes of slopes were not high. The G8 scores were significantly associated with the functioning QOL (RS = 0.68, P < 0.001), global QOL (RS = 0.70, P < 0.001), and 100-symptom QOL (RS = 0.61, P < 0.001) in the mCSPC group (Fig. 1b). Analysis for the association of G8 score and each HRQOL item (Fig. S1) demonstrated that the highest slope magnitude was observed in the global QOL (slope = 2.86) followed by fatigue (slope = 2.28) and sleep (slope = 1.71) QOLs in the M0 group (Fig. S1A-C). Similarly, the highest slope magnitude for G8 score was observed in the global QOL (slope = 7.15) followed by fatigue (slope = 6.46) and role (slope = 6.15) QOLs for frailty in the mCSPC group (Fig. S1D-F).
A G8 score of ≤ 14 was significantly associated with functioning QOL (Fig. 1c; P < 0.001), global QOL (Fig. 1d; P < 0.001), and 100-symptom QOL (Fig. 1e; P < 0.001). Optimal cutoff values for G8 ≤ 14 of functioning QOL, global QOL, and 100-symptom QOL were < 91 (Fig. S2A; AUC, 0.671), < 67 (Fig. S2B; AUC, 0.644), and < 91 (Fig. S2C; AUC, 0.632), respectively. Multivariable logistic regression analyses showed that G8 ≤ 14 was significantly associated with QOL-low (functioning QOL < 91, global QOL < 67, and 100-symptom QOL < 91) (Fig. 1f, Table 2).
Exploratory outcome
The G8 scores (Fig. S3A) and HRQOLs (Fig. S3B) were significantly different among the patients with RARP, RT, and ADT alone in the M0 group. Global QOL was not significantly different between the patients treated with RARP and RT/ADT.
Discussion
Although numerous studies have shown the clinical implications of frailty or HRQOL on unfavorable outcomes in patients with PC [29,30,31,32], there is insufficient evidence available for the association of frailty with HRQOL in those patients. We found that frailty (G8 ≤ 14) was significantly associated with worse baseline HRQOL in patients with PC. In the M0 group, the G8 score correlated with treatment selection, while the G8 score and HRQOL factors had weak correlations. In the mCSPC group, there is a strong correlation between the G8 score and HRQOL, especially in the global QOL (RS = 0.70). Moreover, multivariable logistic regression analyses showed that G8 ≤ 14 was independent factor for worse baseline HRQOL. To the best of our knowledge, this is the first study to evaluate the association of frailty with HRQOL in patients with PC.
The key finding of our study was that patients with frailty had a significantly worse HRQOL in both M0 and mCSPC groups. As frailty and HRQOL were associated with unfavorable outcomes, we confirmed a positive association between them, whereas the magnitudes of slopes in the M0 group were small. Of QLQ-C30 items, global QOL had greater magnitude of slope for the association of frailty in the mCSPC group. It might be due to the total decline of HRQOL in those patients as a result of metastatic PC-related symptoms. Indeed, our results showed all items of symptom QOL were significantly worse in the mCSPC group (Fig. 2d). The presence of metastatic PC-related symptoms might be the key role for worse G8 and QOL scores. Given that the clinical implications of frailty and HRQOL are greater in patients with metastatic disease, management focusing on both frailty and global QOL is necessary. Although we could not address the causal relationship between frailty and HRQOL, it is interesting whether the intervention for frailty can improve HRQOL or not. As the final goal of frailty and HRQOL evaluation is individual care to maintain and improve them, further studies are needed to improve frailty and HRQOL in those patients.
Secondary outcomes: The association of frailty and HRQOL. The association of geriatric 8 (G8) with health-related quality of life (HRQOL) in the localized prostate cancer (the M0 group) (a) and metastatic castration-sensitive prostate cancer (the mCSPC group) (b) were investigated. The effect of frailty (G8 ≤ 14) on the HROQL was investigated in the functioning QOL (c), global QOL (d), and 100–symptom QOL (e). Summary of age, metastasis, and number of comorbidities adjusted multivariable logistic regression model were shown (f)
Our observations suggested that a small decline of QOL scores was associated with frailty. The cutoff of functioning QOL < 91 and 100-symptom QOL < 91 suggested that an answer of “a little (2)” for any two items in the functioning or symptom QOL was related to frailty. Similarly, the cutoff score of global QOL < 67 suggested that an answer of “4 or less” was related to frailty. However, our findings need to be validated because there were no previous studies investigating the association between the frailty and HRQOL. Also, the cutoff value of frailty might be different depending on diseases and stages [6, 15]. Furthermore, AUC values of ROC curve analyses are not high enough (AUC < 0.70) to clearly exhibit predictive accuracy. Therefore, further studies are needed to elucidate the cutoff value of HRQOL for frailty not only in prostate cancer but also in other cancers.
