Abstract
Background
The aim of this study was to elucidate the risk factors for and prognostic value of lateral pelvic lymph node (LPLN) metastasis in advanced rectal cancer patients, including those with stage IV disease.
Methods
The treatment outcomes of 78 patients with advanced rectal cancer, the lower margin of which was located at or below the peritoneal reflection, who underwent curative-intent surgery with bilateral LPLN dissection from 2005 to 2018 were retrospectively analyzed.
Results
In total, 78 rectal cancer patients, including 13 patients with stage IV tumors, 9 patients (11.5%) had LPLN metastasis. A multivariate analysis to identify preoperative clinical factors associated with LPLN metastasis showed that tumor location (below the peritoneal reflection: Rb), LPLN metastasis on preoperative imaging and distant metastasis were independent predictors of LPLN metastasis. In addition, metastasis at the regional lymph nodes in the mesorectum was significantly associated with LPLN metastasis. Both the disease-free survival (DFS) and cancer-specific survival (CSS) of patients with LPLN metastasis were significantly worse in comparison to patients without LPLN metastasis, and the CSS of stage IV patients with LPLN metastasis was significantly worse in comparison to stage IV patients without LPLN metastasis.
Conclusions
Tumor location (Rb), LPLN metastasis on preoperative imaging and distant metastasis were risk factors for LPLN metastasis. The prognosis of rectal cancer patients with LPLN metastasis is poor. There may not be the indication of LPLN dissection in stage IV lower rectal cancer except cases having complaints due to LPLN metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the treatment strategy recommended by the international guidelines for advanced rectal cancer is neoadjuvant chemoradiotherapy (CRT) and total mesorectal excision (TME) [1, 2], data have recently emerged showing that radiotherapy is associated with substantial long-term functional side effects [3, 4]. On the other hand, lateral pelvic lymph node (LPLN) dissection in addition to TME without preoperative CRT in patients with advanced lower rectal cancer is recommended in the Japanese Society for Cancer of the Colon and Rectum guidelines for the treatment of colorectal cancer [5]. The incidence of LPLN metastasis has been demonstrated to be 15–30% [6,7,8]. The Japan Clinical Oncology Group (JCOG) 0212 randomized controlled trial failed to support the non-inferiority of TME alone in comparison to TME with autonomic nerve-sparing LPLN dissection [9]. The JCOG0212 study thus supported the validity of TME with LPLN dissection as the standard Japanese method for the resection of rectal cancer.
The optimal treatment for LPLN metastasis is complete autonomic nerve-sparing LPLN dissection for patients with LPLN metastasis and omission of unnecessary LPLN dissection for patients without LPLN metastasis, as this avoids the postoperative complications and urinary/sexual dysfunction associated with LPLN dissection [10]. Thus, an accurate preoperative diagnosis of LPLN metastasis aimed at omitting LPLN dissection is important. Although previous studies have attempted to identify the risk factors or predictive factors for LPLN metastasis in rectal cancer patients, including preoperative imaging, clinicopathological and molecular biological factors [11,12,13], they have not been applied in the clinical setting for the selection of patients for whom LPLN dissection can be omitted.
The 5-year survival rate of patients with LPLN metastasis is approximately 40% [6,7,8], which is comparable with that of patients with resectable liver or lung metastasis. From this viewpoint, LPLN metastasis should be classified as distant metastasis, and resected if possible. The overall survival of patients with stage IV colorectal cancer has increased significantly from the 1980s to the present, thanks to new cytotoxic molecules (irinotecan and oxaliplatin), the addition of targeted drugs (anti-VEGF and anti-EGFR) to chemotherapy [14,15,16,17,18], effective palliative treatment in advanced lines [19, 20], and the radical resection of metastasis [21, 22]. Thus, the prognostic value of LPLN metastasis in stage IV patients needs to be elucidated. Since most previous studies reporting the treatment outcome of LPLN metastasis in rectal cancer patients excluded stage IV patients, the prognostic value of LPLN metastasis in stage IV patients remains unclear.
In the present study, we analyzed the treatment outcome of LPLN dissection in patients with advanced rectal cancer, including patients with stage IV disease, to identify risk factors for LPLN metastasis and elucidate the prognostic value of LPLN metastasis.
