Abstract
Background
Pleural effusion is one of the most common complications of lung adenocarcinoma and is diagnostically challenging. This study aimed to investigate the diagnostic performance of carcinoembryonic antigen (CEA), cytokeratin fragment (CYFRA) 21-1, and cancer antigen (CA) 19-9 for lung adenocarcinoma-associated malignant pleural effusion (MPE) through a validation study and meta-analysis.
Methods
Pleural effusion samples were collected from 81 lung adenocarcinoma-associated MPEs and 96 benign pleural effusions. CEA, CYFRA 21-1, and CA19-9 were measured by electrochemiluminescence immunoassay. The capacity of tumor markers was assessed with receiver operating characteristic curve analyses and the area under the curve (AUC) was calculated. Standard methods for meta-analysis of diagnostic studies were used to summarize the diagnostic performance of CEA, CYFRA 21-1, and CA19-9 for lung adenocarcinoma-associated MPE.
Results
The pleural levels of CEA, CYFRA 21-1, and CA19-9 were significantly increased in lung adenocarcinoma-associated MPE compared to benign pleural effusion. The cut-off points for CEA, CYFRA 21-1, and CA19-9 were optimally set at 4.55 ng/ml, 43.10 μg/ml, and 12.89 U/ml, and corresponding AUCs were 0.93, 0.85, and 0.81, respectively. The combination of CEA, CYFRA 21-1, and CA19-9 increased the sensitivity to 95.06%, with an AUC of 0.95. Eight studies were included in this meta-analysis. CEA showed the best diagnostic performance with pooled sensitivity, specificity, positive/negative likelihood ratio, and diagnostic odds ratio of 0.75, 0.96, 16.01, 0.23, and 81.49, respectively. The AUC was 0.93.
Conclusions
CEA, CYFRA 21-1, and CA19-9 play a role in the diagnosis of lung adenocarcinoma-associated MPE. The combination of these tumor markers increases the diagnostic accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant pleural effusion (MPE) is a common complication of lung cancer. It was reported that approximately 15% of lung cancer patients present with pleural effusion at the time of initial diagnosis, and up to 50% of patients would develop pleural effusion later in the course of their disease, especially for lung adenocarcinoma [1, 2]. Adenocarcinoma is the most common histological type of lung cancer; approximately half of lung cancer patients are diagnosed with adenocarcinoma, and the incidence of lung adenocarcinoma is rapidly increasing worldwide [3, 4]. Lung adenocarcinoma patients often present with MPE, and the detection of lung adenocarcinoma-associated MPE is important because the presence of pleural effusion means the patient has a poor prognosis as it is classified as stage IV disease [5, 6]. The poor prognosis may be attributable to diagnostic delay and lack of effective therapies. Therefore, there is an increasing need to discover biomarkers or to develop novel methods for the early diagnosis of lung adenocarcinoma-associated MPE.
The diagnosis of MPE remains a clinical challenge. Cytological examination of pleural effusions obtained through thoracentesis is a standard and non-invasive method for the diagnosis of MPE; however, it has a sensitivity of only 40–70% [7], and to identify the source of adenocarcinoma cells, further immunostaining examination may be needed [8]. Thoracoscopic pleural biopsy or image-guided percutaneous pleural biopsy can provide relatively high specificity, but such invasive procedures may not be available in all hospitals and may not be well tolerated by all patients [9]. Consequently, to establish the diagnosis of lung adenocarcinoma-associated MPE in a less-invasive way through tumor marker assays is of great interest to physicians. Classical tumor markers, such as carcinoembryonic antigen (CEA), cytokeratin fragment (CYFRA) 21-1, and cancer antigen (CA) 19-9 play a role in the identification of MPE [10, 11], while their roles in the diagnosis of lung adenocarcinoma-associated MPE have not been fully explained. To date, there is no consensus with regard to whether these tumor markers are effective at detecting lung adenocarcinoma-associated MPE. The present study aimed to investigate the roles of CEA, CYFRA 21-1, and CA19-9 in the diagnosis of lung adenocarcinoma-associated MPE through a validation study and meta-analysis.
