Abstract
Background
Anti-programmed cell death protein-1/ligand-1 (anti-PD-1/PD-L1) therapy is promising for patients with non-small-cell lung cancer (NSCLC); however, clinical trials have focused on patients with a performance status (PS) 0 or 1. This study aimed to evaluate the clinical outcomes and correlation between PD-L1 expression status and tumor response to anti-PD-1/PD-L1 therapy among NSCLC patients with poor PS (i.e., PS ≥ 2).
Methods
In total, 130 patients with NSCLC and PS ≥ 2 treated with anti-PD-1/PD-L1 monotherapy at 12 institutions between January 2016 and August 2019 were retrospectively reviewed. PD-L1 expression status was divided into four groups: < 1%, 1–49%, ≥ 50%, and unknown.
Results
The objective response rate and PS improvement rate were 23 and 21% and were higher in the PD-L1 ≥ 50% group than in other groups (P < 0.01). Median progression-free survival (PFS) was 62 days and was longer in the PD-L1 ≥ 50% group than in other groups (P = 0.03). Multivariate analyses revealed that PD-L1 expression is significantly associated with prolonged PFS (PD-L1 < 1%; reference; 1–49%, hazard ratio [HR] 0.19, 95% confidence interval [CI] 0.04–0.99, P = 0.05; ≥ 50%, HR 0.12, 95% CI 0.02–0.71, P = 0.02; unknown, HR 0.30, 95% CI 0.08–1.22, P = 0.09).
Conclusions
NSCLC patients with poor PS and PD-L1 ≥ 50% are expected to benefit from anti-PD-1/PD-L1 therapy, despite a modest overall response among NSCLC patients with poor PS. Accordingly, PD-L1 expression provides useful information regarding decision-making for anti-PD-1/PD-L1 therapy even in these populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint blockade targeting programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) has drastically altered the therapeutic landscape of advanced non-small-cell lung cancer (NSCLC) [1]. A phase III study of anti-PD-1 or PD-L1 antibody monotherapy, including nivolumab, pembrolizumab, or atezolizumab, among NSCLC patients reported a prolonged overall survival compared with that for docetaxel as second-line treatment [2,3,4,5]. In a randomized phase III clinical trial, survival was significantly longer for patients with PD-L1-positive NSCLC treated with first-line pembrolizumab monotherapy than with platinum-based chemotherapy [6, 7]. However, these clinical trials involved patients with NSCLC and a performance status (PS) of 0 or 1 and excluded those with a poor PS ≥ 2, as defined by the Eastern Cooperative Oncology Group (ECOG) scale. Therefore, the anti-tumor effectiveness and tolerability of anti-PD-1/PD-L1 antibodies among NSCLC patients with poor PS remain unclear.
Approximately 30–40% of advanced NSCLC patients have a poor PS ≥ 2 on the ECOG scale, based on disease burden, comorbidities, or both [8]. NSCLC patients with poor PS are generally intolerant to chemotherapy and are advised high-quality supportive care, except for some patients classified as PS 2, being potential candidates for chemotherapy [8]. Monotherapy with an anti-PD-1/PD-L1 antibody has a lower risk of treatment-related symptoms and hematologic toxicity and is better tolerated than cytotoxic chemotherapy despite a risk of immune-related adverse events [9]. Therefore, physicians occasionally administer anti-PD-1/PD-L1 therapy to NSCLC patients with a poor overall clinical condition.
The PD-L1 expression status of tumor cells, as evaluated via immunohistochemistry, is a potential predictor of the response to anti-PD-1/PD-L1 antibody therapy and is frequently evaluated on the basis of a three cut-point system in routine clinical practice: PD-L1 expression status < 1, 1–49, and ≥ 50% [10]. Since patients with poor PS experience cancer-related symptoms and have a shorter survival, strict patient selection based on predictive biomarkers is essential among them [8, 11]. Clarification of clinical outcomes based on PD-L1 expression among NSCLC patients with poor PS, treated with anti-PD-1/PD-L1 therapy, could facilitate clinical decision-making by physicians.
In this study, we retrospectively investigated the anti-tumor effectiveness and tolerability of anti-PD-1/PD-L1 antibodies among patients with NSCLC and PS ≥ 2. Moreover, based on clinical outcomes, we evaluated the value of PD-L1 expression for identifying patients with poor PS potentially benefiting from this therapeutic approach.