Patients and methods
Patients
We reviewed a total of 78 patients with advanced rectal cancer (clinical stage II/III/IV), the lower margin of which was located at or below the peritoneal reflection, who underwent curative-intent surgery with bilateral LPLN dissection at Kumamoto University Hospital from 2005 to 2018. All of the study participants provided their informed consent, and the study design was approved by the appropriate ethics review boards. All the patients underwent TME or tumor-specific mesorectal excision. In principle, the patients were followed up at 3-monthly intervals for 5 years. Tumor markers (CEA and CA19-9) were examined at every patient visit. CT of the chest and abdomen was performed every 6 months. Colonoscopy was performed 3 times within 5 years after surgery (1, 3, and 5 years after surgery). The median follow-up time was 46.8 months.
Diagnosis and LPLN dissection
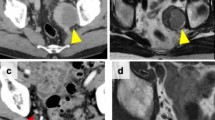
All of the patients underwent preoperative CT with 5-mm-thick sections using intravenous contrast media, and lymph nodes of > 5 mm in diameter were considered positive. Lymph nodes in the lateral pelvic area outside the pelvic plexus and hypogastric nerves along the internal ileac (station 263), external ileac (station 293), and common ileac (station 273) vessels, and in the obturator space (station 283) were considered to be LPLNs. Patients with LPLN metastasis were classified as stage III in this study according to Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma (9th Edition). Lymph nodes in the area lying along the inferior mesenteric vessels were considered regional lymph nodes. The depth of invasion and the tumor location were determined preoperatively by CT, magnetic resonance imaging (MRI) and barium enema. All cancers were biopsied and a pathological diagnosis was obtained before surgery.
All patients underwent bilateral LPLN dissection. LPLN dissection was performed by open surgery until 2016 and laparoscopic surgery thereafter. In open surgery, the lymph nodes at stations 263, 273, 283, and 293 were dissected. Only the lymph nodes at stations 263 and 283 were dissected by laparoscopic surgery, and the dissection of lymph nodes at stations 273 and 293 was omitted in the absence of preoperative lymph node swelling.
Statistical analyses
The factors associated with LPLN metastasis were analyzed by a Chi-squared test and Fisher’s exact test. A logistic regression analysis was performed to identify the factors independently associated with LPLN metastasis. Survival curves were plotted according to the Kaplan–Meier method; the differences between two curves were analyzed using the log-rank test. All statistical analyses were performed using the SPSS software program (version 11; IBM, Chicago, IL, USA). P values of < 0.05 were considered to indicate statistical significance.
Results
The clinicopathological characteristics of a total of 78 patients who underwent surgery for advanced rectal cancer with bilateral LPLN dissection are summarized in Table 1. On preoperative imaging studies such as CT and MRI, 15 patients (19.2%) had LPLN metastasis (cN3), and 13 patients (16.7%) had distant metastasis (liver, n = 6; lung, n = 1; lymph node, n = 2; peritoneal dissemination, n = 1; liver and lung, n = 2; liver and lymph node, n = 1). All stage IV patients underwent curative-intent surgery with bilateral LPLN dissection in combination with synchronous or metachronous surgical resection or radiofrequency ablation (RFA) for distant metastasis. Twenty-six of 78 patients (33.3%) underwent preoperative treatment (systemic chemotherapy, n = 21; chemoradiotherapy, n = 2; liver resection, n = 2; liver RFA, n = 1). LPLN dissection was performed by open surgery in 60 patients (76.9%) and laparoscopic surgery in 18 patients (23.1%). Of the 78 patients in the present study, R0 resection was performed in 74 patients (94.9%) and R1/2 resection was performed in 4 patients (5.1%). Two R1 resections had a positive circumferential resection margin. Two R2 resections had a positive distal margin and unresectable lung metastasis. On pathological examination, although most tumors were differentiated type (tub1, well differentiated; tub2, moderately differentiated; pap, papillary) adenocarcinoma, 3 tumors were mucinous and 1 was poorly differentiated adenocarcinoma. Fourteen of 78 patients (17.9%) had T4b tumors and 9 (11.5%) had LPLN metastasis. Of the 78 patients in the present study, 6 patients (7.7%) experienced local recurrence in the study period (presacral, n = 3; right LPLN, n = 1; anastomotic site, n = 1; perineal area after abdominoperineal resection, n = 1). At the time of primary rectal cancer surgery, 3 of the 6 patients (50%) had LPLN metastasis and the pathological stage of the 6 patients was stage III/IV: 4/2.
Table 2 shows the preoperative clinical factors associated with LPLN metastasis. In the multivariate analysis, tumor location (Rb) (HR 32.1, P = 0.025), LPLN metastasis (cN3) (HR 7.1, P = 0.049) and distant metastasis (cM-positive) (HR 16.3, P = 0.034) were independent predictive factors for LPLN metastasis. Table 3 shows the pathological factors associated with LPLN metastasis. Thirty-one patients had regional LN metastasis with or without LPLN metastasis and regional LN metastasis was significantly associated with LPLN metastasis (P = 0.025).