Patients and methods
Patients
The present study was conducted based on the principles expressed in the Declaration of Helsinki. Ethical approval was obtained from the West China Hospital of Sichuan University Ethics Committee. All patients provided written informed consent for the collection of samples and subsequent analysis at admission.
Pleural effusion samples were collected from 177 consecutive patients presenting with pleural effusion who were admitted to our hospital between February 2011 and October 2012. Among them, there were 81 patients with lung adenocarcinoma. Lung adenocarcinoma was diagnosed by (1) cytological examination with the presence of adenocarcinoma cells and showed positive immunocytochemical staining for thyroid transcription factor-1 in MPE cell blocks; (2) tumors that were histologically diagnosed from primary adenocarcinomas of the lung through biopsy; (3) additional ultrasound or radiological examinations excluded the tumor from other places. Additionally, 96 benign pleural effusions (BPEs) were collected as controls.
Pleural effusion collection and tumor marker examination
All included subjects underwent a standard thoracocentesis procedure within 24 h after admission. All pleural effusion samples were collected and transported to the Department of Laboratory Medicine, West China Hospital within 30 min of collection. CEA, CYFRA 21-1, and CA 19-9 in pleural effusions were detected by an electrochemiluminescence immunoassay (Roche Cobas 8000 modular analyser series; Roche Diagnostics, USA). Serum levels of tumor markers were also detected in some of these patients with MPE. Generally, measurement of these tumor markers will be completed within 4 h. In addition, pleural total protein, glucose, and lactate dehydrogenase (LDH) levels were also measured. The laboratory studies were blinded to the etiologies of the pleural effusions.
Statistical analysis
Data were expressed as the mean ± SD. Differences in data were analyzed by the nonparametric Mann–Whitney U‑test. The receiver operating characteristic curves and areas under the curve (AUCs) were calculated to determine the overall diagnostic value of each marker in pleural fluid. The sensitivity, specificity, positive predictive value, and negative predictive value for diagnosing lung adenocarcinoma-associated MPE were also calculated. In addition, we evaluated the ability of a combination of two or all three tumor markers to distinguish lung adenocarcinoma-associated MPE from BPE. Correlations were performed using the bivariate Pearson’s correlation test. Statistical analysis was carried out using SPSS 18.0 software (Chicago, IL, USA). A value of p < 0.05 was considered significant.
Meta-analysis
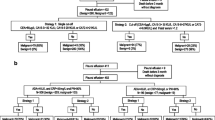
Standard methods recommended for meta-analyses of diagnostic accuracy studies were used [12]. To identify studies that evaluated the use of CEA, CYFRA 21-1, and CA 19-9 to diagnose lung adenocarcinoma-associated MPE, we searched in PubMed and EMBASE up to January 1, 2016. The search terms were ‘pleural effusion or pleural fluid or hydrothorax’, ‘carcinoembryonic antigen or CEA or cytokeratin fragment 21-1 or CYFRA 21-1 or cancer antigen 19-9 or CA 19-9’, ‘lung adenocarcinoma or pulmonary adenocarcinoma’ and ‘sensitivity or specificity or accuracy’. We also checked diagnostic studies on malignant pleural effusion to identify potential studies. Only diagnostic studies using these tumor markers for diagnosing lung adenocarcinoma-associated MPE on humans and published in the English language were included in the present meta-analysis. Two reviewers independently assessed the final set of articles and retrieved related data. The methodological quality of included studies was evaluated using the Quality Assessment for Studies of Diagnostic Accuracy [13].
The following measures of test accuracy, together with 95% confidence intervals (95% CIs) were calculated for each study—sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). The sensitivity and specificity for the single test threshold identified for each study was used to plot a summary receiver operating characteristic (SROC) curve. Spearman’s rank correlation was used to test for threshold effects. The heterogeneity effect was measured using the Q test and the inconsistency index (I2). A random-effects meta-analysis was carried out to take into account inter-study variability. Deeks’ funnel plots were used to detect potential publication bias. All meta-analyses were performed using two statistical software programs—Meta-DiSc for Windows (XI, Cochrane Colloquium, Barcelona, Spain) and Stata (version 12, Stata Corporation, College Station, TX, USA). All statistical tests were two-sided, and significance was set at p < 0.05.