Patients and methods
Patients
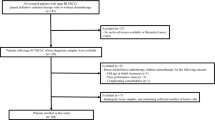
NSCLC patients with PS ≥ 2, receiving anti-PD-1/PD-L1 antibody monotherapy between January 2016 and August 2019 at the Kumamoto University Hospital and 11 general hospitals in Kumamoto or Miyazaki (Miyazaki Higashi Hospital, Kumamoto Regional Medical Center, Saiseikai Kumamoto Hospital, Japanese Red Cross Kumamoto Hospital, Kumamoto Chuo Hospital, Miyazaki Prefectural Nobeoka Hospital, Tamana Central Hospital, Omuta Tenryo Hospital, Kumamoto Rosai Hospital, Kumamoto Saishun Medical Center, and Minamata City General Hospital & Medical Center) were included herein. Nivolumab, pembrolizumab, or atezolizumab were administered as the anti-PD/PD-L1 antibody. We recorded the following data upon initiation of anti-PD-1/PD-L1 monotherapy: age, sex, ECOG PS, smoking, histology, epidermal growth factor receptor (EGFR) mutations/anaplastic lymphoma kinase (ALK) status, PD-L1 expression, stage at diagnosis, treatment, and adverse events. PD-L1 expression was evaluated via the PD-L1 Immunohistochemistry 22C3 pharmDx assay by SRL, Inc. (Tokyo, Japan), BML, Inc. (Tokyo, Japan), and LSI Medience Corporation (Tokyo, Japan). PD-L1 expression was assessed in tumor cells and divided into four groups: < 1%, 1–49%, ≥ 50%, and unknown. This study was approved and registered as IRB number 1750 by our institutional review board.
Clinical assessment and outcome parameter
The highest PS status during anti-PD-1/PD-L1 therapy was defined as the best PS. The time to PS improvement was defined as the time from initial administration of anti-PD-1/PD-L1 monotherapy to the date of the first documented improvement to the best PS. The best tumor response during treatment of anti-PD-1/PD-L1 antibody was assessed using the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST ver1.1). The time to response (TTR) was defined as the time from initial administration of anti-PD-1/PD-L1 monotherapy to the confirmation of a response. Progression-free survival (PFS) was defined as the time from the initial administration of anti-PD-1/PD-L1 monotherapy to disease progression, based on assessments of RECIST ver. 1.1, death of any cause, or censoring date of last follow-up. Overall survival (OS) was defined as the time from the initial administration of anti-PD-1/PD-L1 monotherapy to death of any cause or censoring date of last follow-up. The worst adverse events during the treatment course were estimated using the Common Terminology Criteria for Adverse Events version 4.0.
Statistical analyses
The chi-squared test or Fisher’s exact test was used to compare clinical factors between categorical variables. Estimated PFS or OS were analyzed using the Kaplan–Meier method and compared among groups, using the log-rank test. A cumulative incidence analysis (Gray’s test) was performed to verify whether PD-L1 expression influences PS considering cancer-specific deaths as competing risks. Stratified Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for each PD-L1 expression category. Variables (age [< 70/ ≥ 70 years], sex, smoking history, histology [squamous/non-squamous], stage at diagnosis [advanced/postoperative recurrence], driver mutation status, treatment line [1st/2nd/3rd line or later], brain metastasis status, liver metastasis status, history of radiotherapy) that violated the proportional hazards assumption were used as stratification factors. Statistical analyses were conducted using JMP (version 10; SAS, Cary, NC, USA), SPSS (version 23.0; IBM, Armonk, NY, USA), and R version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered significant.