The impact of each QOL item for frailty needs to be debated. Our additional analysis for each HRQOL item demonstrated that the highest slope magnitude for G8 score was observed in the global QOL (slope = 2.86) followed by fatigue and sleep QOLs in the M0 group (Fig. S1C). Similarly, the highest slope magnitude for G8 score was observed in the global QOL (slope = 7.15) followed by fatigue and role QOLs for frailty in the mCSPC group (Fig. S1F). These results suggested that a decline in global and fatigue QOLs might be a sign of frailty in patients with PC. As the questionnaire of global QOL included only two simple questions, it might be useful for frailty screening. However, further larger studies are warranted to identify the positive association of frailty with HRQOL in those patients.
The limitations in this study include the retrospective study design, small sample size, selection bias, and unmeasurable confounding factors. We could not exclude the impact of the diagnosis of PC on QLQ-C30 because we included patients with post-prostate biopsy (after PC diagnosis) and prior to prostate biopsy (before PC diagnosis) at the time of the HRQOL valuation. Also, we could not include the potential confounding factors, such as other cancers, mental disease, and dementia, which might be associated with frailty and HRQOL. Moreover, the optimal cut-off level of G8 has not been established in Japanese patients with PC. Results may not be applied to other countries because of racial, regional, and insurance system differences. We excluded 224 patients, representing about 1/3 of the total, may lead selection bias because HRQOL questionnaires are often refused by patients with poor conditions. There are some overlapping questions between G8 score and EORTC QLQ-C30. Also, the G8 might not be optimal to evaluate frailty in localized prostate cancer because majority of patients do not experience weight loss, mobility and neuropsychological problems at the diagnosis. The difference of frailty and HRQOL among the RARP, RT and ADT alone might be just looking at the selection bias. However, visualization of potential selection bias using frailty and HRQOL are important for patient management. Despite these limitations, the study demonstrates the positive association between the frailty and HRQOL in patients with localized and metastatic PC. Our results suggested that frailty is one of the signs of worse HRQOL. Furthermore, we found the global QOL (2 simple questions) might be a useful predictor for frailty. As frailty is the key factor for treatment selection and intensity, frailty evaluation is important for the management of PC patients. Further study is needed to validate our findings.
Conclusion
The baseline frailty and HRQOL were significantly different between the localized and metastatic disease. The baseline frailty was significantly associated with worse HRQOL in patients with PC. The global QOL had a highest association with G8 score in mCSPC patients.
