Abstract
Background
Inhibition of Notch by γ-secretase inhibitor (GSI) has been shown to have an antitumor effect in Notch-expressing non-small cell lung cancer (NSCLC) and to induce apoptosis through modulation of Bcl-2 family proteins. In particular, Bim, a BH3-only member of the Bcl-2 family of proteins, has an important role in the induction of apoptosis in NSCLC when cells are treated with GSI. ABT-737, a BH3-only mimetic, targets the pro-survival Bcl-2 family and also induces apoptosis.
Methods
The Notch-expressing NSCLC cell lines H460, A549, H1793, and HCC2429 were used. The combined antitumor effect of GSI and ABT-737 was evaluated using the MTT proliferation assay in vitro and in xenograft mouse models. The expression of the Notch pathway and Bcl-2 family was analyzed using Western blotting analysis when cells were treated with a single drug treatment or a combination treatment.
Results
GSI XX or ABT-737 alone inhibited cell proliferation in a dose-dependent manner, and combination drug treatment showed a synergistic antitumor effect in vitro. In vivo, this drug combination significantly suppressed tumor proliferation compared to the single drug treatment. Phospho-Bcl-2 was downregulated and Bax was upregulated by both the single and combination drug treatments. Bim was induced by a single drug treatment and was enhanced by combination treatment. Combination treatment-induced apoptosis was decreased by Bim inhibition, suggesting that the antitumor effect of the drug combination was dependent on Bim.
Conclusion
Based on our data, we propose that the combination treatment is a promising strategy for NSCLC therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common cause of malignancy-related death in the world, and despite advances in treatment, its incidence rate is still increasing [1, 2]. To improve the poor prognosis of patients with NSCLC, further studies that specifically examine the development of new therapeutic strategies are required.
The Notch receptor is a single-pass transmembrane protein that regulates cell-fate determination in multicellular organisms. There are four Notch receptors, which bind two families of ligands, Jagged and Delta-like, in mammals [3, 4]. Upon ligand binding, the Notch receptor undergoes a number of proteolytic cleavages. The final cleavage by the γ-secretase complex results in release of the Notch intracellular domain (NICD), which forms a nuclear complex with the transcription factor CSL (CBF, Sel, Lag-1) and induces the expression of target genes such as Hairy and enhancer of split (Hes) and Hairy/enhancer of split related with the YRPW (Hey) gene family [3, 4]. Several studies have highlighted the aberrant activation of Notch pathways in the tumorigenesis of many cancers [5–8]. We have reported that γ-secretase inhibitor (GSI) downregulates NICD expression and inhibits the growth of lung cancer both in vitro and in vivo [9].
The role of the Notch pathway in tumorigenesis is also related to the regulation of apoptosis. Deregulation of apoptosis has been demonstrated to lead to cancer development, proliferation, and treatment resistance [10, 11]. The mitochondria-mediated apoptotic pathway is largely regulated by Bcl-2 family proteins, with members of this family possessing at least one of four conserved motifs that are known as Bcl-2 homology domains (BH1–BH4) [12]. This Bcl-2 family has been divided into three subfamilies: (1) pro-survival family members, (2) pro-apoptotic family members that contain multiple Bcl-2 homology domains, and (3) BH3-only proteins such as Bim that exhibit sequence homology only in the BH3 domain [12–14]. We demonstrated that Notch3 modulates apoptosis in Notch-expressing NSCLC through Bcl-2 family proteins, especially through Bim [15].
ABT-737, a small-molecule BH3 domain mimetic that functions as a Bcl-2 inhibitor, has been shown to bind with high affinity to Bcl-2 and Bcl-xL, thereby freeing Bax or Bak to trigger permeabilization of the mitochondrial membrane and caspase-3 activation and subsequently cell death [16]. It has exhibited preclinical activity as a single agent in several cancer cells [17–19]. Although the combination of GSI and ABT-737 showed synergistic antitumor effects in multiple myeloma and breast cancer [20, 21], there have been no reports regarding the combined effect of GSI and ABT-737 in NSCLC. Because regulation of the Bcl-2 family is also important for the suppression of Notch-expressing NSCLC, in this study we evaluated the antitumor effect of the combination of GSI and ABT-737 in vitro and in vivo and its association with the Bcl-2 family in Notch-expressing NSCLC.
