Abstract
Background
Anti-programmed cell death receptor (PD)-1 antibody treatment results in better prognosis than standard chemotherapy in patients with non-small cell lung cancer (NSCLC), especially those with high PD-ligand 1 (PD-L1) expression. However, several studies have reported a lack of antitumor effect of PD-1 antibody, even in patients with high PD-L1 expression. Therefore, reliable predictors of treatment response are urgently needed. The albumin–globulin ratio (AGR) is associated with prognosis in several cancers. We aimed to determine whether AGR is a predictive biomarker of anti-PD-1 antibody response in patients with NSCLC.
Patients and methods
Seventy-four NSCLC patients treated with anti-PD-1 antibody were retrospectively enrolled. Patients with driver mutations were excluded.
Results
The mean AGR was significantly higher in the disease control (DC) group than in the progressive disease (PD) group (p < 0.001). Receiver operating characteristic curve analysis revealed an AGR cutoff value for dividing patients into the DC or PD groups of 1.17. Multivariate logistic regression analysis showed that a high AGR (≥1.17, cutoff value) was an independent predictor of DC (p = 0.001). Progression-free survival (PFS) and overall survival (OS) were significantly longer in the high-AGR group than in the low-AGR group (p = 0.008, p = 0.002, respectively). Multivariate Cox regression analysis of PFS and OS showed that high AGR was an independent prognostic factor (p = 0.020, p < 0.001, respectively).
Conclusion
Pretreatment serum AGR may be a useful predictor for DC and prognostic factor of anti-PD-1 antibody in patients with NSCLC. The clinical utility of AGR still needs to be confirmed in a prospective analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-programmed cell death receptor-1 (PD-1) antibodies block the interaction of PD-1 on T cells with its ligand, PD-L1, on tumor cells [1,2,3]. Three large phase III trials have demonstrated the superiority of the antitumor effects of two anti-PD-1 antibodies, nivolumab and pembrolizumab, compared to that of standard second-line chemotherapy with docetaxel [4,5,6]. In these clinical trials, anti-PD-1 antibody treatment was more effective in patients with high PD-L1 expression in lung cancer tissues than in those with low or no PD-L1 expression. Therefore, PD-L1 expression is considered a predictor of the effect of anti-PD-1 antibody [4, 5, 7, 8]. In addition, PD-L1 expression was identified as prognostic factor in combination therapy involving anti-PD-1 antibody and chemotherapy [9, 10]. However, a clinical study reported a lack of antitumor effect of anti-PD-1 antibody in some patients with high PD-L1 expression [8]. Therefore, the identification of factors that can more accurately predict the effect of anti-PD-1 antibody is urgently needed. Furthermore, a readily detectable serum biomarker would be very useful in clinical practice.
The neutrophil–lymphocyte ratio (NLR), C-reactive protein (CRP) level, and lactate dehydrogenase (LDH) level have been reported to be serum predictive biomarkers of the effect of anti-PD-1 antibody against several types of cancers, including non-small cell lung cancer (NSCLC) [11,12,13,14,15]. Furthermore, the platelet–lymphocyte ratio (PLR) [14, 15] and CRP albumin ratio [16] have been reported to be predictors of the effect of anti-PD-1 antibody in NSCLC patients. These biomarkers reflect the degree of systemic inflammation or immunocompetence and are associated with the antitumor effect of anti-PD-1 antibody. From these observations, biomarkers associated with inflammation may be good predictors of the anti-PD-1 antibody response.
Albumin and globulin are the major protein constituents of serum. A low serum albumin level indicates malnutrition and is a prognostic factor in various types of cancer. Furthermore, globulin, which consists mainly of immunoglobulins, plays an important role in immunity and inflammation. In previous reports, a low albumin–globulin ratio (AGR; albumin/globulin) has been shown to predict poor outcomes of various carcinomas, including lung cancer [17,18,19]. However, whether AGR is a predictor of the antitumor effect of anti-PD-1 antibody has not yet been investigated. Therefore, we aimed to retrospectively investigate whether AGR is predictive of the effect of anti-PD-1 antibody treatment in NSCLC patients.