References
Culp MB, Soerjomataram I, Efstathiou JA et al (2020) Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 77(1):38–52. https://doi.org/10.1016/j.eururo.2019.08.005
Kimura T, Egawa S (2018) Epidemiology of prostate cancer in Asian countries. Int J Urol 25(6):524–531. https://doi.org/10.1111/iju.13593
Ito K, Oki R, Sekine Y et al (2019) Screening for prostate cancer: history, evidence, controversies and future perspectives toward individualized screening. Int J Urol 26(10):956–970. https://doi.org/10.1111/iju.14039
Isharwal S, Johanning JM, Dwyer JG et al (2017) Preoperative frailty predicts postoperative complications and mortality in urology patients. World J Urol 35(1):21–26. https://doi.org/10.1007/s00345-016-1845-z
Suskind AM, Walter LC, Jin C et al (2016) Impact of frailty on complications in patients undergoing common urological procedures: a study from the American college of surgeons national surgical quality improvement database. BJU Int 117(5):836–842. https://doi.org/10.1111/bju.13399
Soma O, Hatakeyama S, Okamoto T et al (2019) Multicenter prospective study validating the efficacy of a quantitative assessment tool for frailty in patients with urological cancers. Med Oncol 36(10):88. https://doi.org/10.1007/s12032-019-1313-x
Xue QL (2011) The frailty syndrome: definition and natural history. Clin Geriatr Med 27(1):1–15. https://doi.org/10.1016/j.cger.2010.08.009
Soma O, Hatakeyama S, Imai A et al (2020) Relationship between frailty and lower urinary tract symptoms among community-dwelling adults. Low Urin Tract Symptoms 12(2):128–136. https://doi.org/10.1111/luts.12292
Kodama H, Hatakeyama S, Momota M et al (2020) Effect of frailty and comorbidity on surgical contraindication in patients with localized prostate cancer (FRART-PC Study). Urol Oncol. https://doi.org/10.1016/j.urolonc.2020.06.019
Momota M, Hatakeyama S, Soma O et al (2020a) Frailty is a predictor of moderate to severe pain after robot-assisted laparoscopic prostatectomy: a case-control study (FRAP study). BJUI Compass 1(3):100–107. https://doi.org/10.1002/bco2.17
Levy I, Finkelstein M, Bilal KH et al (2017) Modified frailty index associated with Clavien-Dindo IV complications in robot-assisted radical prostatectomies: a retrospective study. Urol Oncol 35(6):425–431. https://doi.org/10.1016/j.urolonc.2017.01.005
Taylor BL, Xia L, Guzzo TJ et al (2019) Frailty and greater health care resource utilization following major urologic oncology surgery. Eur Urol Oncol 2(1):21–27. https://doi.org/10.1016/j.euo.2018.06.005
Molina-Garrido MJ, Guillen-Ponce C (2017) Use of geriatric assessment and screening tools of frailty in elderly patients with prostate cancer. Rev Aging Male 20(2):102–109. https://doi.org/10.1080/13685538.2016.1277516
Droz JP, Albrand G, Gillessen S et al (2017) Management of prostate cancer in elderly patients: recommendations of a task force of the international society of geriatric oncology. Eur Urol 72(4):521–531. https://doi.org/10.1016/j.eururo.2016.12.025
Momota M, Hatakeyama S, Soma O et al (2020b) Geriatric 8 screening of frailty in patients with prostate cancer. Int J Urol 27(8):642–648. https://doi.org/10.1111/iju.14256
Decoster L, Van Puyvelde K, Mohile S et al (2015) Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol 26(2):288–300. https://doi.org/10.1093/annonc/mdu210
Fukushi K, Narita T, Hatakeyama S et al (2017a) Difference in toxicity reporting between patients and clinicians during systemic chemotherapy in patients with urothelial carcinoma. Int J Urol 24(5):361–366. https://doi.org/10.1111/iju.13318
Husson O, de Rooij BH, Kieffer J et al (2020) The EORTC QLQ-C30 summary score as prognostic factor for survival of patients with cancer in the “real-world”: results from the population-based PROFILES registry. Oncologist 25(4):e722–e732. https://doi.org/10.1634/theoncologist.2019-0348
Shirk JD, Saigal CS (2017) From QOL to QALYs: Comparing nononcologic outcomes in prostate cancer survivors across treatments. Urol Oncol 35(2):69–75. https://doi.org/10.1016/j.urolonc.2016.07.019
Van Hemelrijck M, Sparano F, Moris L et al (2020) Harnessing the patient voice in prostate cancer research: systematic review on the use of patient-reported outcomes in randomized controlled trials to support clinical decision-making. Cancer Med 9(12):4039–4058. https://doi.org/10.1002/cam4.3018
Chen MN, Ho KY, Hung YN et al (2019) Pre-treatment quality of life as a predictor of distant metastasis-free survival and overall survival in patients with head and neck cancer who underwent free flap reconstruction. Eur J Oncol Nurs 41:1–6. https://doi.org/10.1016/j.ejon.2019.05.003
