Abstract
Whether consolidation chemotherapy (CCT) after chemoradiotherapy (CRT) helps in the treatment of locally advanced non-small cell lung cancer (LA-NSCLC) is controversial. The aim of this meta-analysis was to evaluate the impact of CCT on overall survival (OS), progression-free survival (PFS), overall response rate (ORR) and toxicities in patients with inoperable LA-NSCLC. PubMed, Embase, The Cochrane Library, WanFang, VIP, and CNKI were searched to identify any relevant publications. After screening the literature and completing quality assessment and data extraction, the meta-analysis was performed using RevMan5.3 software. Ultimately, 5 eligible studies with a total of 1036 patients were selected for the present meta-analysis. The results of the analysis indicated that treatment of LA-NSCLC patients with CRT followed by CCT improved OS (pooled HR 0.85; 95% CI 0.73–0.99; P = 0.03), but did not improve PFS (pooled HR 0.78; 95% CI 0.60–1.02; P = 0.07) and ORR (P = 0.26). Although it could increase the risk of grade ≥3 infection (P = 0.04), it may not increase the risk of grade ≥3 radiation pneumonitis (P = 0.09) during the CCT period. CCT after concurrent CRT may provide additional benefits in the treatment of LA-NSCLC. Although this therapeutic strategy did not prolong PFS, further assessment is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with approximately 1.4 million deaths annually [1]. Among new cases of lung cancer, 85% are non-small cell lung cancer (NSCLC) [2]. Approximately one-third of NSCLC patients present with locally advanced disease (stages IIIa/IIIb), and surgery may not be suitable for these patients. A number of randomized clinical trials and meta-analyses of LA-NSCLC support the conclusion that concurrent CRT improved overall survival (OS) compared to radiotherapy (RT) alone and/or sequential chemoradiotherapy (CRT) [3–5]. However, the prognosis is still poor, with high rates of local and distant failure and five-year overall survival rates ranging between 15 and 25% [6]. Therefore, more effective therapy or other systemic antitumor agents are required to further improve the current level of response and survival. The addition of consolidation chemotherapy (CCT) is another potential approach, and several randomized studies and systematic reviews/meta-analyses have already indicated the efficacy of CCT [7, 8]. Cortesi et al. reported that CCT was safely administered without severe adverse events or any influence on the final treatment response rate [9]. However, a recent report on the pooled analysis of 41 studies failed to provide evidence that CCT yielded a significant survival benefit for patients with LA-NSCLC [10]. A clinical trial by Hanna et al. demonstrated that consolidation docetaxel after cisplatin/etoposide with RT (PE/XRT) results in increased toxicities but does not further improve survival compared with PE/XRT alone in patients with stage III inoperable NSCLC [11]. Another Phase II study said the addition of consolidation paclitaxel after CRT resulted in increased toxicity without improving survival [12]. Therefore, the optimal treatment strategy is still controversial. The objectives of this systematic review and meta-analysis were to determine the survival benefit of CCT and to determine whether CCT is superior to CRT in unresectable LA-NSCLC patients.
Materials and methods
Search strategy
We searched the online PubMed, Embase, Cochrane Library, WanFang, VIP, and CNKI databases to identify relevant studies in the published literature. The systematic search on PubMed was performed using the following MesH and free text terms: chemoradiotherapy, carcinoma, non-small cell lung and consolidation chemotherapy. For Embase, the search method was exp chemoradiotherapy/or chemoradiotherapy.mp., exp non-small cell lung cancer/or lung non small cell cancer/or non small cell lung cancer.mp. and exp consolidation chemotherapy or/consolidation chemotherapy.mp. The search strategy for the Cochrane Library was MeSH descriptor: [carcinoma, non-small-cell lung] explode all trees; MeSH descriptor: [chemoradiotherapy] explode all trees; and MeSH descriptor: [consolidation chemotherapy] explode all trees. We also searched Chinese databases, such as CNKI, using the key words chemoradiotherapy, non-small cell lung cancer and consolidation chemotherapy. The search was performed without any language limitations.