Patients and methods
Participants and study design
A total of 85 patients with advanced NSCLC who received anti-PD-1 antibody (nivolumab or pembrolizumab) at Hiroshima University Hospital between September 2015 and April 2018 were enrolled. It has been reported that anti-PD-1 antibody shows no significant antitumor effect in NSCLC patients with driver mutations [20,21,22]. Therefore, we analyzed the remaining 74 patients after excluding 11 patients with driver mutations. This retrospective analysis was approved by the Hiroshima University Institutional Review Board (No. E939). All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. To obtain consent of the patients, the opt-out method was applied in this retrospective study.
Data collection
Patient characteristics and clinical data from before the administration of anti-PD-1 antibody were obtained. We collected data on age, sex, Eastern Cooperative Oncology Group performance status (PS), smoking history, histologic type, PD-L1 tumor proportion score (TPS), prior chemotherapy lines, AGR, progression-free survival (PFS), and overall survival (OS). We categorized the smoking history as follows: never smoker or former/current smoker. Response to anti-PD-1 antibody was determined using the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 criteria [23], and the date of progression and date of death or last follow-up were specified. PD-L1 expression was assessed in formalin-fixed tumor samples using commercially available PD-L1 IHC 22C3 pharmDx assay (Dako North America).
Data analysis
Data are summarized as the number of subjects, median (range), or mean ± standard deviation. Comparisons between two groups were performed using Pearson's χ2 test or the Mann–Whitney nonparametric U test. The relationship of each serum biomarker with AGR was determined using a Spearman correlation (r) (Supplementary Table 2). The optimal cutoff values for pretreatment AGR and for C-reactive protein-to-albumin ratio (CRP/Alb) were estimated by receiver operating characteristic (ROC) curve analysis. The cutoff value of NLR was set to 5.0, as previously reported in several studies [11, 13, 14]. Univariate and multivariate logistic regression analyses were performed to determine risk factors for progressive disease (PD). In addition, univariate and multivariate Cox regression analyses of PFS and OS were performed to determine prognostic factors. Parameters with a p value less than 0.1 in the univariate analysis were selected for inclusion in multivariable analysis. Survival curves were estimated by Kaplan–Meier analysis, and the log-rank test was utilized to examine the significance of differences in survival distributions between groups. Generally, results with p values of ≤ 0.05 were considered to be statistically significant for all analyses. All statistical analyses were performed using JMP®14 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline patient characteristics and tumor response
Seventy-four patients who were diagnosed with NSCLC at our hospital and were administered nivolumab or pembrolizumab were analyzed. The clinical characteristics of the 74 patients with NSCLC are shown in Table 1. The median age was 66.5 years; males represented 70.3% (52/74) of patients, those with a PS of 0–1 represented 90.5% (67/74), and current or former smokers represented 82.4% (61/74). Squamous cell carcinoma patients accounted for 17.6% (13/74), and non-squamous cell carcinoma accounted for 82.4% (61/74) of cases. PD-L1 TPS was measured in 73.0% of patients (54/74), and those with PD-L1 TPS ≥ 1% accounted for 87.0% (47/54) of patients. Anti-PD-1 antibody was administered as first-line treatment in 5.4% (4/74) of cases, second-line in 43.2% (32/74) of cases, and third-line or greater in 51.4% (38/74) of cases. The antitumor effect of anti-PD-1 antibody was evaluated in terms of complete response (0%), partial response (36.5%; 27/74), stable disease (14.9%; 11/74), and PD (48.6%; 36/74). The disease control (DC) rate was 51.4% (38/74). Supplementary Table 1 shows baseline values of NLR and CRP/Alb, and Supplementary Table 2 shows the correlation coefficient of each serum biomarker. The correlation coefficients (r, 95% CI) were as follows: AGR vs NLR: − 0.284 and − 0.481 to − 0.060, p = 0.014: CRP/Alb vs AGR: − 0.624 and − 0.746 to − 0.461, p < 0.001: CRP/Alb vs NLR: 0.637 and 0.478‒0.755, p < 0.001.