Figure 1 shows the results of a Kaplan–Meier analysis for DFS and CSS after surgery stratified by LPLN metastasis in all 78 patients. The median follow-up time was 46.8 months. Both the DFS (Fig. 1a) and CSS (Fig. 1b) of patients with LPLN metastasis were significantly worse in comparison to patients without LPLN metastasis (log-rank test: P = 0.0011 and P = 0.011, respectively). Figure 2 shows the DFS and CSS after surgery of the 65 patients with clinical stage II–III rectal cancer. The median follow-up time was 47.6 months. Clinical stage II/III patients with LPLN metastasis showed significantly poorer DFS in comparison to patients without LPLN metastasis (Fig. 2a, Log-rank test: P < 0.0083). Figure 3 shows the DFS and CSS after surgery in the 13 patients with clinical stage IV rectal cancer. The median follow-up time was 28.2 months. Consistent with the CSS analysis in all 78 patients, clinical stage IV patients with LPLN metastasis showed significantly poorer CSS in comparison to patients without LPLN metastasis (Fig. 3b, Log-rank test: P < 0.001).
Of the 78 patients enrolled in the present study, there were 5 stage IV patients with LPLN metastasis. The sites of distant metastasis were as follows: liver, n = 1; lung, n = 1; both liver and lung, n = 2; paraaortic lymph node, n = 1. Preoperatively, all 5 patients were treated with oxaliplatin-based systemic chemotherapy and R0 resection was performed in 4 patients. One patient with lung metastasis was supposed to undergo resection of lung metastasis after rectal cancer surgery. However, multiple lymph node metastases were detected after primary surgery and systemic chemotherapy was performed. All 4 patients who underwent R0 resection received adjuvant chemotherapy (5-FU based, n = 2; irinotecan + 5-FU, n = 1). However, 2 patients experienced early recurrence (one had multiple lymph node metastases and one had liver metastasis) and it seemed to be associated with worse cancer-specific survival, as shown in Fig. 3b.
Discussion
In the present study, we retrospectively analyzed the treatment outcomes of surgery with LPLN dissection for rectal cancer patients, including patients with stage IV disease to identify risk factors for LPLN metastasis and elucidate the prognostic value of LPLN metastasis. In the overall population of 78 rectal cancer patients, including 13 patients with stage IV tumors, 9 patients (11.5%) had LPLN metastasis. A multivariate analysis to identify preoperative clinical factors associated with LPLN metastasis showed that tumor location (Rb), LPLN metastasis on preoperative imaging (cN3) and distant metastasis (cM-positive) were independent predictive factors of LPLN metastasis. In addition, metastasis at the regional LNs in the mesorectum was significantly associated with LPLN metastasis. Both the DFS and CSS of patients with LPLN metastasis were significantly worse than those of patients without LPLN metastasis, and the CSS of stage IV patients with LPLN metastasis was also significantly worse in comparison to stage IV patients without LPLN metastasis.
In previous studies, the incidence of LPLN metastasis was demonstrated to be 15–30% [6,7,8]. On the other hand, the rate of LPLN metastasis in the present study was 11.5%, which was lower than that of previous reports. One possible reason for the low LPLN metastasis rate is that 26 of 78 patients (33.3%) received preoperative treatments such as systemic chemotherapy. Reinforced and/or stronger systemic chemotherapy in the preoperative period (neoadjuvant chemotherapy: NAC) has therapeutic potential in terms of obtaining carcinogenic control in patients with advanced rectal cancer [23,24,25,26]. Both systemic NAC (for carcinogenic control) and the surgical dissection of LPLN (for local curability) may have therapeutic potential in the treatment of advanced rectal cancer. Whether the combination of systemic NAC and intentional dissection of LPLN truly results in adequate local curability, a reduction of distant metastasis, and a favorable long-term outcome, should be clarified in a large prospective randomized trial.
In the present study LPLN dissection was performed by open surgery until 2016 and laparoscopic surgery thereafter. Laparoscopic LPLN dissection is a technically complex and challenging procedure. Although the operative time of laparoscopic rectal cancer surgery with LPLN dissection was significantly longer in comparison to open surgery, laparoscopic surgery was associated with significantly less blood loss, and the postoperative complication rate of the two groups was similar in the present study (data not shown). Consistent with our findings, some previous studies have demonstrated the feasibility of laparoscopic LPLN dissection for rectal cancer [27,28,29]. In addition, the feasibility of additional laparoscopic LPLN dissection after preoperative CRT in patients with advanced lower rectal cancer was demonstrated [30]. Although laparoscopic LPLN dissection in advanced rectal cancer patients, even after preoperative treatment, seems technically feasible, the oncological safety needs to be clarified.