Results
Patient characteristics
Of the 177 patients, 81 (40 male, 41 female, mean age 60 years) were diagnosed with lung adenocarcinoma-associated MPE. The 96 controls with BPE comprised 68 males and 28 females with a median age of 58 years. Therefore, the two groups did not differ significantly in terms of age or sex.
Of the 81 cases of lung adenocarcinoma-associated MPE, the cytology examination was positive in 26 cases (32.09%). Of the 96 patients with BPE in the control group, there were 46 cases of tuberculous pleural effusion, 22 cases of heart failure, 20 cases of parapneumonic effusion, four cases of hepatic pleural effusion and four cases of transudates without evidence of malignancy. The clinical characteristics and biochemical results of the study population are summarized in Table 1.
Levels of tumor markers and diagnostic accuracy
The median levels of CEA, CYFRA 21-1, and CA 19-9 were significantly higher in lung adenocarcinoma-MPE patients than in BPE controls (293.59 ± 397.89 vs 2.92 ± 15.65 ng/ml for CEA; 154.77 ± 174.49 vs 25.03 ± 40.98 μg/ml for CYFRA 21-1; and 127.00 ± 281.85 vs 5.83 ± 8.61 U/ml for CA19-9; all p values <0.001).
There were 81, 71 and 43 patients with paired serum/pleural effusion results for CEA, CYFRA 21-1, and CA 19-9 measurements, respectively. Pleural levels of CEA, CYFRA 21-1, and CA 19-9 were significantly higher than in corresponding serum levels (293.59 ± 397.89 vs 31.78 ± 58.43 ng/ml for CEA; 163.97 ± 179.51 vs 12.24 ± 17.20 μg/ml for CYFRA 21-1; and 219.81 ± 363.43 vs 21.39 ± 25.44 U/ml for CA19-9; all p values <0.001). Pleural levels of tumor markers correlated positively with serum levels of tumor markers (r = 0.365, p = 0.000 for CEA, r = 0.280, p = 0.018 for CYFRA 21-1, and r = 0.592, p = 0.000 for CA 19-9).
As shown in Fig. 1, CEA showed the best discriminate ability; at a cut-off value of 4.55 ng/ml, the sensitivity, specificity, positive predictive value, and negative predictive value were 83.95, 96.88, 95.77 and 90.56%, respectively, and the AUC was 0.93. The cut-off value, sensitivity, specificity, positive predictive value, negative predictive value, and AUC values of the three tumor markers are shown in Table 2. For the 55 patients diagnosed by histopathological examination, the AUCs of pleural CEA, CYFRA 21-1, and CA 19-9 were 0.928, 0.817 and 0.838, respectively.
Diagnostic performance of tumor marker combinations
Next, we investigated the diagnostic performance of different tumor marker combinations. Regarding the combination of two markers, the CEA and CYFRA 21-1 pair showed the best diagnostic accuracy with an AUC of 0.95, a sensitivity of 87.65%, and a specificity of 93.75%. When the three tumor markers were combined together, the sensitivity increased to 95.06%, specificity was 87.50%, and the AUC increased to 0.95 (Fig. 2).
Meta-analysis
Eight studies (including the present study) that evaluated the diagnostic accuracy of lung adenocarcinoma-MPE using pleural concentrations of CEA, CYFRA 21-1, and CA 19-9 were included in this meta-analysis [14–20]. All the lung adenocarcinoma-MPEs were diagnosed with the presence of adenocarcinoma cells in cytological or biopsy specimens, which is considered the gold standard for MPE studies. The QUADAS scores of included studies were all >10, suggesting the reliability of our results. The clinical summary and quality assessment of included studies are shown in Table 3.
CEA showed the highest diagnostic accuracy for lung adenocarcinoma-MPE with pooled sensitivity, specificity, PLR, NLR, and DOR of 0.75 (95% CI 0.71–0.79), 0.96 (95% CI 0.94–0.97), 16.01 (95% CI 8.95–28.65), 0.23 (95% CI 0.15–0.35), and 81.49 (95% CI 34.55–192.24), respectively. The AUC was 0.93, with a Q value of 0.86. The SROC curves of CEA, CYFRA 21-1, and CA 19-9 are shown in Fig. 3. The pooled data for each tumor marker are summarized in Table 4.