Comparison of patient characteristics between DC and PD groups
The clinical characteristics of patients who obtained DC and those with PD are shown in Table 2. The proportion of patients with mean AGR was higher in the DC group than in the PD group (p < 0.001).
AGR analysis
The optimal cutoff value of AGR for predicting DC was determined to be 1.17 according to the ROC curve (Fig. 1). The area under the curve (AUC) of AGR was 0.724 (p = 0.003). Based on the cutoff value, the high AGR (≥ 1.17) group included 32 patients (43.2%; 32/74), and the low AGR (< 1.17) group included 42 patients (56.8%; 42/74). The clinical characteristics of patients in the high AGR (≥1.17) or low (<1.17) groups are shown in Supplementary Table 3. There was no significant difference in the patient background information of both groups.
Univariate and multivariate logistic regression analysis for DC
The results of the univariate and multivariate logistic regression analyses are shown in Table 3. In the univariate logistic regression models, good PS (0–1), continuous value of AGR, and high AGR (≥ 1.17, cutoff value) were significant predictors of DC (odds ratio [95% CI]: 0.135 [0.007–0.850], p = 0.031, 0.115 [0.021–0.504], p = 0.003; 0.217 [0.077–0.573], p = 0.002, respectively). In addition, as shown in supplementary figure 4, continuous value of NLR and CRP/Alb, low NLR (≤ 5.0), and low CRP/Alb (≤ 0.24) were also significant predictors of DC in the univariate logistic regression models.
In the multivariate logistic regression model, continuous value of AGR or high AGR was an independent predictor of DC (0.034 [0.022–0.574], p < 0.001: 0.193 [0.063–0.533], p = 0.001, respectively). Furthermore, multivariate analysis including AGR, NLR, and CRP/Alb showed that high AGR was a significant predictor of DC (0. 248 [0.058–0.935], p = 0.039) (Supplementary figure 4).
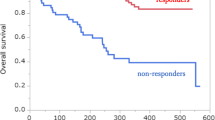
Survival analysis
Kaplan–Meier curves of PFS and OS stratified by pretreatment AGR (≥ 1.17 or < 1.17, cutoff value) are shown in Fig. 2. PFS and OS were significantly longer in the high-AGR group than in the low-AGR group (p = 0.008, p = 0.002, respectively). The median PFS in the high-AGR group and low-AGR group was 310 days and 67 days, respectively. The median OS in the high-AGR group and low-AGR group was not reached and 304 days, respectively.
As shown in supplementary figure 1, in the cases with PD-L1 expression of 50% or more, the PFS and OS in the AGR-high group were also significantly longer than those in the AGR-low group.
Univariate and multivariate Cox regression analysis for PFS and OS
The results of univariate and multivariate Cox regression analyses of PFS are shown in Table 4. In the univariate Cox regression analyses, continuous and cutoff values of AGR were significant predictors (hazard ratio (HR) [95% Cl]: 0.208 [0.079–0.536], p = 0.001; 0.467 [0.258–0.819], p = 0.008, respectively). In multivariate analysis, continuous and cutoff values of AGR were also independent predictors (0.089 [0.074–0.649], p = 0.007; 0.446 [0.216–0.881], p = 0.020, respectively). On the other hand, multivariate analysis including AGR, NLR, and CRP/Alb showed that AGR was not a significant predictor (Supplementary figure 5).
The results of univariate and multivariate Cox analyses for OS are shown in Table 5. Univariate Cox regression analysis showed that continuous and cutoff values of AGR were significant prognostic factors (0.048 [0.013–0.172], p < 0.001; 0.222 [0.082–0.508], p < 0.001, respectively). Multivariate Cox regression analysis showed that continuous and cutoff values of AGR were also significant prognostic factors (0.046 [0.012–0.164], p < 0.001; 0.211 [0.078–0.484], p < 0.001, respectively). In addition, multivariate analysis including AGR, NLR, and CRP/Alb showed continuous and cutoff values of AGR were significant prognostic factors (0.115 [0.017–0.711], p = 0.020; 0.301 [0.102–0.802], p = 0.016, respectively) (Supplementary figure 6).