Our data demonstrated that the tumor location (Rb), LPLN metastasis on preoperative imaging (cN3) and distant metastasis (cM-positive) were independent predictive factors of LPLN metastasis. In the preoperative diagnosis of the LPLN status, we considered lymph nodes of > 5 mm in diameter on CT to be metastasis-positive [11]. The sensitivity and specificity of the LPLN diagnosis in the present study were 66.7% and 87.0%, respectively. These results were comparable to a previous study using CT reported by Fujita et al. (62% and 90%, respectively) [11], and a study using MRI reported by Matsuoka et al. (67% and 83%, respectively) [31]. Preoperative clinicopathological factors such as sex, tumor location, depth of invasion, LPLN status, mesorectal LN status, tumor differentiation and tumor size were reported to be factors associated with LPLN metastasis [8, 11, 32]. Two of the 3 risk factors for LPLN metastasis that we identified (tumor location [Rb] and cN3) were consistent with previous reports. The remaining factor of distant metastasis has never been reported. The fact that rectal cancer patients with distant metastasis are likely to have synchronous LPLN metastasis is in some ways unsurprising. However, the indication of LPLN dissection in surgically resectable stage IV rectal cancer patients is controversial.
Recently, Miyake et al. reported a novel method to predict LPLN metastasis targeting lymph node micrometastasis (sensitivity: 100%, specificity: 86%) [12]. They found micrometastasis in the regional lymph nodes using a novel one-step nucleic acid amplification (OSNA) assay, and demonstrated that none of the patients without regional lymph node micrometastasis had LPLN metastasis. The method is simple, less invasive and time-saving; thus, the authors concluded that the OSNA assay may be useful for identifying advanced rectal cancer patients who should undergo LPLN dissection. Our finding that regional lymph node metastasis was also significantly associated with LPLN metastasis supports Miyake’s conclusion, and further validation analyses targeting micrometastasis at the regional lymph nodes’ are desired.
The prognosis of stage II/III rectal cancer patients with LPLN metastasis is poor. The 5-year survival rate of patients with LPLN metastasis has been reported to be approximately 40% [6,7,8]. The 5-year CSS rate of 40% in the present study is relatively good despite the fact that 17% of our cohort had stage IV disease. This may be due to the development of multidisciplinary approaches such as NAC, neoadjuvant CRT and treatments for recurrent diseases. Our data showed that the CSS of patients with LPLN metastasis was significantly worse than that of patients without LPLN metastasis, and the finding was seen in not only the overall patient population but also in patients with stage IV disease. Especially, the prognosis of stage IV patients with LPLN metastasis was quite poor. Therefore, aggressive adjuvant therapy and intensive postoperative follow-up are requited for such patients. Based on these results, we think there may not be the indication of LPLN dissection in stage IV lower rectal cancer except cases having complaints due to LPLN metastasis. Especially, in stage IV rectal cancer patients accompanied by LPLN metastasis in preoperative imaging, whose prognosis is expected as quite poor, the indication of surgery (especially invasive treatments such as resection of distant metastasis or LPLN dissection, even resection of primary lesion) should be determined carefully. LPLN dissection should be omitted for such patients to avoid unnecessary invasive treatment. Intensive systemic therapy may be a superior option to surgery for some patients.
The present study was associated with some limitations including the retrospective nature of the design, the small population (especially, the number of stage IV patients was small), and the fact that it was performed in a single institute. The number of events (LPLN metastasis in the present study) in our analysis was too small to perform an appropriate multivariate analysis to identify the risk factors for LPLN metastasis. In addition, systemic chemotherapy (especially regarding adjuvant therapy and treatment after recurrence) drastically developed during the study period from 2005 to 2018. In our institution, resectable locally advanced rectal cancer (regardless of LPLN metastasis) was generally considered an indication for surgery without neoadjuvant therapy. However, most of the stage IV rectal cancer patients in the present study were treated with preoperative systemic chemotherapy and the regimen changed during the study period. Thus, the development of systemic therapy might have influenced the results of our retrospective analysis. However, we believe that this study, which demonstrated the prognostic value of LPLN metastasis in stage IV rectal cancer patients for the first time, is valuable.