Inclusion/exclusion criteria
The inclusion criteria, which were set before searching online, were (1) randomized clinical trials comparing the survival between a group receiving combined CRT and another group receiving CRT followed by CCT; (2) the studies involved patients with unresectable LA-NSCLC; (3) in studies that had multiple arms, at least two of the arms fulfilled the previous requirements; and (4) the hazard ratios (HRs) and 95% confidence intervals (CIs) of the patients who underwent CRT followed by CCT and those who received combined CRT could be calculated from the survival data in the article, including OS and progression-free survival (PFS). The definition of PFS is the time from random assignment until the first documentation of disease progression or death by any cause or last follow-up, whichever came first. Disease progression was determined based on a radiological examination, histologic examination, or both. Based on a report published in the ‘International Journal of Clinical Oncology’ [13], treatment responses were evaluated using the Response Evaluation Criteria for Solid Tumors (RECIST) version, as progression of disease, stable disease, partial response (PR) and complete response (CR).
The exclusion criteria were non-randomized controlled trials; trials that did not include survival data as endpoints; and trials that were only published as an abstract, letter, or conference paper. We also excluded trial arms in which surgery or induction chemotherapy was offered in addition to concurrent CRT.
Study selection
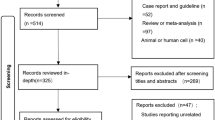
The full texts of the gathered articles were carefully reviewed to identify eligible studies. Any disagreement concerning study selection was resolved by discussion and consensus with another author. In total, we collected 341 studies from the systematic search. Then, 286 irrelevant articles were excluded after title and abstract review, and 55 studies were considered potentially eligible for inclusion. After analyzing the full text articles, 50 studies were excluded because 7 studies were review articles, 4 studies were letters, 10 studies were conference abstracts, 10 articles were single-arm clinical trials, and 17 studies were eliminated due to inadequate data for meta-analysis. Finally, 5 eligible studies were selected for the present meta-analysis according to our criteria. Five included OS data, and 4 included PFS data. A flowchart depicting the study selection is shown in Fig. 1.
Quality assessment
The risk of bias in individual studies was assessed independently by 2 authors. Characteristics assessed by the 2 authors included concealment of a randomization sequence and the number of patients lost to follow-up.
Data extraction
Two authors independently extracted data from the 5 eligible studies. The simplest method consisted of the direct collection of the HR and the 95% CI for OS and PFS from the original article. If these statistics were not available, we extracted univariate Cox hazard regression analysis or log-rank P values and Kaplan–Meier survival curves of the survival outcomes instead. All of the studies included in the analysis also contained the following data—first author’s name, year published, country of origin, tumor grade, number of patients, CRT regimen, CCT regimen, median OS, and median PFS. Any disagreement about data extraction was resolved by discussion and consensus with another author. The data extracted from all 5 included trials are shown in Table 1.
Statistical analysis
The primary endpoint was OS, defined as the time from random assignment until death by any cause. The secondary endpoint was PFS, defined as the time from random assignment until first progression or death by any cause. Statistical analyses were performed using RevMan (Review Manager Version 5.3 for Windows, Cochrane Collaboration, Oxford, UK, 2014). Log hazard ratio (logHR) and standard error (SE) were statistically combined for the quantitative aggregation of the survival results, but the two statistical variables were not explicitly stated in all of the studies. Therefore, based on methods developed by Parmar et al. [14], Williamson et al. [15], and Tierney et al. [16], we calculated the logHR and SE using the primary data as described above—HR and their 95% CI, log-rank P value, or Kaplan–Meier survival curves. We used random-effects models and fixed-effects models for statistical analysis. An observed HR >1 indicated a worse outcome for the positive group relative to the negative group, and if the 95% CI did not overlap 1, the value was considered statistically significant.
Results
Description of trials and patients
The trial characteristics and the treatment schedules are listed in Table 1. There were 1036 patients with unresectable LA-NSCLC in the 5 selected clinical trials, with 530 patients having received CRT followed by CCT and 506 patients having received only CRT. The 5 studies were published from 2008−2015, and the participants were from Turkey, Korea, China, and the USA. The tumor stages of the patients were IIIa or IIIb. In 4 trials, the chemotherapy regimen concurrent with RT was etoposide plus cisplatin, while the other was docetaxel plus cisplatin. The median total radiation dose delivered was 60 Gy in fractionated doses of 2 Gy per day or 1.8 Gy per day. The regimens for CCT were 3 cycles of platinum, taxanes or a combination of both.
Overall survival
The main meta-analysis results for OS are shown in Fig. 2a. OS data were described in 5 trials, in which 530 patients received CRT followed by CCT, and 506 patients received CRT only. The meta-analysis revealed a significant difference in OS in stage IIIa or IIIb unresectable LA-NSCLC patients who received CRT followed by CCT compared to those who received CRT only (pooled HR 0.85; 95% CI 0.73–0.99; P = 0.03; Z = 2.31). According to our result, LA-NSCLC patients may benefit from CCT. There was evidence of significant heterogeneity between trials (χ 2 = 8.11; df = 4.0; I 2 = 51%; P = 0.09).