Frantellizzi V, De Feo MS, Di Rocco A et al (2020) Baseline quality of life predicts overall survival in patients with mCRPC treated with (223)Ra-dichloride. Hell J Nucl Med 23(1):12–20. https://doi.org/10.1967/s002449912001
Suppanuntaroek S, Hatakeyama S, Fujita N et al (2020) Influence of pretreatment quality of life on prognosis in patients with urothelial carcinoma. Int J Clin Oncol 25(2):362–369. https://doi.org/10.1007/s10147-019-01563-2
Quinten C, Kenis C, Hamaker M et al (2020) The added value of geriatric assessment in evaluating a patient’s health-related quality-of-life: a study in ≥70-year-old early-stage invasive breast cancer patients. Eur J Cancer Care (Engl). https://doi.org/10.1111/ecc.13278
Biesma B, Wymenga ANM, Vincent A et al (2011) Quality of life, geriatric assessment and survival in elderly patients with non-small-cell lung cancer treated with carboplatin-gemcitabine or carboplatin-paclitaxel: NVALT-3 a phase III study. Ann Oncol 22(7):1520–1527. https://doi.org/10.1093/annonc/mdq637
Knight J, Ayyash K, Colling K et al (2020) A cohort study investigating the relationship between patient reported outcome measures and pre-operative frailty in patients with operable, non-palliative colorectal cancer. BMC Geriatr 20(1):311. https://doi.org/10.1186/s12877-020-01715-4
Kobayashi K, Takeda F, Teramukai S et al (1998) A cross-validation of the European organization for research and treatment of cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer 34(6):810–815. https://doi.org/10.1016/s0959-8049(97)00395-x
Fukushi K, Narita T, Hatakeyama S et al (2017b) Quality-of-life evaluation during platinum-based neoadjuvant chemotherapies for urothelial carcinoma. Int J Clin Oncol 22(2):366–372. https://doi.org/10.1007/s10147-016-1071-0
Amiya Y, Yamada Y, Sugiura M et al (2017) Outcomes of patients older than 75 years with non-metastatic prostate cancer. Asian J Urol 4(2):102–106. https://doi.org/10.1016/j.ajur.2016.12.001
Nguyen-Nielsen M, Moller H, Tjonneland A et al (2020) Patient-reported outcome measures after treatment for prostate cancer: results from the Danish prostate cancer registry (DAPROCAdata). Cancer Epidemiol 64:101623. https://doi.org/10.1016/j.canep.2019.101623
Cheung AS, de Rooy C, Hoermann R et al (2017) Quality of life decrements in men with prostate cancer undergoing androgen deprivation therapy. Clin Endocrinol (Oxf) 86(3):388–394. https://doi.org/10.1111/cen.13249
Joly F, Oudard S, Fizazi K et al (2020) Quality of life and pain during treatment of metastatic castration-resistant prostate cancer with cabazitaxel in routine clinical practice. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2020.02.003
Acknowledgements
We thank Yuki Fujita and Atsuko Sakuraba for their invaluable help with the data collection.
Funding
This study was supported by a grant-in-aid for scientific research (18K09157, 19H05556, 20K09517, 20K18082, 20K18130, 20K18107) from the Japan Society for the Promotion of Science and a research grant from the 31st Japanese Society of Geriatric Urology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Ethics statement
All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent to participate
Opt-out approach (written consent was not required in exchange for public disclosure of study).
Consent for publication
All authors agreed with the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2020_1798_MOESM1_ESM.pdf
Supplementary file1 (PDF 120 kb) Fig. S1. Supplemental figures: The association of G8 and HRQOL. The association of G8 and functioning QOL items (A) and symptom QOL items (B) in the M0 group were evaluated. Magnitude of slopes in the M0 group was evaluated (C). The association of G8 and functioning QOL items (D) and 100 symptom QOL items (E) in the M1 group were evaluated. Magnitude of slopes in the M0 group was evaluated (F). Fig. S2. Supplemental figures: The optimal cutoff value of QOL scores for G8 ≤14. The optimal cutoff value of QOL scores for G8 ≤14 was evaluated using receiver operating characteristic (ROC) curve and the area under the curve (AUC) in the functioning QOL (A), global QOL (B), and 100 symptom QOL (C). Fig. S3. Exploratory outcomes: Comparison of G8 and QOL scores among the treatments and disease stages. The G8 (A) and QOL (B) scores in the M0 group were compared among the RARP, RT, and ADT alone.
About this article
Cite this article
Hamaya, T., Hatakeyama, S., Momota, M. et al. Association between the baseline frailty and quality of life in patients with prostate cancer (FRAQ-PC study). Int J Clin Oncol 26, 199–206 (2021). https://doi.org/10.1007/s10147-020-01798-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01798-4