SROC curve of CEA, CYFRA 21-1, and CA 19-9 for the diagnosis of lung adenocarcinoma-associated malignant pleural effusion. The size of each solid circle represents the size of each study included in the present meta-analysis. The regression SROC curve indicates the overall diagnostic accuracy. a CEA, b CYFRA 21-1, c CA 19-9
Although we identified significant heterogeneity in the meta-analysis of all three tumor markers (data not shown), we did not perform a meta-regression analysis to investigate potential covariates due to the limited numbers of studies included. No publication biases were identified for the three tumor markers, and the p values of slope coefficient were 0.63, 0.52 and 0.15 for CEA, CYFRA 21-1, and CA 19-9, respectively.
Discussion
As lung adenocarcinoma is characterized by high invasiveness and metastasis capability, patients with lung adenocarcinoma are more likely to present pleural effusion [6, 21]. The early and accurate diagnosis of lung adenocarcinoma-associated MPE may play an important role in the management of patients, by guiding sequent targeted therapy. Our study carried out a clinical study and confirmed that pleural levels of CEA, CYFRA 21-1, and CA 19-9 were significantly increased in lung adenocarcinoma-associated MPEs, and further meta-analysis confirmed that these tumor markers may be useful for distinguishing lung adenocarcinoma-associated MPEs from BPEs.
CEA has been used as a tumor marker for MPEs for a long time. A recently published meta-analysis indicated that the overall sensitivity and specificity of CEA in the diagnosis of MPE were 54.9 and 96.2%, indicating good specificity but poor sensitivity [11]. In addition, CEA is also used to label lung adenocarcinoma cells in pleural effusion through immunocytochemical examinations [22]. In this study, CEA showed a sensitivity of 83.95% and a specificity of 96.88% in the diagnosis of lung adenocarcinoma-associated MPE, suggesting CEA may be more sensitive in the detection of lung adenocarcinoma in pleural effusion. Such results were consistent with other reports. In a report by Huang et al. using a cut-off value of 6 μg/l, the sensitivity and specificity of CEA in the diagnosis of lung adenocarcinoma-associated MPE were 87.8 and 96.8%, respectively [14]. When compared with other causes of MPE, the pleural level of CEA also plays a specific role in the identification of lung adenocarcinoma-associated MPE. In a retrospective study of 251 cases of MPE, lung adenocarcinoma-associated MPE showed the highest pleural levels of CEA than other causes of MPE, including lung squamous cell carcinoma, mesothelioma, small-cell lung cancer, lymphoma/leukemia, and breast cancer [23]. These findings support CEA as a sensitive tumor marker for lung adenocarcinoma-associated MPE.
In our meta-analysis, eight studies (522 cases and 523 controls) that examined the diagnostic accuracy of CEA for lung adenocarcinoma-associated MPE were included. The results showed that the pooled specificity of CEA was 0.96, also suggesting a low rate of misdiagnosis; however, the sensitivity was 0.75, suggesting a high rate of missed diagnoses (25%). Thus, the clinical utility of CEA to screen lung adenocarcinoma-associated MPE is limited. To improve the clinical applicability of these results, DOR, PLR/NLR, and AUC were generated. A DOR of 81.49 suggested a high diagnostic ability of CEA. The PLR of CEA was >10, suggesting that a positive test result for these antigens would indicate a relatively high chance of having lung adenocarcinoma-associated MPE. However, the NLR was 0.23, indicating that a negative CEA measurement result presents a 23% likelihood of being a false negative, which is not sufficiently low to rule out lung adenocarcinoma-associated MPE. The AUC was 0.93, suggesting a high overall diagnostic accuracy of CEA.