Results
Patient characteristics
Between January 2016 and August 2019, 130 NSCLC patients with PS ≥ 2, receiving nivolumab, pembrolizumab, or atezolizumab, were recruited from 12 institutions. The patient characteristics at the start of anti-PD-1/ PD-L1 monotherapy are summarized in Table 1. The median age at the initial anti-PD-1/PD-L1 monotherapy was 68 years. Among 130 patients, 93 (72%) were male, 104 (77%) were smokers, 90 (69%) had non-squamous type, 105 (81%) were at an advanced stage at diagnosis, 17 (13%) had liver metastasis, 25 (19%) had brain metastasis, 56 (43%) had history of radiotherapy, and 94 (72%) were classified as PS 2. EGFR mutations and ALK fusions were detected in 17 (13%) and two patients (2%), respectively. Among 91 patients (70%) examined through immunohistochemical analysis for PD-L1, 17 (13%), 20 (15%), and 54 patients (42%) were classified as PD-L1 < 1, 1–49, and ≥ 50%, respectively. Nivolumab, pembrolizumab, and atezolizumab were administered to 59 (45%), 60 (46%), and 11 patients (8%), respectively. Anti-PD-1/PD-L1 therapy was administered as first-line treatment to 34 patients (26%), as second-line treatment to 45 patients (35%), and as third-line treatment to 51 patients (39%).
Treatment responses
The objective response rate (ORR) and median TTR were 23% (95% CI 17–31%) and 50 days (95% CI 43–73 days), respectively, for all patients (Table 2). The ORR for the PD-L1 ≥ 50% group was higher than those of the PD-L1 < 1%, 1–49%, and unknown groups (37, 18, 0, 18%, respectively, P < 0.01). The median TTRs for the PD-L1 < 1%, 1–49%, ≥ 50%, and unknown groups were 49 days, not available (owing to a lack of responders), 51, and 50 days, respectively (P = 0.84).
Improvement of PS
PS improved in 27 patients (21%). The rate of PS improvement in the PD-L1 ≥ 50% group was higher than that in the PD-L1 < 1%, 1–49%, and unknown groups (37, 12, 0, 13%, respectively, P < 0.01, Fig. 1a, supplementary Table 1). The time to PS improvement was 44 days (95% CI 42–78 days). The times to the improvement of the best PS in the PD-L1 < 1%, 1–49%, ≥ 50%, and unknown groups were 68 days (95% CI 58–77 days), not available (owing to a lack of improvement), 39 days (95% CI 24–57 days), and 93 days (95% CI 36–191 days), respectively (P < 0.01, Fig. 1b).
Progression and OS
The median follow-up time from the initial administration of anti-PD-1/PD-L1 antibodies was 141 days. In all patient populations, median PFS and OS were 62 days (95% CI 43–78 days) and 168 days (95% CI 95–231 days), respectively (Fig. 2a, b). The median PFS was longer in the PD-L1 ≥ 50% group than in the PD-L1 < 1%, 1–49%, and unknown groups (89 days [95% CI 55–189 days], 45 days [95% CI 29–129 days], 41 days [95% CI 26–68 days], and 58 days [95% CI 34–67 days], respectively, P = 0.03, Fig. 2c). The median OS did not differ among PD-L1 groups (Fig. 2d).
Efficacy according to PS
ORR, PFS, and OS according to PS are shown in supplementary Table 2 and supplementary Fig. 1. There was no significant difference in ORR, PFS, and OS between PS2 and PS 3 or 4 patients (ORR 23% [95% CI 16–33%] vs. 22% [95% CI 12–38%], P = 1.00, median PFS 63 days [95% CI 50–75 days] vs. 45 days [95% CI 29–85 days], P = 0.61, median OS 176 days [95% CI 102–249 days] vs. 81 days [95% CI 22–139 days], P = 0.35). With respect to the PD-L1 ≥ 50% group, ORR, PFS, and OS did not differ between patients with PS 2 and PS 3 or 4 (ORR 44% [95% CI 28–60%] vs. 27% [95% CI 13–48%], P = 0.29, median PFS 90 days [95% CI 13–168 days] vs. 81 days [95% CI 0–163 days], P = 0.92, median OS 231 days [95% CI 5–457 days]vs. 81 days [95% CI 0–221 days], P = 0.58, supplementary Table 3).
Multivariate analysis of PFS and OS in different PD-L1 expression groups
We performed multivariate analyses of PFS and OS stratified based on PD-L1 expression (Table 3). PD-L1 expression was significantly associated with an improved PFS (PD-L1 < 1%; reference; PD-L1 1–49%, HR = 0.19 [95% CI 0.04–0.99], P = 0.05; PD-L1 ≥ 50%, HR = 0.12, [95% CI 0.02–0.71], P = 0.02; unknown, HR = 0.30, 95% CI [0.08–1.22], P = 0.09). Multivariate analysis of OS displayed no significant differences among PD-L1 groups.