In conclusion, the independent risk factors for LPLN metastasis in advanced rectal cancer include tumor location (Rb), LPLN metastasis on preoperative imaging (cN3) and distant metastasis (cM-positive). The prognosis of rectal cancer patients with LPLN metastasis is poor, not only in the overall rectal cancer patient population but also in patients with stage IV disease. There may not be the indication of LPLN dissection in stage IV lower rectal cancer except cases having complaints due to LPLN metastasis to avoid unnecessary invasive treatment.
References
Heald RJ (1988) The 'Holy Plane' of rectal surgery. J R Soc Med 81:503–508
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Bruheim K, Guren MG, Skovlund E et al (2010) Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 76:1005–1011
Rothenberger DA, Akbari R, Baxter NN (2004) Are we overtreating some patients with rectal cancer? Oncology 18:1789–1796
Watanabe T, Muro K, Ajioka Y et al (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23:1–34
Fujita S, Yamamoto S, Akasu T et al (2003) Lateral pelvic lymph node dissection for advanced lower rectal cancer. Br J Surg 90:1580–1585
Ueno M, Oya M, Azekura K et al (2005) Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg 92:756–763
Sugihara K, Kobayashi H, Kato T et al (2006) Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 49:1663–1672
Fujita S, Mizusawa J, Kanemitsu Y et al (2017) Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg 266:201–207
Hojo K, Sawada T, Moriya Y (1989) An analysis of survival and voiding, sexual function after wide iliopelvic lymphadenectomy in patients with carcinoma of the rectum, compared with conventional lymphadenectomy. Dis Colon Rectum 32:128–133
Fujita S, Yamamoto S, Akasu T et al (2009) Risk factors of lateral pelvic lymph node metastasis in advanced rectal cancer. Int J Colorectal Dis 24:1085–1090
Miyake Y, Mizushima T, Hata T et al (2017) Inspection of perirectal lymph nodes by one-step nucleic acid amplification predicts lateral lymph node metastasis in advanced rectal cancer. Ann Surg Oncol 24:3850–3856
Yano H, Saito Y, Takeshita E et al (2007) Prediction of lateral pelvic node involvement in low rectal cancer by conventional computed tomography. Br J Surg 94:1014–1019
Douillard JY, Oliner KS, Siena S et al (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369:1023–1034
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Saltz LB, Clarke S, Diaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Van Cutsem E, Kohne CH, Lang I et al (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019
Van Cutsem E, Tabernero J, Lakomy R et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30:3499–3506
Grothey A, Van Cutsem E, Sobrero A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312
Mayer RJ, Van Cutsem E, Falcone A et al (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372:1909–1919
Adam R, Wicherts DA, de Haas RJ et al (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27:1829–1835
Tomlinson JS, Jarnagin WR, DeMatteo RP et al (2007) Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25:4575–4580
Hasegawa J, Nishimura J, Mizushima T et al (2014) Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol 73:1079–1087
Hasegawa S, Goto S, Matsumoto T et al (2017) A multicenter phase 2 study on the feasibility and efficacy of neoadjuvant chemotherapy without radiotherapy for locally advanced rectal cancer. Ann Surg Oncol 24:3587–3595
Schrag D, Weiser MR, Goodman KA et al (2014) Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 32:513–518
Uehara K, Hiramatsu K, Maeda A et al (2013) Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II trial. Jpn J Clin Oncol 43:964–971
Furuhata T, Okita K, Nishidate T et al (2015) Clinical feasibility of laparoscopic lateral pelvic lymph node dissection following total mesorectal excision for advanced rectal cancer. Surg Today 45:310–314
Liang JT (2011) Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol 18:153–159
Nagayoshi K, Ueki T, Manabe T et al (2016) Laparoscopic lateral pelvic lymph node dissection is achievable and offers advantages as a minimally invasive surgery over the open approach. Surg Endosc 30:1938–1947
Ogura A, Akiyoshi T, Nagasaki T et al (2017) Feasibility of laparoscopic total mesorectal excision with extended lateral pelvic lymph node dissection for advanced lower rectal cancer after preoperative chemoradiotherapy. World J Surg 41:868–875
Matsuoka H, Nakamura A, Masaki T et al (2007) Optimal diagnostic criteria for lateral pelvic lymph node metastasis in rectal carcinoma. Anticancer Res 27:3529–3533
Ueno H, Mochizuki H, Hashiguchi Y et al (2007) Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg 245:80–87
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hiyoshi, Y., Miyamoto, Y., Kiyozumi, Y. et al. Risk factors and prognostic significance of lateral pelvic lymph node metastasis in advanced rectal cancer. Int J Clin Oncol 25, 110–117 (2020). https://doi.org/10.1007/s10147-019-01523-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01523-w