Progression-free survival
The main meta-analysis results for PFS are shown in Fig. 2b. PFS data were described in 4 trials, in which 448 patients received CRT followed by CCT and 422 patients received CRT only. The meta-analysis revealed no significant difference in PFS in stage IIIa or IIIb unresectable LA-NSCLC patients who received CRT followed by CCT compared to those who received CRT only (pooled HR 0.78; 95% CI 0.60–1.02; P = 0.07; Z = 1.84). The random-effects model was used, and there appeared to be evidence of significant heterogeneity between trials (τ 2 = 0.04; χ 2 = 6.07; df = 3; I 2 = 55%; P = 0.08).
Overall response rate
Data regarding the overall response rate (ORR) were available in two trials with 623 patients. We defined ORR as CR plus PR. CCT followed by CRT compared with CRT alone had no statistically significant effect on ORR (RR 1.04, 95% CI 0.91–1.19, P = 0.26, Fig. 3a). As there was no heterogeneity in the ORR of the effect across the included studies (I 2 = 0%, P = 0.51), we carried out data analysis by the fixed-effects model.
a Forest plots of studies evaluating risk ratios of ORR for CCT followed by CRT compared to CRT alone; b forest plots of studies evaluating risk ratios of grade ≥3 radiation pneumonitis during the CCT period in a fixed-effects model; c forest plots of studies evaluating risk ratios of grade ≥3 infection during the CCT period in a fixed-effects model
Toxicity
During the CRT period, only three trials involving 810 patients provided information on grade 3 or 4 adverse events. Neutropenia accounted for 20.24%, with esophagitis being the next most common event (12.21%). Infection, fatigue, anemia, thrombocytopenia, febrile neutropenia and radiation pneumonitis accounted for 6.78, 6.18, 5.80, 5.67, 4.44 and 0%, respectively (Table 2). During the CCT period, grade ≥3 non-hematologic toxic effects were analyzed. Data for radiation pneumonitis and esophagitis were available for 3 included trials, but esophagitis cannot be pooled. Data for infection were available for 2 included trials (Table 3). According to the pooled analysis for toxic effects, grade ≥3 radiation pneumonitis showed no statistical significance between the two arms (RR 0.44, 95% CI 0.18–1.12, P = 0.09, Fig. 3b). However, adding CCT after CRT may increase the risk of grade ≥3 toxic events of infection compared with CRT alone (RR 0.33, 95% CI 0.12–0.96, P = 0.04, Fig. 3c). There was significant heterogeneity between the trials in the toxicity analysis for infection but no significant heterogeneity for radiation pneumonitis.
Discussion
Due to its high incidence and mortality rate, more effective treatment is urgently needed for the treatment of NSCLC. According to the National Comprehensive Cancer Network guidelines, the treatment for unresectable LA-NSCLC is definitive CRT. The combination of systemic chemotherapy and thoracic RT has been established as the standard of care for patients with stage III NSCLC, as it prolongs OS [21]. Concurrent CRT is superior to a sequential approach, as shown by phase III trials in stage III NSCLC [22, 23]. However, no significant progress in the strategy of treatment has been made recently [24]. Currently, little is known about the efficacy of CCT after concurrent CRT. The outcome of LA-NSCLC is relatively poor, with a high possibility of residual disease after definitive CRT. Thus, some clinical trials have gradually investigated the role of additional CCT. Against this background, we present a meta-analysis to evaluate the efficacy and toxicity of CCT followed by CRT versus CRT alone in the treatment of LA-NSCLC.
We identified five trials that evaluated the efficacy and toxicity of CCT followed by CRT versus CRT alone for LA- NSCLC. We comprehensively searched literature regardless of published year and language. Our meta-analysis showed that the addition of CCT after concurrent CRT significantly prolonged OS (pooled HR 0.85; 95% CI 0.73–0.99; P = 0.03). In accordance with our meta-analysis, a number of pilot studies have been reported, some of which have shown encouraging results [25, 26], and have suggested that concurrent CRT followed by CCT is clinically viable, based on median survival in patients with unresectable stage III NSCLC. Furthermore, the results of S9504, a Southwest Oncology Group (SWOG) phase II trial, found consolidation docetaxel after concurrent CRT in stage IIIB NSCLC was feasible and generally tolerable, and the results compared favorably with the predecessor trial S9019 [27, 28]. Therefore, according to our meta-analysis, patients may benefit from the addition of CCT after concurrent CRT with prolonged OS.
