Abstract
Background
The indications for neoadjuvant chemotherapy (NAC) in resectable colorectal liver metastases (CRLMs) remain unclear. Tumor burden score (TBS) is a prognostic tool based on tumor size and number of tumors. However, its utility in the NAC setting for initially resectable CRLM has never been investigated.
Methods
TBS is a distance from the origin on a Cartesian plane to the coordinates (x, y) = (tumor size in centimeter, number of tumors). TBS < 3 was defined as “TBS-low”, whereas TBS ≥ 3 as “TBS-high”. Between 2008 and 2018, 102 patients who underwent hepatectomy for resectable CRLM were retrospectively analyzed using the Kaplan–Meier method and Cox proportional hazards regression models.
Results
Among the TBS-low (n = 46) and TBS-high (n = 56) groups, baseline patient characteristics were mostly similar except for TBS-related parameters. NAC was more frequently administered in the TBS-high group (p = 0.038). The overall survival (OS) rates were similar between the two groups. Subgroup analysis showed that NAC was associated with non-significantly improved 5-year OS in the TBS-high group [76.1% with NAC and 54.9% without NAC (p = 0.093)]. In multivariate analysis, NAC was an independent prognostic factor for favorable OS only in the TBS-high group, while adjuvant chemotherapy (AC) was associated with improved OS only in the TBS-low group.
Conclusion
In patients with resectable CRLM, the TBS-high population had a survival benefit from NAC, while the TBS-low population benefited from AC. TBS may serve as an indicator for patients who will benefit from NAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is a dominant metastatic site from colorectal cancer, and hepatic resection is the only potential curative treatment in patients with colorectal liver metastases (CRLMs). Long-term survival after surgery for CRLM has improved considerably, especially in the last 2 decades, with 5-year overall survival (OS) reaching up to 58% due to technical improvement and perioperative multimodal treatments [1,2,3,4]. However, whether to start treatment with upfront surgery or neoadjuvant chemotherapy (NAC) for resectable CRLM remains controversial.[5, 6] The EORTC 40,983 trial reported improved disease-free survival [7], but no OS benefit with perioperative FOLFOX4 therapy for 1–4 resectable CRLMs [8]. On the other hand, the survival benefit of NAC in high-risk resectable CRLM patients has been documented in retrospective series [9,10,11].
To predict survival after resection of CRLMs, numerous prognostic factors have been advocated to date [12,13,14,15,16,17,18]. One of the most widely accepted risk scores is the Fong Clinical Risk Score [17]: disease-free interval < 12 months, number of metastases > 1, preoperative CEA level > 200 ng/mL, largest liver metastasis > 5 cm, and lymph node-positive primary tumor are counted as 1 point each, and a score of more than 2 points is considered high risk. Importantly, this clinical risk score was developed in only patients who underwent upfront surgery. In fact, the prognostic accuracy of risk scores has not always been reproducible by external cohort validation in the era of currently developed chemotherapy [19].
The tumor burden score (TBS), reported by Sasaki et al., is a newly developed model that translates the size and number of CRLMs into one variable using the Pythagorean theorem and has better prognostic discriminatory power than traditional tumor morphologic categorization [20]. The concept of the TBS was described as “Metro-ticket” paradigm; as the longer trip on the Metro results in higher cost, increments in size and number of CRLMs result in worse prognosis. Specifically, the hazard ratio (HR) for the OS of TBS ≥ 3 to < 9, and TBS ≥ 9 were 1.66 and 2.60, respectively with referent TBS < 3. In addition, the discriminatory ability in predicting outcomes among patients treated with preoperative chemotherapy was clearly advocated [20].
There was nobody who raised any objections to introduce systemic chemotherapy for patients with marginally resectable or initially unresectable liver metastases. However, indication of preoperative chemotherapy for initially resectable liver metastases remains controversial. The objective of this study was to assess the TBS in patients who underwent liver resection for initially resectable CRLM in our center and to verify whether the TBS could become a tool for NAC indication, especially for high-risk patients.