Materials and methods
Cell lines and inhibitors
The NSCLC cell lines H460, A549, H1793, and H1395 were obtained from the American Type Culture Collection. HCC2429 was established as previously described [22]. H1395 cells do not express Notch, whereas the other four cell lines have been shown previously to have high Notch3 expression [9]. Cell lines were maintained in RPMI supplemented with 10% fetal calf serum at 37°C in a humid environment in 5% CO2. γ-Secretase inhibitor XX (GSI XX) was obtained from Calbiochem (San Diego, CA, USA). ABT-737 (S1002) was obtained from Sellek Chemicals (Houston, TX, USA).
MTT proliferation assay
All cell lines were seeded into a 96-well plate at a density of 4000 cells per well and were incubated overnight. The cells were treated with increasing concentrations of GSI XX, ABT-737, or a combination of GSI XX and ABT-737 and then incubated for 4 days. The MTT assay was performed according to the manufacturer’s recommendation. In this assay, the absorption of light was determined at 560 nm using a microplate reader (Varioskan Flash; Thermo Fisher Scientific). IC50 data of the single treatment of each cell line were determined (Table 1). All experiments were performed in quintuplicate.
CI isobologram assessment of the combination of GSI XX and ABT-737
All cell lines were seeded into a 96-well plate at a density of 4000 cells per well and were incubated overnight. GSI XX and ABT-737 were administered to each cell line at a constant concentration ratio, which was determined by the IC50 data of the single drug treatment (Table 1). The MTT assay was performed, and the combined cytotoxicity of GSI XX and ABT-737 in each cell line was analyzed by a Fa-CI isobologram plot using the computer software Compusyn (Biosoft, Cambridge, UK); the Chou–Talalay method for the combination of two drugs based on the median effect equation has been previously described [23]. The concentration–effect curve for each drug alone was determined based on experimental observations using the median effect principle and was compared with the effect achieved with a combination of the two drugs to derive a combination index (CI) value. The CI value offers quantitative definitions for the additive effect (CI = 1), synergism (CI < 1), and antagonism (CI > 1) of drug combinations.
Antibodies and Western blotting analysis
H460 and HCC2429 cells were seeded at a density of 1.0 × 105 cells per well into a 6-well plate and incubated overnight. H460 cells were treated with control, or with 15 μM GSI XX and/or 7.5 μM ABT-737. HCC2429 cells were treated with control, or with 7.5 μM GSI XX and/or 1 μM ABT-737. The administered drug concentration was based on the IC50 dose for each cell line. Cells were harvested at 72 h after treatment. These harvested proteins were applied for Western blotting analysis. Notch3 was detected using a rabbit polyclonal antibody from Allele Biotechnology and Pharmaceuticals (ABP-PAB-10683; San Diego, CA, USA) at 1:500 dilution. Anti-pro-survival Bcl-2 family antibodies and polyclonal rabbit antibodies to phospho-Bcl-2 (2827), Bcl-2 (2870), Bcl-xL (2764), and Mcl-1 (4572) were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-pro-apoptotic Bcl-2 family antibodies, and polyclonal rabbit antibodies to phospho-Bad (5284), Bad (9239), Bax (5023), Bik (4592), BID (2002), Bak (6947), and Puma (4975) were obtained from Cell Signaling Technology. The polyclonal rabbit antibody to Bim (B7929) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The polyclonal rabbit antibody to Noxa was obtained from Biovision (Mountain View, CA, USA). The polyclonal rabbit antibody to PARP (9542) was purchased from Cell Signaling Technology. All analyses were stained with Ponceau S, and equal protein loading was confirmed.
Real-time RT-PCR
H460 and HCC2429 cells were seeded at a density of 1.0 × 105 cells per well into a 6-well plate and were incubated overnight. The cells were treated with control, or with GSI XX and/or ABT-737. At 72 h after treatment, total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. RNA was reverse transcribed into cDNA by using TaqMan reverse transcription reagents with random hexamers obtained from Applied Biosystems (Life Technologies, Carlsbad, CA, USA). Expression of Hey1 and GAPDH mRNA was determined by the quantitative real-time polymerase chain reaction (RT-PCR) with the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) according to the manufacturer’s instructions, using Hey1 and GAPDH reagents obtained from Applied Biosystems. The mean relative expression levels were compared to expression of an internal control (GAPDH). All PCR amplifications were performed using a MicroAmp optical 96-well reaction plate with a TaqMan Fast Universal PCR Master Mix and with the TaqMan Gene Expression Assay kit (Applied Biosystems).