Safety
Adverse events of any grade and grade ≥ 3 were observed in 69 patients (53%) and 20 patients (15%), respectively. Treatment-related adverse events commonly include fever (23%), liver dysfunction (18%), pneumonitis (14%), skin toxicity (10%), and diarrhea (10%). Grade ≥ 3 adverse events, including fever, pneumonitis, skin toxicity, liver dysfunction, and diarrhea, occurred in 2 (2%), 5 (4%), 1 (1%), 4 (3%), and 3 (2%) patients, respectively. Moreover, adverse events of any grade and grade ≥ 3 showed no significant difference between PS2 and PS 3 or 4 patients (any grade; 53% vs. 52%, P = 1.00, grade ≥ 3; 13% vs. 22%, P = 0.19, supplementary Table 4). Treatment-related death occurred in three patients. Two patients with PS 2 and one patient with PS 4 died due to pneumonitis and septic shock, respectively (Table 4).
Discussion
To our knowledge, this is the largest retrospective study on the effectiveness and tolerability of anti-PD-1/PD-L1 therapy among NSCLC patients with PS ≥ 2.
Clinical studies on anti-PD-1/PD-L1 therapy among previously treated NSCLC patients with PS of 0 or 1 have reported median PFS and OS values of 2.3–4.0 months and 9.2–13.8 months, respectively [2,3,4,5]. Several retrospective studies analyzing actual clinical data have reported that a poor PS (≥ 2) is a negative predictive factor for PFS and a prognostic factor among NSCLC patients treated with anti-PD-1 antibodies [12,13,14,15,16,17]. Moreover, these studies have reported median PFS and OS values of 1.2–1.7 and 2.7–7.5 months among NSCLC patients with poor PS treated with anti-PD-1 antibodies [12,13,14,15,16,17]. Herein, the median PFS and OS were 62 and 168 days, being shorter than those of previous clinical trials and similar to estimates based on clinical data obtained from NSCLC patients with poor PS treated with anti-PD-1 antibodies. Hence, the efficacy of anti-PD-1/PD-L1 antibody therapy among NSCLC patients with poor PS might be relatively modest compared with that for patients with a good PS.
Among NSCLC patients with poor PS, several clinical guidelines have recommended carboplatin-based or single-agent chemotherapy for PS 2 and palliative care for PS 3–4 [18, 19]. A subgroup analysis of several randomized trials indicated that median PFS and OS are 1.6–5.8 and 3.0–11.5 months among NSCLC patients with PS of 2 treated with single-agent or combination chemotherapy, consistent with our results among patients with poor conditions, even though approximately 30% of patients were classified as PS 3 or 4 [20, 21]. A meta-analysis of randomized clinical trials including patients with advanced cancer reported that anti-PD-1/PD-L1 antibodies are better tolerated than chemotherapy, as evident from the lower incidence of any all-grade (67.6% versus 82.9%) or high-grade adverse events (11.4% versus 35.7%) [9]. Accordingly, anti-PD-1/PD-L1 monotherapy might be carefully considered a treatment alternative for patients with poor PS.
A clinical trial reported that PD-L1 expression ≥ 50% on tumor cells is associated with better tumor shrinkage of pembrolizumab in NSCLC patients, consistent with the results of our study limited to NSCLC patients with poor PS [10, 22]. Furthermore, NSCLC patients with poor PS and PD-L1 ≥ 50% had higher PS improvement rates and earlier time to PS improvement than patients in the other PD-L1 groups. For patients with poor PS, tumor response and improvement of PS are essential for symptom relief because PS is associated with cancer-related symptoms. Thus, NSCLC patients with PD-L1 ≥ 50% could benefit from anti-PD-1/PD-L1 therapy in terms of anti-tumor response and early cancer-related symptom relief.