Discussion
This is the first study to show that AGR is a predictor of the antitumor effect of anti-PD-1 antibody in patients with NSCLC. Albumin and globulin, constituting the AGR, are the main protein components of human serum. Globulin, which is the denominator in the AGR, comprises a large number of immunity-related proteins, such as immunoglobulins, CRP, interleukins, tumor necrosis factor (TNF), and transforming growth factor-β (TGF-β), and is reported to be increased in patients with chronic inflammation due to malignant tumors [24]. These inflammatory cytokines are reportedly associated with tumor progression and resistance to chemotherapy through their effects on the proliferation of cancer cells and tumor angiogenesis [25, 26]. Therefore, we hypothesized that a low level of globulin, i.e., a high AGR, would be a predictive factor for the antitumor effect of anti-PD-1 antibody. In contrast, serum albumin, which is the numerator in the AGR, is indispensable for the physiological activities of the human body and is known to be an indicator of nutritional status. Low albumin in the serum, reflecting a state of malnutrition, would weaken cellular and humoral immunity, phagocytic functions, and other defense mechanisms in patients with cancer. Furthermore, it has been reported that albumin levels may decrease owing to inflammation or development of malignancy, and a low albumin level is known to be a predictor of poor outcomes in patients with malignant tumors [27,28,29]. Therefore, those with high levels of albumin, namely patients with high AGR, are considered to benefit from anti-PD-1 antibody treatment. However, serum albumin levels may change not only due to malignancy but also as a result of various other causes, such as stress, liver failure, and aging. Thus, these factors may limit the clinical application of albumin. From these observations, we hypothesized that the AGR, which consists of both albumin and globulin, would be a good serum biomarker of the antitumor effect of anti-PD-1 antibody.
In this study, AGR was found to be associated not only with the antitumor response of NSCLC to anti-PD-1 antibody but also with PFS and OS. Furthermore, this association was also observed in cases with PD-L1 expression of 50% or more. High AGR has been previously reported to be a prognostic factor in lung cancer, and the result of our study, in which the high AGR group had a longer OS than the low AGR group, is consistent with those of previous studies [17,18,19, 27, 30]. However, 20% of the patients with low AGR showed a long-term PFS of over 500 days. Based on this result, we consider that AGR does not have the ability to completely divide patients into responders and non-responders for ICI treatment.
To examine whether AGR is superior to the previous prognostic biomarker for ICI treatment, we performed univariate and multivariate analyses for DCR, PFS, and OS including NLR and CRP/Alb, which have been previously reported as prognostic factors of ICI treatment in patients with NSCLC [11, 13, 14, 16] (Supplementary figure 4‒6). Univariate analysis revealed that NLR or CRP/Alb was also a significant predictive factor for disease control and prognostic factor for PFS and OS in our cohort. Subsequently, the multivariate analyses showed that NLR and CRP/Alb were significant predictive factors of DC. On the other hand, only AGR was a significant prognostic factor for OS, and NLR was a significant factor for PFS. The reason of these results may be attributable to the lack of statistical power due to the small sample size or to the fact that each factor is a confounding factor (Supplementary Table 2). From these observations, we could not conclude that AGR is the best predictive or prognostic biomarker for ICI treatment, and further study is needed.
In previous studies, CRP and NLR were reported to be serum biomarkers of the antitumor effect of anti-PD-1 antibody in NSCLC patients [13,14,15,16, 31]. One study showed that the effect of anti-PD-1 antibody was significantly attenuated in the high-CRP group compared to that in the low-CRP group [32]. This may be attributed to the fact that IL-6 production from cancer cells is associated with high CRP levels. In fact, IL-6 is known to enhance the proliferation of cancer cells and is reported to inhibit PD-L1 expression [33, 34]. Several studies have shown that NLR is a predictive factor for the effect of anti-PD-1 antibody. Lymphocytes, which form part of the NLR, play an important role in the immune response of PD-L1 on tumor cells. Neutrophils suppress lymphocyte activity by producing several chemokines and cytokines. This explains the mechanism by which NLR is considered to predict antitumor response [35, 36]. However, the CRP level and NLR reflect only inflammation, whereas AGR includes albumin, which is associated with nutritional status, immunocompetence, and cancer prognosis. Thus, AGR is expected to be superior to CRP and NLR in predicting antitumor response.