CYFRA 21-1 is a fragment of cytokeratin 19 which provides a useful marker for epithelial malignancies, distinctly reflecting ongoing cell activity. Accelerated CK19 degradation occurs in neoplastically transformed epithelial cells as a result of increased protease activity of caspase 3, a regulator of the apoptosis cascade, and fragments are released into circulation. Therefore, increased CYFRA 21-1 is recognized as typical tumor marker [24]. Studies have reported that the sensitivity of CYFRA 21-1 is higher in lung squamous cell carcinoma than in lung adenocarcinoma [25]. The diagnostic sensitivity of CYFRA 21-1 in this study containing only lung adenocarcinoma cases would be not expected to be too high. In this study, the sensitivity of CYFRA 21-1 was only 66.67%. CA 19-9 is an isolated Lewis antigen of the MUC1 protein. Serum CA19-9 measurement plays an important role in the diagnosis of pancreatic cancer [26]. Growing studies suggest that CA19-9 also plays a role in determining MPE with an overall sensitivity of 37.6%, and specificity of 98% [11]. Our study found that pleural CA19-9 levels were significantly increased in lung adenocarcinoma-associated MPE, although the sensitivity was only 60.49%. These results suggest that clinical value of CYFRA 21-1 and CA19-9 in the screening of lung adenocarcinoma-associated MPE is limited, and the interpretation of diagnostic results of CYFRA 21-1 and CA19-9 should be objective.
Examination of a combination of multiple tumor biomarkers to diagnose MPE has become an area of interest for clinicians [14, 16, 27]. In this study, the combination of CYFRA 21-1 or CA19-9 with CEA increases the diagnostic accuracy, and the CEA and CYFRA 21-1 pair showed the highest diagnostic accuracy, with an AUC of 0.95. The combination of the three tumor markers showed the highest sensitivity of 95.1%, with an AUC of 0.95, but the specificity was decreased at 87.5%. We suggest that the examination of combined tumor markers should be recommended in the identification of lung adenocarcinoma-associated MPE.
In clinical practice, tumor marker test results may not replace pathological evidence when establishing the diagnosis of lung adenocarcinoma-associated MPE. One of the aims of this study is to help clinicians decide when to obtain a cytological/histological specimen by invasive measures to investigate a possible diagnosis of malignancy if the tumor markers test results are positive and, more importantly, in patients with pseudo-negative cytology of their pleural effusions [16]. When the results suggest a high possibility of lung adenocarcinoma, more suitable management should be planned. Additionally, although increasing novel diagnostic markers or techniques are used for diagnosing MPE, such as microRNAs and CD66c [15, 19, 20], tumor markers like CEA, CYFRA 21-1, and CA 19-9 still have obvious advantages. These markers are currently used tumor markers for MPE that can be detected in most hospitals and are almost affordable for every patient. Other novel markers, however, still have a long way to go from bench to bedside.
There were several limitations in this study. First, this is a small-scale single-center study, and our meta-analysis only included eight studies. More prospective studies on a larger scale should be performed in multiple centers with different populations to validate our findings. We also observed that all included studies were performed in East Asia; however, studies should be performed to check whether there are ethnic differences among Western and Eastern countries. Additionally, we did not compare the pleural levels of tumor markers in lung adenocarcinoma-associated MPE with other causes of MPE. Further studies should be pay attention to discriminating lung adenocarcinoma-associated MPE from other causes of MPE.
Conclusion
Taken together, measurement of pleural CEA, CYFRA 21-1, and CA 19-9 levels plays a role in the diagnosis of lung adenocarcinoma-associated MPE. The combination of these tumor markers increases the diagnostic accuracy.