We further evaluated the clinical significance of PD-L1 expression in NSCLC patients with poor PS to predict the efficacy of anti-PD-1/PD-L1 therapy. Multivariate analyses herein revealed that PD-L1 expression is significantly correlated with better PFS. This finding is consistent with those of previous reports. A phase I study on pembrolizumab therapy for advanced NSCLC patients reported that PD-L1 expression ≥ 50% on tumor cells is associated with longer PFS in both previously treated and untreated patients than that for a value of < 50% [10, 23]. Moreover, a clinical study on advanced NSCLC patients treated with anti-PD-1 antibodies reported that PD-L1 upregulation is associated with longer PFS [15, 24]. Thus, our findings indicate that even in NSCLC patients with poor PS, PD-L1 expression is positively correlated with the anti-tumor activity of anti-PD-1/PD-L1 antibodies and can be a clinically useful biomarker.
Multivariate analysis of OS did not display a significant correlation with PD-L1 expression among patients with poor PS. However, a long-term follow-up clinical trial for nivolumab or pembrolizumab among advanced NSCLC patients with good PS revealed that PD-L1 expression ≥ 50% was associated with longer OS [25, 26]. The lack of a correlation between PD-L1 expression and OS herein might be attributed to variations in demographic factors among groups, a relatively small sample size, and short follow-up periods. Thus, prospective studies on larger cohorts and longer follow-up periods are required to confirm the correlation between PD-L1 expression and survival benefit among NSCLC patients with poor PS.
With respect to adverse events, clinical trials of anti-PD1/PD-L1 monotherapy for NSCLC patients have reported rates of any-grade treatment-related adverse events of 58–70.9% for PS 0 or 1 and 7–37% for grade ≥ 3 [2,3,4,5,6,7]. Our results for any-grade and grade ≥ 3 adverse events were consistent with the results of these clinical trials, suggesting that the administration of anti-PD-1/PD-L1 therapy to NSCLC patients with poor PS is feasible and does not have a higher rate of treatment-related adverse events than that for patients with good PS. However, further investigation is warranted for the safety of anti-PD-1/PD-L1 therapy because of the heterogeneity of populations, with various factors affecting immune-related adverse events, and lack of clinical information for patients with poor PS [27].
This study has several limitations. First, this was a retrospective review including a heterogeneous population, and a selection bias could not be avoided. Second, our study primarily included patients with pretreated NSCLC and poor PS, and data on anti-PD-1/PD-L1 therapy in first-line settings were insufficient. Third, only PD-L1 expression was assessed as a predictive marker for the response to anti-PD-1/PD-L1 therapy. Various candidate predictive biomarkers for anti-PD-1/PD-L1 therapy have been identified, such as the tumor mutation burden, microsatellite instability, and tumor-infiltrating CD8 + T cells; therefore, further studies are required to identify alternative biomarkers [28]. Finally, this study analyzed NSCLC patients with poor PS, including both PS2 and PS3 or 4 patients. Since clinical guidelines for NSCLC patients with PS3 or 4 recommend palliative care, clinicians should carefully consider the administration of anti-PD-1/ PD-L1 antibody for these populations.
In conclusion, anti-PD-1/PD-L1 therapy among NSCLC patients and patients with PS ≥ 2 had modest efficacy with acceptable toxicity. PD-L1 expression was correlated with prolonged PFS after anti-PD-1/PD-L1 therapy even in these populations, providing important information for clinical physicians. Anti-PD-1/PD-L1 therapy can even be beneficial in cases of poor PS and PD-L1 ≥ 50%, providing a potential therapeutic strategy for this critical patient subset.