In this study, good PS was also a prognostic factor for anti-PD-1 antibody response. PS is generally known to be a prognostic factor in NSCLC, and the effect of chemotherapy is reduced in patients with poor PS [37]. In addition, poor PS is an unfavorable prognostic factor regardless of the expression of PD-L1 in tumors [31]. While the reason for this is unclear, the results of our study are consistent with this phenomenon.
We are aware of several limitations of our study. First, this study was a single-center retrospective analysis and included a small number of subjects. Therefore, a prospective multicenter study is warranted to verify our findings. Second, the treatment line under which PD-1 antibody was administered was not uniform across all patients. Thus, it is necessary to determine whether AGR is a prognostic factor for the antitumor effect of anti-PD-1 antibody in patients receiving the same treatment line. Third, in this study, PD-L1 expression of tumors was not measured in 28% of all patients. This may have led to underestimation of the association of PD-L1 expression with antitumor effect using ICI treatment.
Conclusion
In summary, the results of this study provide evidence that pretreatment serum AGR serves as a useful predictor for disease control and prognostic factor of anti-PD-1 therapy in patients with NSCLC. However, we are not able to conclude that AGR is the best biomarker, when AGR is compared to NLR. Although the clinical utility of AGR still needs to be confirmed in a prospective analysis, anti-PD-1 antibody treatment is considered for NSCLC patients with high AGR in addition to high PD-L1 expression.
References
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. https://doi.org/10.1056/NEJMoa1200690
Wang C, Thudium KB, Han M et al (2014) In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2:846–856. https://doi.org/10.1158/2326-6066.CIR-14-0040
Brahmer JR, Drake CG, Wollner I et al (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28:3167–3175. https://doi.org/10.1200/JCO.2009.26.7609
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Herbst RS, Baas P, Kim D-W et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (Lond Engl) 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Wu Y-L, Lu S, Cheng Y et al (2019) Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: checkmate 078 randomized phase III clinical trial. J Thoracic Oncol. https://doi.org/10.1016/j.jtho.2019.01.006
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051. https://doi.org/10.1056/NEJMoa1810865
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Ameratunga M, Chénard-Poirier M, Moreno Candilejo I et al (2018) Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur J Cancer (Oxf Engl 1990) 89:56–63. doi: 10.1016/j.ejca.2017.11.012
Buder-Bakhaya K, Hassel JC (2018) Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-a review from the melanoma perspective and beyond. Front Immunol 9:1474. https://doi.org/10.3389/fimmu.2018.01474
Fukui T, Okuma Y, Nakahara Y et al (2018) Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2018.04.021
Diem S, Schmid S, Krapf M et al (2017) Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (Amsterdam, Netherlands) 111:176–181. https://doi.org/10.1016/j.lungcan.2017.07.024
Svaton M, Zemanova M, Skrickova J et al (2018) Chronic inflammation as a potential predictive factor of nivolumab therapy in non-small cell lung cancer. Anticancer Res 38:6771–6782. https://doi.org/10.21873/anticanres.13048
Inoue T, Tamiya M, Tamiya A et al (2018) Analysis of early death in Japanese patients with advanced non-small-cell lung cancer treated with Nivolumab. Clin Lung Cancer 19:e171–e176. https://doi.org/10.1016/j.cllc.2017.09.002
Chi J, Xie Q, Jia J et al (2018) Prognostic value of albumin/globulin ratio in survival and lymph node metastasis in patients with cancer: a systematic review and meta-analysis. J Cancer 9:2341–2348. https://doi.org/10.7150/jca.24889