Patients and methods
Patient population
One hundred sixty-nine consecutive patients who underwent hepatectomy for CRLM between January 2008 and December 2018 were identified from the Nagoya University Hospital prospective database and analyzed retrospectively. Of those, repeated hepatectomy (n = 46), initially unresectable (n = 8), and borderline resectable (n = 13) cases were excluded. A total of 102 patients were included in this study.
A complete set of demographic data on age, sex, and clinicopathological findings, including the site and TNM status of the primary tumor and pretreatment serum carcinoembryonic antigen (CEA) level, was collected. Liver metastatic status, including timing, size and number of tumors, was determined by imaging studies. Synchronous CRLM was defined as having a disease-free interval of zero. Chemotherapeutic status and perioperative outcomes were also collected. The response to chemotherapy was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). An R0 resection was defined as no microscopic tumor invasion at the margin of the specimen. Pathologically complete response patients were counted as zero tumor number and zero diameter. OS was calculated from the date of treatment, either NAC or surgery, to the date of death or last follow-up. This study was approved by the Nagoya University Hospital Institutional Review Board (approval number 2019-0233).
Preoperative assessment and treatment indication
Each patient was assessed at a multidisciplinary pretreatment conference for resectability upon diagnosis of CRLM. Chest to abdominopelvic, contrast enhanced dynamic thin slice (0.75-mm slice thickness) computed tomography (CT), gadoxetic acid enhanced magnetic resonance imaging of liver, 18F-fluorodeoxyglucose positron emission tomography CT, and indocyanine green (ICG) clearance tests were routinely performed. Patients were then allocated to either NAC or upfront surgery by the physician’s choice, considering the trend of time and patient background. Patients were more likely to be allocated to NAC when they had previously reported high-risk features (i.e., synchronous metastasis, multiple metastases, or large metastases). Adjuvant chemotherapy (AC) was also administered according to the physician’s choice.
Definition of resectable CRLM
The definition of “resectable” CRLM is controversial, especially in recent decades, and institutional discrepancies are also non-negligible. In our institute and in this study, those who meet the following criteria were defined as resectable: patients who were medically fit, patients who were evaluated by a liver surgeon as being “technically feasible”, patients in whom both the inflow and outflow of the liver were preserved or would be preserved after vascular reconstruction, patients in whom remnant hepatic function was maintained according to the ICG clearance test, and patients with an absence of unresectable extrahepatic metastasis. Otherwise, resection was not limited by the tumor size, number of metastases, tumor location, metastatic timing, concomitant resection of the primary site, or chemotherapeutic status.
Tumor burden score
TBS for CRLM is a newly developed prognostic model [20] based on both maximum tumor size and number of lesions. The TBS is defined as the distance from the origin on a Cartesian plane (0, 0) to the (x, y) coordinates of the point, where the maximum tumor size (cm) is on the x-axis and the number of liver lesions is on the y-axis. The distance was calculated using the Pythagorean theorem according to the following formula:
TBS values were originally categorized into three “zones” with incremental worsening of OS [zone 1: TBS < 3, zone 2: TBS ≥ 3 to < 9, and zone 3: TBS ≥ 9]. In the current study, a TBS < 3 was defined as “TBS-low”, and a TBS ≥ 3 was defined as “TBS-high” based on a significant OS difference in the external cohorts validated in the original study [20].
Statistical analysis
Continuous variables are expressed as medians with ranges. Categorical variables are presented as whole numbers and percentages. Comparisons between groups were performed using the Chi-square test, Fisher’s exact test, or the Wilcoxon signed-rank test where applicable. OS was estimated by the Kaplan–Meier method, and the log-rank test was used to assess the difference. Cox proportional hazards regression models were used to evaluate the prognostic factors. Variables with a p value < 0.120 on univariate analysis were included in the multivariate analysis. Survival estimates were written as hazard ratios (HRs) with 95% confidence intervals (95% CIs). Statistical analyses were implemented with JMP 10.0.2 software (SAS Institute).