siRNA transfection
H460 and HCC2429 were seeded at a density of 1.0 × 105 cells per well into a 6-well plate the day before transfection. The Bim siRNA sequence was ON-TARGET plus SMART obtained from Thermo Fisher Scientific. Cells were transfected with 100 pmol siRNA in Opti-MEM medium (Invitrogen) using 100 pmol lipofectamine 2000 (Invitrogen). Medium was exchanged after 6 h and the cells were then incubated overnight. Cells were treated with control, or with GSI XX (H460, 15 μM; HCC2429, 7.5 μM) and/or ABT-737 (H460, 7.5 μM; HCC2429, 1 μM) and were harvested at 48 h after treatment. To confirm the efficiency of siRNA transfection and assess the apoptosis, Bim protein expression and PARP expression with or without treatment were measured at 72 h after transfection by Western blotting analysis. Nonspecific siRNAs against the target sequence, ON-TARGET plus Non-targeting pool, were used as controls.
Apoptosis analysis
H460 and HCC2429 cells were seeded at a density of 1.0 × 105 cells per well into a 6-well plate, incubated overnight, and subsequently treated with control or with GSI XX (H460, 15 μM; HCC2429, 7.5 μM) and/or ABT-737 (H460, 7.5 μM; HCC2429, 1 μM). The cells were incubated for 48 h and then stained with FITC-conjugated Annexin V and propidium iodide (PI), using the Annexin V-FITC Apoptosis Detection kit (Calbiochem). The percentage of apoptotic cells (Annexin V-positive and PI-negative population) was determined using flow cytometry (BD FACSCalibur; Becton, Dickinson, Frankin Lakes, NJ, USA).
In vivo tumorigenicity
All animal husbandry and experiments were performed under protocols approved by the Institutional Animal Care Committee at Hokkaido University School of Medicine. H460 and HCC2429 cells (1.0 × 106 cells) were diluted in 100 μl phosphate-buffered saline (PBS) and injected subcutaneously into the right posterior leg of athymic, 5-week-old, female nude mice (nu+/nu+). When the tumors were palpable, the mice were randomly assigned to one of four groups: control group, the GSI XX or the ABT-737 single drug treatment group, or the combination of GSI XX and ABT-737 group. Each group consisted of eight mice. In GSI XX and/or ABT-737 treatment, 200 μg/kg GSI XX was administered intraperitoneally (i.p.) on days 1, 2, 3, and 8, 9, 10, as previously described [9] and/or 100 μg/kg ABT-737 was administered by i.p. on days 1 and 14, as previously described [20]. The tumors were then measured every 2 days using digital calipers. Tumor volume (TV) was determined using the formula TV = (length) × (width) × (height)/2 [24]. Tumor growth rate (%TV) on day X was calculated as (TV on day X/TV on day 1) × 100, as previously described [9].
Statistical analysis
Statistical analysis was carried out using Microsoft Excel software. Significance between the control and observation arms of both in vitro and in vivo assays was determined using analysis of variance (ANOVA). Statistical significance was established at p < 0.05.
Results
Combined antitumor effect of GSI XX and ABT-737 on Notch-expressing lung cancer cell lines
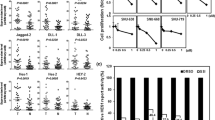
To investigate the antitumor effect of GSI XX, ABT-737, and combination therapy, we used the MTT assay to measure cell viability following drug treatment of four lung cancer cell lines (H460, HCC2429, H1793, A549), which were previously shown to have high Notch3 expression [9]. GSI XX or ABT-737 alone inhibited the growth of the lung cancer cells in a dose-dependent manner (Fig. 1a, b). The IC50 of GSI XX or ABT-737 varied according to the lung cancer cell line (Table 1). The lung cancer cells were then treated with a combination of GSI XX and ABT-737 at a constant ratio, with doses determined based on the IC50 of the single treatment, and the combined antitumor effect was evaluated. The combination index was less than 1 for all four cell lines, indicating that combination therapy showed a synergistic antitumor effect (Fig. 2a). On the other hand, H1395 cells, which do not express Notch, showed resistance to GSI XX (Table 1). Moreover, combination index following treatment of H1395 cells with a combination of GSI XX and ABT-737 was about 1, which suggested an additive, but not a synergistic, antitumor effect of this combination (Fig. 2b). To determine the antitumor effect of combination treatment in vivo, we utilized xenograft models. Treatment with GSI XX or ABT-737 alone inhibited tumor growth compared with control. Furthermore, the combination treatment of GSI XX and ABT-737 significantly delayed tumor growth compared with either single agent alone (Fig. 2c). No body weight loss was observed (data not shown), indicating that all treatments were well tolerated.