References
Assi HI, Kamphorst AO, Moukalled NM et al (2018) Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 124(2):248–261. https://doi.org/10.1002/cncr.31105
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London, England) 389(10066):255–265. https://doi.org/10.1016/s0140-6736(16)32517-x
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England) 387(10027):1540–1550. https://doi.org/10.1016/s0140-6736(15)01281-7
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New Eng J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Eng J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. https://doi.org/10.1016/s0140-6736(18)32409-7
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Eng J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Govindan R, Garfield DH (2004) Treatment approaches in patients with advanced non-small cell lung cancer and poor performance status. Semin Oncol 31(6 Suppl 11):27–31. https://doi.org/10.1053/j.seminoncol.2004.10.006
Nishijima TF, Shachar SS, Nyrop KA et al (2017) Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist 22(4):470–479. https://doi.org/10.1634/theoncologist.2016-0419
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med 372(21):2018–2028. https://doi.org/10.1056/NEJMoa1501824
Gridelli C, Ardizzoni A, Le Chevalier T et al (2004) Treatment of advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an European Experts Panel. Ann Oncol 15(3):419–426. https://doi.org/10.1093/annonc/mdh087
Adachi Y, Tamiya A, Taniguchi Y et al (2020) Predictive factors for progression-free survival in non-small cell lung cancer patients receiving nivolumab based on performance status. Cancer Med 9(4):1383–1391. https://doi.org/10.1002/cam4.2807
Fujimoto D, Yoshioka H, Kataoka Y et al (2018) Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung cancer (Amsterdam, Netherlands) 119:14–20. https://doi.org/10.1016/j.lungcan.2018.02.017
Ksienski D, Wai ES, Croteau N et al (2019) Pembrolizumab for advanced nonsmall cell lung cancer: efficacy and safety in everyday clinical practice. Lung cancer (Amsterdam, Netherlands) 133:110–116. https://doi.org/10.1016/j.lungcan.2019.05.005
Morita R, Okishio K, Shimizu J et al (2020) Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: a multicenter retrospective observational study in Japan. Lung cancer (Amsterdam, Netherlands) 140:8–18. https://doi.org/10.1016/j.lungcan.2019.11.014
Tamiya M, Tamiya A, Inoue T et al (2018) Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: a retrospective multicenter trial. PLoS ONE 13(2):e0192227. https://doi.org/10.1371/journal.pone.0192227
Taniguchi Y, Tamiya A, Isa SI et al (2017) Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res 37(10):5857–5862. https://doi.org/10.21873/anticanres.12030
Network. NCC (2019) Non-Small Cell Lung Cancer (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed December 16, 2019.
Planchard D, Popat S, Kerr K et al (2019) Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30(5):863–870. https://doi.org/10.1093/annonc/mdy474
Lilenbaum R, Villaflor VM, Langer C et al (2009) Single-agent versus combination chemotherapy in patients with advanced non-small cell lung cancer and a performance status of 2: prognostic factors and treatment selection based on two large randomized clinical trials. J Thorac Oncol 4(7):869–874. https://doi.org/10.1097/JTO.0b013e3181a9a020
Zinner R, Visseren-Grul C, Spigel DR et al (2016) Pemetrexed clinical studies in performance status 2 patients with non-small cell lung cancer (Review). Int J Oncol 48(1):13–27. https://doi.org/10.3892/ijo.2015.3219
Zhang B, Liu Y, Zhou S et al (2020) Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: a meta-analysis. Int Immunopharmacol 80:106214. https://doi.org/10.1016/j.intimp.2020.106214
Incorvaia L, Fanale D, Badalamenti G et al (2019) Programmed death ligand 1 (PD-L1) as a predictive biomarker for pembrolizumab therapy in patients with advanced non-small-cell lung cancer (NSCLC). Adv Ther 36(10):2600–2617. https://doi.org/10.1007/s12325-019-01057-7
Lin SY, Yang CY, Liao BC et al (2018) Tumor PD-L1 expression and clinical outcomes in advanced-stage non-small cell lung cancer patients treated with nivolumab or pembrolizumab: real-world data in Taiwan. J Cancer 9(10):1813–1820. https://doi.org/10.7150/jca.24985
Garon EB, Hellmann MD, Rizvi NA et al (2019) Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 37(28):2518–2527. https://doi.org/10.1200/jco.19.00934
Gettinger S, Horn L, Jackman D et al (2018) Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol 36(17):1675–1684. https://doi.org/10.1200/jco.2017.77.0412
Passaro A, Spitaleri G, Gyawali B et al (2019) Immunotherapy in non–small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol 37(22):1863–1867. https://doi.org/10.1200/jco.18.02118
Evans M, O'Sullivan B, Smith M et al (2018) Predictive markers for anti-PD-1/PD-L1 therapy in non-small cell lung cancer-where are we? Transl Lung Cancer Res 7(6):682–690. https://doi.org/10.21037/tlcr.2018.06.09
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Jodai, T., Saruwatari, K., Ikeda, T. et al. Clinical outcomes and predictive value of programmed cell death-ligand 1 expression in response to anti-programmed cell death 1/ligand 1 antibodies in non-small cell lung cancer patients with performance status 2 or greater. Int J Clin Oncol 26, 78–86 (2021). https://doi.org/10.1007/s10147-020-01789-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01789-5