Results
Patient characteristics and perioperative outcomes
A total of 102 patients were allocated to either NAC or upfront surgery according to physician choice and divided into TBS-low (n = 46) and TBS-high (n = 56) groups (Fig. 1). The baseline characteristics and perioperative outcomes of all patients and stratified by TBS groups are shown in Table 1, with a median follow-up of 3.4 (range 0.1–10.9) years. In summary, the patients’ backgrounds were basically similar except that significantly more patients were allocated to NAC in the TBS-high group than in the TBS-low group (66.1% vs 45.7%, p = 0.038). The number of CRLMs, size of the largest CRLM, proportion of bilobar metastases, and extent of hepatic resection were higher in the TBS-high group than in the TBS-low group. The postoperative morbidity rates and pathological R0 resection rates were similar between the two groups. The pathologically identified number of tumors and size of the largest metastasis were significantly larger in the TBS-high group than in the TBS-low group. The rate of pathological complete remission was non-significantly higher in the TBS-low group.
Neoadjuvant and adjuvant chemotherapy
Of the 58 patients (56.9%) who received NAC, 54 patients (93.1%) received combined cytotoxic agents with fluorouracil and oxaliplatin. Two patients received fluorouracil plus irinotecan, one patient received fluorouracil only, and one patient received triplet therapy. Targeted biological agents with bevacizumab, panitumumab, or cetuximab were administered in 42 (72.4%) patients. The median treatment duration was 12 weeks.
AC was administered in 22 patients (50.0%) of those who underwent upfront surgery, and 9 patients (29.3%) of those who received NAC.
Oncologic outcomes
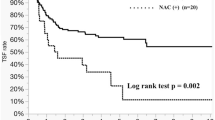
In the total cohort, recurrence was observed in 60 (58.8%) patients. The liver was the most dominant site for recurrence (32.4%), followed by the lung (24.5%). Sites of recurrence, re-resection rates were similar between the two TBS groups (Table 1). The 3- and 5-year OS rates were 79.2% and 64.3%, respectively. The 5-year OS was 55.2% in the TBS-low group and 69.2% in the TBS-high group (p = 0.286) (Fig. 2). In the subgroup survival analysis, patients who received NAC had a non-significantly improved OS in the TBS-high group (the 5-year OS was 76.1% in the NAC group and 54.9% in the upfront surgery group, p = 0.093) but not in the TBS-low group (5-year OS was 49.2% in the NAC group and 60.1% in the upfront surgery group, p = 0.338) (Fig. 3a, b). On the other hand, patients who received AC had a non-significant favorable increase in OS in the TBS-low group (5-year OS was 69.0% in the AC group and 47.8% in the non-AC group, p = 0.085) but not in the TBS-high group (5-year OS was 56.8% in the AC group and 72.4% in the non-AC group, p = 0.324) (Fig. 3c, d). In multivariate survival analyses, age ≥ 65 years (p = 0.003), CEA ≥ 200 (p = 0.048), and upfront surgery (p = 0.009) were independent risk factors for poor OS in the TBS-high group, while lymph node-positive status of the primary tumor (p = 0.038) and non-AC treatment (p = 0.022) were independent risk factors for poor OS in the TBS-low group (Table 2).
Kaplan–Meier curves of each TBS group by treatment settings. a NAC did not improve overall survival in the TBS-low group (p = 0.338). b Patients who received NAC had a non-significant improvement in overall survival in the TBS-high group (p = 0.093). c Patients who received AC had a non-significant improvement in overall survival in the TBS-low group (p = 0.085). d AC did not improve overall survival in the TBS-high group (p = 0.324)
Recurrence status after hepatectomy in the TBS-high group is shown in Table 3. There was no difference in the rate, sites and re-resection of recurrences among the NAC and upfront surgery groups.