Lv G-Y, An L, Sun X-D et al (2018) Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta Int J Clin Chem 476:81–91. doi: 10.1016/j.cca.2017.11.019
Yao Y, Zhao M, Yuan D et al (2014) Elevated pretreatment serum globulin albumin ratio predicts poor prognosis for advanced non-small cell lung cancer patients. J Thoracic Dis 6:1261–1270. https://doi.org/10.3978/j.issn.2072-1439.2014.07.13
Lee CK, Man J, Lord S et al (2017) Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thoracic Oncol 12:403–407. https://doi.org/10.1016/j.jtho.2016.10.007
Fujimoto D, Yoshioka H, Kataoka Y et al (2018) Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung Cancer (Amsterdam, Netherlands) 119:14–20. https://doi.org/10.1016/j.lungcan.2018.02.017
Kobayashi K, Nakachi I, Naoki K et al (2018) Real-world efficacy and safety of nivolumab for advanced non-small-cell lung cancer: a retrospective multicenter analysis. Clin Lung Cancer 19:e349–e358. https://doi.org/10.1016/j.cllc.2018.01.001
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxf Engl 1990) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12:223–226. https://doi.org/10.1097/MCO.0b013e32832a7902
Aggarwal BB, Shishodia S, Sandur SK et al (2006) Inflammation and cancer: How hot is the link? Biochem Pharmacol 72:1605–1621. https://doi.org/10.1016/j.bcp.2006.06.029
Lin W-W, Karin M (2007) A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Investig 117:1175–1183. https://doi.org/10.1172/JCI31537
Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9:69. https://doi.org/10.1186/1475-2891-9-69
Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D et al (2007) Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol 14:381–389. https://doi.org/10.1245/s10434-006-9093-x
Ikeda S, Yoshioka H, Ikeo S et al (2017) Serum albumin level as a potential marker for deciding chemotherapy or best supportive care in elderly, advanced non-small cell lung cancer patients with poor performance status. BMC Cancer 17:797. https://doi.org/10.1186/s12885-017-3814-3
He J, Pan H, Liang W et al (2017) Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: a systematic review and meta-analysis. J Cancer 8:4002–4010. https://doi.org/10.7150/jca.21141
Oya Y, Yoshida T, Kuroda H et al (2017) Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget 8:103117–103128. https://doi.org/10.18632/oncotarget.21602
Akamine T, Takada K, Toyokawa G et al (2018) Association of preoperative serum CRP with PD-L1 expression in 508 patients with non-small cell lung cancer: a comprehensive analysis of systemic inflammatory markers. Surg Oncol 27:88–94. https://doi.org/10.1016/j.suronc.2018.01.002
Chen M-F, Chen P-T, Chen W-C et al (2016) The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget 7:7913–7924. https://doi.org/10.18632/oncotarget.6861
Liu H, Shen J, Lu K (2017) IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun 486:239–244. https://doi.org/10.1016/j.bbrc.2017.02.128
Petrie HT, Klassen LW, Kay HD (1985) Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol (Baltimore, MD 1950) 134:230–4
Moses K, Brandau S (2016) Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol 28:187–196. https://doi.org/10.1016/j.smim.2016.03.018
Ettinger DS, Wood DE, Aisner DL et al (2017) Non-small cell lung cancer, Version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Comprehens Cancer Netw JNCCN 15:504–535
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N.H. and M.O. received research funding from ONO PHARMACEUTICAL CO. LTD., CHUGAI PHARMACEUTICAL CO. LTD., Astra Zeneca K.K.. M.O. received honoraria from ONO PHARMACEUTICAL CO. LTD., CHUGAI PHARMACEUTICAL CO. LTD., Astra Zeneca K.K..
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Nakanishi, Y., Masuda, T., Yamaguchi, K. et al. Albumin–globulin ratio is a predictive biomarker of antitumor effect of anti-PD-1 antibody in patients with non-small cell lung cancer. Int J Clin Oncol 25, 74–81 (2020). https://doi.org/10.1007/s10147-019-01539-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01539-2