In addition, our results revealed that the addition of CCT did not improve PFS (pooled HR 0.78, 95% CI 0.60−1.02, P = 0.07) and ORR (pooled RR 1.04, 95% CI 0.91–1.19, P = 0.26). Although PFS data were described in 4 trials, only the data from Mutlu et al. [17] confirmed the use of CCT according to PFS. In our meta-analysis, it was noted that the trial by Liu et al. [19] reported conflicting results between OS and PFS. Patients in the CCT group achieved significant survival prolongation compared to those in the non-CCT group (median OS 27 vs 16 months; 5-year OS 30.4 vs 22.5%; P = 0.012). However, median PFS and 5-year PFS were 12 months and 21.8% in the CCT group and 9 months and 21.4% in the non-CCT group, respectively (P = 0.291). This may be an important factor influencing our PFS results.
Whether CCT increases toxicity is also a concern. A phase I and phase II trial by Horinouch et al. [29] indicated that the higher pulmonary toxicities in the CCT group might be related to consolidation docetaxel. A relatively higher frequency of pulmonary complications was also reported in the experimental arm of the previous phase III trial that examined docetaxel as a consolidation therapy after concurrent CRT [30]. Our meta-analysis found the opposite result. Grade ≥3 radiation pneumonitis showed no statistical significance between the two arms (P = 0.09) during the CCT period. Of these studies, the trial by Ahn et al. [19] determined that CRT with weekly docetaxel plus cisplatin (DP) is a feasible regimen with acceptable toxicity. The trial by Liu et al. [19] found that CCT may prolong survival without increasing treatment-related toxicity. In addition, we noted that the incidence of neutropenia was the most common event, accounting for 20.24%, followed by esophagitis (12.21%) during the CRT period. Adding CCT after CRT may increased the risk of grade ≥3 toxic events of infection compared with CRT alone (P = 0.04). Our analysis may not be able to detect other small differences in toxicity because many included studies lacked effective data.
At present, there is still no clear evidence that CRT followed by CCT improves survival in patients with LA-NSCLC compared to patients receiving CRT alone. The role of additional CCT is controversial, and few ongoing trials have explored the significance of CCT. Therefore, our meta-analysis may have guiding significance for the treatment of LA-NSCLC. To the best of our knowledge, CCT has been routinely incorporated in many ongoing trials.
This study has several limitations. As the meta-analysis is based on the results of published articles and several steps of integration, certain biases are therefore inevitable. Heterogeneity was found in the meta-analysis for OS of the prognostic role of CCT (I 2 = 51%, P = 0.09). The heterogeneity mainly originated from the studies by Ahn et al. [11], Jalal et al. [20], and Hanna et al. [11]. These studies exhibited conflicting survival outcomes. Although the patient characteristics, trial phase, study period, and tumor grade of the trials did not significantly differ between CRT followed by CCT and CRT alone, we cannot exclude the possibility that other differences may have affected our conclusions. For example, CRT and CCT regimens as well as the number of patients receiving each treatment may have varied. Moreover, publication bias may also have been a problem. Although we searched systematically, some factors may still have introduced bias. For example, positive results tend to be accepted by journals, and unpublished papers and abstracts were excluded because the required data were not available. Fortunately, the funnel plot showed no significant publication bias, suggesting that the statistics obtained approximate the actual results. The funnel plots are shown in Fig. 4a, b.