Discussion
Various prognostic predictors after resection of CRLM have been advocated to date [13, 15, 17, 18], and virtually all of them include tumor size and number of tumors as independent prognostic factors. The cutoff values, however, were set somewhat arbitrarily, making it difficult to estimate the extent of prognostic risk. As such, the TBS for CRLM, reported by Sasaki et al., is a newly developed prognostic tool that utilizes a continuum of tumor size and number of tumors, which better predicted survival than each dichotomous factor. (5-year OS of 604 patients, TBS < 3 (n = 174), TBS ≥ 3 to < 9 (n = 363), and TBS ≥ 9 (n = 67) were 68.9%, 49.4%, and 25.5%, respectively.) [20] Interestingly in the present study, the TBS alone did not distinguish OS. Furthermore, the 5-year OS of 69.2% in the TBS-high group was relatively favorable for high-risk CRLM compared with the previously reported 5-year OS value of up to 58% [1, 2, 4, 20]. Given that the background characteristics of the two groups in our study were mostly similar in terms of previously reported risk factors such as primary tumor nodal status and preoperative CEA levels, we hypothesized that NAC was responsible in the TBS-high group for a favorable OS. In the multivariate analysis, NAC in the TBS-high group turned out to be an independent favorable prognostic factor, as did age < 65 and CEA level < 200. In the TBS-low group, on the other hand, NAC was not associated with survival. We suggest that TBS alone could be utilized as a clinical tool for NAC indication in patients with initially resectable CRLMs.
Ayez et al. reported in a retrospective series that patients with a high Fong Clinical Risk Score were associated with improved OS after NAC [10], and a prospective randomized study comparing NAC followed by surgery vs surgery alone in high-risk resectable CRLM is ongoing [21]. Likewise, Hokuto et al. reported that CRLM patients with a primary colorectal cancer N-stage of N2-3 should be administered NAC as first-line therapy [9]. These studies suggested that patients with high-risk profiles might benefit from preoperative treatment, which was consistent with the current study. The reason why NAC improve the survival in high-risk CRLM is interesting but unintelligible, as the recurrence rate, sites and re-resection after recurrence in the TBS-high population were similar between the NAC and the upfront surgery groups in this study. The higher dose intensity in NAC setting compared to the AC setting might be a considerable reason, eliminating pre-existing micrometastasis of the remaining liver and other organs, especially in patients with higher tumor burden.
On the other hand, an optimal regimen of NAC also remains unclear. Combined cytotoxic agents with fluorouracil and oxaliplatin have been widely accepted; however, the efficacy of triplet regimen or additional targeted agents should be further investigated. Although Sasaki et al. also demonstrated that the TBS model was able to stratify prognosis among those who had PD/SD or PR/CR response after NAC [20], this was not reproducible in our cohort (data not shown). It might be partly due to the differences in the chemotherapy agents.
Another interesting finding from the present study was that AC was responsible for the favorable OS according to the multivariate analysis in the TBS-low group but not in the TBS-high group. The FFCD 9002 trial, which investigated adjuvant fluorouracil and folinic acid vs surgery alone after resection of CRLM [22], demonstrated a non-significant, but favorable OS in the AC group. Most of the patients in this study had only 1 or 2 CRLMs (87%) and tumor size less than 5 cm (74%), thus considered to be relatively low risk in terms of the TBS. Our group demonstrated similar favorable survival in the multicenter phase II trial of adjuvant S-1 for relatively low-risk patients [23]. Although the role of AC after curative resection of CRLM is still undetermined [24], some existing evidence and our study suggest that patients with low TBS benefit from AC rather than NAC.
It is important to note that initially resectable CRLMs might become inoperable after NAC because of progressive disease or severe adverse events. Mukai et al. reported that 4 out of 61 (6.6%) patients who underwent NAC with S1 and oxaliplatin plus bevacizumab for CRLM became inoperable for such reasons [25]. In the EORTC 40,983 trial, the rate of progression under chemotherapy was reported to be 7%. Not high but non-negligible rates of failures must be taken into consideration, which also supports NAC being limited to high-risk populations.
This single-center, limited sample size, retrospective, non-randomized, and per-protocol study might be biased due to its nature. In addition, we did not include RAS and BRAF mutation status in the current study because approximately half of the data were missing and thus considered inappropriate. Although another prognostic risk score (the GAME score) was recently proposed that included KRAS mutation status as an independent prognostic factor [26], the addition of cetuximab to resectable CRLM resulted in decreased progression-free survival, and KRAS mutation status is not an indicator for NAC in resectable CRLM in current clinical practice [27]. The role of RAS and BRAF mutation status in preoperatively predicting which treatment should be used needs further investigation.