References
Agalioti T, Giannou AD, Stathopoulos GT (2015) Pleural involvement in lung cancer. J Thorac Dis 7:1021–1030
Thomas JM, Musani AI (2013) Malignant pleural effusions: a review. Clin Chest Med 34:459–471
Zugazagoitia J, Enguita AB, Nuñez JA et al (2014) The new IASLC/ATS/ERS lung adenocarcinoma classification from a clinical perspective: current concepts and future prospects. J Thorac Dis 6:S526–S536
Tang Y, He Z, Zhu Q et al (2014) The 2011 IASLC/ATS/ERS pulmonary adenocarcinoma classification: a landmark in personalized medicine for lung cancer management. J Thorac Dis 6:S589–S596
Kasapoglu US, Arınç S, Gungor S et al (2015) Prognostic factors affecting survival in non-small cell lung carcinoma patients with malignant pleural effusions. Clin Respir J. doi:10.1111/crj.12292
Wu SG, Yu CJ, Tsai MF et al (2013) Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J 41:1409–1418
Marel M, Stastny B, Melínová L et al (1995) Diagnosis of pleural effusions. Experience with clinical studies, 1986–1990. Chest 107:1598–1603
Porcel JM, Palma R, Bielsa S et al (2015) TTF-1 and napsin A on cell blocks and supernatants of pleural fluids for labeling malignant effusions. Respirology 20:831–833
Lombardi G, Zustovich F, Nicoletto MO et al (2010) Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol 33:420–423
Shi HZ, Liang QL, Jiang J et al (2008) Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology 13:518–527
Nguyen AH, Miller EJ, Wichman CS et al (2015) Diagnostic value of tumor antigens in malignant pleural effusion: a meta-analysis. Transl Res 166:432–439
Pang C, Wu Y, Wan C et al (2016) Accuracy of the bronchoalveolar lavage enzyme-linked immunospot assay for the diagnosis of pulmonary tuberculosis: a meta-analysis. Medicine (Baltimore) 95:e3183
Whiting PF, Weswood ME, Rutjes AW et al (2006) Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 6:9
Huang WW, Tsao SM, Lai CL et al (2010) Diagnostic value of Her-2/neu, Cyfra 21-1, and carcinoembryonic antigen levels in malignant pleural effusions of lung adenocarcinoma. Pathology 42:224–228
Han HS, Yun J, Lim SN et al (2013) Downregulation of cell-free miR-198 as a diagnostic biomarker for lung adenocarcinoma-associated malignant pleural effusion. Int J Cancer 133:645–652
Hsieh TC, Huang WW, Lai CL et al (2013) Diagnostic value of tumor markers in lung adenocarcinoma-associated cytologically negative pleural effusions. Cancer Cytopathol 121:483–488
Lv M, Mou Y, Wang P et al (2013) Diagnostic and predictive role of cell-free midkine in malignant pleural effusions. J Cancer Res Clin Oncol 139:543–549
Wang Y, Chen Z, Chen J et al (2013) The diagnostic value of apolipoprotein E in malignant pleural effusion associated with non-small cell lung cancer. Clin Chim Acta 421:230–235
Shin YM, Yun J, Lee OJ et al (2014) Diagnostic value of circulating extracellular miR-134, miR-185, and miR-22 levels in lung adenocarcinoma-associated malignant pleural effusion. Cancer Res Treat 46:178–185
Son SM, Han HS, An JY et al (2015) Diagnostic performance of CD66c in lung adenocarcinoma-associated malignant pleural effusion: comparison with CEA, CA 19-9, and CYFRA 21-1. Pathology 47:123–129
Botana-Rial M, De Chiara L, Valverde D et al (2012) Prognostic value of aberrant hypermethylation in pleural effusion of lung adenocarcinoma. Cancer Biol Ther 13:1436–1442
Wu GP, Zhang SS, Fang CQ et al (2008) Immunocytochemical panel for distinguishing carcinoma cells from reactive mesothelial cells in pleural effusions. Cytopathology 19:212–217
Wang XF, Wu YH, Wang MS et al (2014) CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause. Asian Pac J Cancer Prev 15:363–368
Wang YX, Hu D, Yan X (2013) Diagnostic accuracy of Cyfra 21-1 for head and neck squamous cell carcinoma: a meta-analysis. Eur Rev Med Pharmacol Sci 17:2383–2389
Ono A, Takahashi T, Mori K (2013) Prognostic impact of serum CYFRA 21-1 in patients with advanced lung adenocarcinoma: a retrospective study. BMC Cancer 13:354
Huang Z, Liu F (2014) Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: a meta-analysis. Tumour Biol 35:7459–7465
Antonangelo L, Sales RK, Corá AP et al (2015) Pleural fluid tumour markers in malignant pleural effusion with inconclusive cytologic results. Curr Oncol 22:e336–e341
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (81300032), and Projects in the Science and Technology Pillar Program from the Department of Science and Technology of Sichuan province (2015SZ0151). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no competing interests exist.
Additional information
M. Feng, J. Zhu, L. Liang contributed equally to this study.
About this article
Cite this article
Feng, M., Zhu, J., Liang, L. et al. Diagnostic value of tumor markers for lung adenocarcinoma-associated malignant pleural effusion: a validation study and meta-analysis. Int J Clin Oncol 22, 283–290 (2017). https://doi.org/10.1007/s10147-016-1073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1073-y