In conclusion, this meta-analysis showed that CCT after concurrent CRT may provide additional benefits in the treatment of LA-NSCLC. However, the therapy schedule may not improve PFS and ORR. The toxicities are controversial and further assessment is warranted. More clinical studies are necessary to establish appropriate CCT regimens and other novel treatment strategies to prolong survival and increase the cure rate of patients with stage III inoperable LA-NSCLC.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Molina JR, Yang P, Cassivi SD et al (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584–594
Furuse K, Fukuoka M, Kawahara M et al (1999) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17:2692–2699
Aupérin A, Le Péchoux C, Rolland E et al (2010) Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 28:2181–2190
Curran WJ Jr, Paulus R, Langer CJ et al (2011) Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 103:1452–1460
Ramnath N, Dilling TJ, Harris LJ et al (2013) Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143(Suppl):e314S–e3140S
Soon YY, Stockler MR, Askie LM et al (2009) Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol 27:3277–3283
Zhang X, Zang J, Xu J et al (2011) Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: a systematic review and meta-analysis. Chest 140:117–126
Cortesi E, Moscetti L, Nelli F et al (2007) Induction therapy with paclitaxel and carboplatin followed by hyperfactionated radiotherapy plus weekly concurrent chemotherapy and subsequent consolidation therapy in unresectable locally advanced non-small-cell lung cancer. Tumori 93:133–137
Tsujino K, Kurata T, Yamamoto S et al (2013) Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol 8:1181–1189
Hanna N, Neubauer M, Yiannoutsos C et al (2008) Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and US Oncology. J Clin Oncol 26:5755–5760
Davies AM, Chansky K, Lau DH et al (2006) Phase II study of consolidation paclitaxel after concurrent chemoradiation in poor-risk stage III non-small-cell lung cancer: SWOG S9712. J Clin Oncol 20;24(33):5242–5246
Yamamoto N, Goto K, Nishio M et al (2016) Erratum to: Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR, mutation-positive non-small-cell lung cancer. Int J Clin Oncol 1–9
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17(24):2815–2834
Williamson PR, Smith CT, Hutton JL et al (2002) Aggregate data meta-analysis with time-to-event outcomes. Stat Med 21(22):3337–3351
Tierney JF, Stewart LA, Ghersi D et al (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8(1):16
Mutlu H, Arslan D, Gündüz Ş et al (2014) The optimal treatment modality in patients with T4N2M0 non-small cell lung cancer: the best choice may be definitive chemoradiotherapy followed by consolidation chemotherapy. Chemotherapy 60:107–111
Ahn JS, Ahn YC, Kim JH et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-0414. J Clin Oncol 2015; 33:2660–2666
Liu L, Bi N, Ji Z et al (2015) Consolidation chemotherapy may improve survival for patients with locally advanced non-small-cell lung cancer receiving concurrent chemoradiotherapy—retrospective analysis of 203 cases. BMC Cancer 15:715
Jalal SI, Riggs HD, Melnyk A et al (2012) Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol 23:1730–1738
Jett JR, Scott WJ, Rivera MP et al (2003) Guidelines on treatment of stage IIIB non-small cell lung cancer. Chest 123:221S–225S
Furuse K, Fukuoka M, Kawahara M et al (1999) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17:2692–2699
Fournel P, Robinet G, Thomas P et al (2005) Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienned’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 23:5910–5917
Decker RH, Lynch TJ (2012) Unmet challenges in the use of novel agents in locally advanced non-small-cell lung cancer. J Clin Oncol 30:582–584
Bastos BR, Hatoum GF, Walker GR et al (2010) Efficacy and toxicity of chemoradiotherapy with carboplatin and irinotecan followed by consolidation docetaxel for unresectable stage III non-small cell lung cancer. J Thorac Oncol 5:533–539
Eroglu C, Orhan O, Unal D, et al. Concomitant chemoradiotherapy with docetaxel and cisplatin followed by consolidation chemotherapy in locally advanced unresectable non-small cell lung cancer. Ann Thorac Med 201;8(2): 109, 3
Gandara DR, Chansky K, Albain KS et al (2006) Long-term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage IIIB non-small-cell lung cancer: a phase II Southwest Oncology Group study (S9504). Clin Lung Cancer 8(2):116–121
Albain KS, Crowley JJ, Turrisi AT et al (2002) Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol 20(16):3454–3460
Horinouchi H, Sekine I, Sumi M et al (2013) Long-term results of concurrent chemoradiotherapy using cisplatin and vinorelbine for stage III non-small-cell lung cancer. Cancer Sci 104:93–97
Takigawa N, Kiura K, Segawa Y et al (2006) Second primary cancer in survivors following concurrent chemoradiation for locally advanced non-small-cell lung cancer. Br J Cancer 95:1142–1144
Acknowledgements
The present study was funded by The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology. The authors would like to thank the American Journal Experts Service Center for their excellent language editing service.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
About this article
Cite this article
Wang, X., Ding, X., Kong, D. et al. The effect of consolidation chemotherapy after concurrent chemoradiotherapy on the survival of patients with locally advanced non-small cell lung cancer: a meta-analysis. Int J Clin Oncol 22, 229–236 (2017). https://doi.org/10.1007/s10147-016-1074-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1074-x