In conclusion, in patients with initially resectable CRLM, the TBS-high population had a survival benefit from NAC, while the TBS-low population benefited from AC. The TBS might have the potential to be a useful indicator for NAC.
References
House MG, Ito H, Gönen M et al (2010) Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1600 patients during two decades at a single institution. J Am Coll Surg 210:744–752
Kopetz S, Chang GJ, Overman MJ et al (2009) Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 27:3677–3683
Khatri VP, Petrelli NJ, Belghiti J (2005) Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol 23:8490–8499
Choti MA, Sitzmann JV, Tiburi MF et al (2002) Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 235:759–766
Khoo E, O’Neill S, Brown E et al (2016) Systematic review of systemic adjuvant, neoadjuvant and perioperative chemotherapy for resectable colorectal-liver metastases. HPB (Oxford) 18:485–493
Nigri G, Petrucciani N, Ferla F et al (2015) Neoadjuvant chemotherapy for resectable colorectal liver metastases: what is the evidence? results of a systematic review of comparative studies. Surgeon 13:83–90
Nordlinger B, Sorbye H, Glimelius B et al (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–1016
Nordlinger B, Sorbye H, Glimelius B et al (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215
Hokuto D, Nomi T, Yasuda S et al (2018) Risk factors for unresectable recurrence after up-front surgery for colorectal liver metastasis. World J Surg 42:884–891
Ayez N, van der Stok EP, Grünhagen DJ et al (2015) The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: clinical risk score as possible discriminator. Eur J Surg Oncol 41:859–867
Tanaka K, Adam R, Shimada H et al (2003) Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. BJS 90:963–969
Beppu T, Sakamoto Y, Hasegawa K et al (2012) A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a project study for hepatic surgery of the Japanese society of hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci 19:72–84
Konopke R, Kersting S, Distler M et al (2009) Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int 29:89–102
Rees M, Tekkis PP, Welsh FK et al (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247:125–135
Nagashima I, Takada T, Adachi M et al (2006) Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: comparison of our scoring system to the positive number of risk factors. World J Gastroenterol 12:6305–6309
Iwatsuki S, Dvorchik I, Madariaga JR et al (1999) Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 189:291–299
Fong Y, Fortner J, Sun RL et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230(3):309–318
Nordlinger B, Guiguet M, Vaillant JC et al (1996) Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie Cancer 77:1254–1262
Zakaria S, Donohue JH, Que FG et al (2007) Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg 246:183–191
Sasaki K, Morioka D, Conci S et al (2018) The tumor burden score: a new, “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg 267:132–141
Ayez N, van der Stok EP, de Wilt H et al (2015) Neo-adjuvant chemotherapy followed by surgery versus surgery alone in high-risk patients with resectable colorectal liver metastases: the CHARISMA randomized multicenter clinical trial. BMC Cancer 15:180
Portier G, Elias D, Bouche O et al (2006) Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 24:4976–4982
Kato T, Uehara K, Maeda A et al (2015) Phase II multicenter study of adjuvant S-1 for colorectal liver metastasis: survival analysis of N-SOG 01 trial. Cancer Chemother Pharmacol 75:1281–1288
Mitry E, Fields AL, Bleiberg H et al (2008) Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 26:4906–4911
Mukai T, Uehara K, Goto H et al (2017) Phase II trial of neoadjuvant chemotherapy with S-1 and oxaliplatin plus bevacizumab for colorectal liver metastasis (N-SOG 05 trial). Jpn J Clin Oncol 47:597–603
Margonis GA, Sasaki K, Gholami S et al (2018) Genetic and morphological evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg 105:1210–1220
Primrose J, Falk S, Finch-Jones M et al (2014) Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol 15:601–611
Funding
No financial support was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors had any declaration or interests.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
This study was retrospective observational study. Therefore, informed consent was not obtained from all individual participants included in the study and it was approved by the Nagoya University Hospital Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yonekawa, Y., Uehara, K., Mizuno, T. et al. The survival benefit of neoadjuvant chemotherapy for resectable colorectal liver metastases with high tumor burden score. Int J Clin Oncol 26, 126–134 (2021). https://doi.org/10.1007/s10147-020-01793-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01793-9