GSI XX or ABT-737 inhibited cell proliferation in a dose-dependent manner. The cells were treated with increasing concentrations of control or GSI XX and/or ABT-737. Treated cells were then incubated for 4 days. The MTT assay was performed and IC50 data of a single treatment of each cell line were determined. a IC50 of GSI XX in Notch-expressing NSCLC cell lines and H1395 Notch-negative cell line. GSI XX inhibited the growth of NSCLC in a dose dependent manner. *p < 0.05 vs. control. b IC50 of ABT-737 in Notch-expressing NSCLC cell lines. ABT-737 inhibited the growth of NSCLC cells in a dose-dependent manner. *p < 0.05 vs. control
The combination of GSI XX and ABT-737 showed a synergistic antitumor effect in vitro and inhibition of tumor proliferation in vivo. a The Fa-CI plot of isobologram analysis of GSI XX and ABT-737 treatment in four Notch-expressing lung cancer cell lines. The CI value was less than 1, indicating a synergistic effect. b The Fa-CI plot of isobologram analysis of GSI XX and ABT-737 treatment of H1395, a Notch-negative lung cancer cell line. For H1395, the CI value was around 1, which suggested an additive, but not a synergistic, antitumor effect of this combination. c H460 and HCC2429 cells (1.0 × 106 cells) were injected subcutaneously into the right posterior leg of athymic, 5-week-old, female nude mice (nu+/nu+). In GSI XX and/or ABT-737 treatment, 200 μg/kg GSI XX was administered intraperitoneally (i.p.) on days 1, 2, 3, and 8, 9, 10, and/or 100 μg/kg ABT-737 was administered by i.p. on days 1 and 14. The combination of GSI XX and ABT-737 significantly inhibited the growth of Notch-expressing lung cancer compared to control or single treatment in in vivo xenograft models (n = 8)
Combination of GSI XX and ABT-737 modulates the expression of Bcl-2 family proteins
We previously showed that Notch inhibition by GSI induced apoptosis through Bcl-2 family proteins [15]. ABT-737 also modulates Bcl-2-related proteins and induces apoptosis [26]. We first used Western blotting to evaluate the expression of the Bcl-2 family in H460 and HCC2429 cells treated with GSI XX, ABT-737 alone, or the combination treatment. The expression of the pro-survival Bcl-2 proteins phospho-Bcl-2 and BclxL was suppressed by single or combination drug treatment (Fig. 3a). Conversely, the expression of the pro-apoptotic protein BAK was upregulated by treatment (Fig. 3b). As we had previously observed that the BH3-only protein, Bim, was induced and was necessary for induction of apoptosis by Notch inhibition [15], the expression of BH3-only proteins was also evaluated following single or combination drug treatment. Bim was upregulated in both cell lines by singe drug treatment, and this upregulation was enhanced by the combination drug treatment (Fig. 3c). Although Noxa was reportedly induced by Notch inhibition [20, 21], the expression of Noxa and that of other BH3-only proteins were not affected by any treatment (Fig. 3c; Supplementary Fig. 1). We next evaluated the expression of Notch following single or combination drug treatment. NICD3 and Hey1 were downregulated by GSI, as we reported previously (Fig. 4a, b) [9]. NICD suppression seems to be attenuated slightly in the combination compared to GSI XX monotherapy in H460 (Fig. 4a). Although there were no reports whether ABT-737 affected Notch expression, ABT-737 might have some influence on Notch expression. However, no difference of Hey-1 mRNA suppression between GSI and the combination was seen (Fig. 4b), indicating that the attenuation of NICD suppression by combination treatment in H460 did not have as much impact on the Notch pathway downregulation.
Bcl-2 protein expression was regulated by treatment. H460 cells were treated with control, or with 15 μM GSI XX and/or 7.5 μM ABT-737. HCC2429 cells were treated with control, or with 7.5 μM GSI XX and/or 1 μM ABT-737. The administered drug concentration was based on the IC50 dose for each cell line. Cells were harvested at 72 h after treatment. a The protein expression of prosurvival Bcl-2 members was analyzed in H460 and HCC2429 cells by Western blotting. The expression of phospho-Bcl-2 and Bcl-xL was downregulated by treatment, but the expression of Mcl-1 was not affected. b The protein expression of proapoptotic Bcl-2 family members was evaluated by Western blotting. BAX expression was upregulated by treatment. c Expression of the BH3-only proteins, Bim and Noxa, was evaluated by Western blotting. Bim expression was induced by single treatment and enhanced by combination treatment. The expression of Noxa was unchanged by drug treatment
NICD3 and Hey-1 were suppressed by GSI XX or combination. The NSCLC cell lines were treated as in Fig. 2. a The expression of NICD3 was analyzed by Western blotting. NICD3 expression was inhibited by GSI XX or combination of both cell lines. b The mRNA of Hey-1 was analyzed using real-time RT-PCR. Hey-1 mRNA was suppressed by GSI XX or combination in both cell lines. Results are the mean ± SD. *p < 0.05 vs. control
Bim is necessary for the apoptosis induced by drug treatment
Because GSI or ABT-737 was reported to induce apoptosis through Bcl-2-related proteins, we evaluated the expression of poly-ADP ribose polymerase (PARP) and the percentage of apoptotic cells following the single or combination drug treatment. Cleaved PARP was induced by single drug treatment and enhanced by combination drug treatment (Fig. 5a). Moreover, apoptosis was induced by single drug treatment and increased by combination drug treatment (Fig. 5b). We previously reported that induction of apoptosis by Notch3 inhibition depends on Bim [15]. Therefore, to evaluate whether Bim was important in the antitumor effect of the combination of GSI XX and ABT-737, we used Bim siRNA to suppress Bim expression in H460 and HCC2429 cells. The treatment-induced upregulation of Bim was mitigated by Bim siRNA (Fig. 6a). The loss of Bim abrogated the induction of PARP in the cells treated with the single or combination drug treatment (Fig. 6a). Furthermore, induction of apoptosis by the single or combination drug treatment was impaired by inhibition of Bim expression (Fig. 6b).
Combination of GSI XX and ABT-737 enhanced apoptosis. H460 and HCC2429 cells were treated with control, or with GSI XX (H460, 15 μM; HCC2429, 7.5 μM) and/or ABT-737 (H460, 7.5 μM; HCC2429, 1 μM). and were incubated for 48 h. Then cells were assayed by Western blotting of PARP (a) and were stained with FITC-conjugated Annexin V and propidium iodide (PI) (b). a Combination therapy increased the expression of cleaved PARP compared to single treatment. b Combination therapy induced apoptosis in a significantly higher number of cells compared to single treatment
Combination-induced apoptosis was dependent on Bim. H460 and HCC2429 were were transfected with 100 pmol siRNA in Opti-MEM medium using 100 pmol Lipofectamine 2000. Cells were treated with control, or with GSI XX (H460, 15 μM; HCC2429, 7.5 μM) and/or ABT-737 (H460, 7.5 μM; HCC2429, 1 μM) and were harvested at 48 h after treatment. Bim protein expression and PARP with or without treatment was measured at 72 h after transfection by Western blotting analysis (a). Cells were stained with FITC-conjugated Annexin V and propidium iodide (PI), (b). a The inhibition of Bim expression by Bim siRNA abrogated the elevated expression of cleaved PARP that was induced by combination. b Combination-induced apoptosis was impaired by Bim siRNA
Discussion
In the current study, we showed that the combination therapy of GSI XX and ABT-737 enhanced the inhibition of tumor growth of Notch-expressing NSCLC cells both in vitro and in vivo compared with a single drug treatment. Treatment with the combination modulated the expression of both pro-survival Bcl-2 family proteins and proapoptotic Bcl-2 family proteins. Furthermore, the combined antitumor effect was dependent on Bim, which has previously been shown to operate in tumor suppression and initiation of apoptosis [11, 12]. These data provide a rationale for modulating Bcl-2 family proteins and inducing apoptosis in Notch-expressing NSCLC, suggesting that this combined treatment might be a promising new therapy.
In our data, Notch inhibition induced Bim upregulation and phosphor Bcl-2 upregulation. Bim is regulated by the ERK/MAPK pathway and is required to mediate epithelial growth factor receptor (EGFR) inhibitor-induced apoptosis in lung cancer cells [25]. Bim can bind and neutralize pro-apoptotic Bcl-2 family members, such as Bcl-2, which inhibit pro-survival Bcl-2 family members such as Bax and Bak [12–14]. Moreover, it has already been shown that the Notch pathway cross-talked with the MAPK pathway [26]. We revealed that loss of Notch activity resulted in the downregulation of phospho-ERK and the upregulation of Bim [9, 15]. We also showed that loss of Notch and EGFR inhibition induced Bim significantly compared to Notch suppression only [15]. Those data suggest that Notch modulates Bim through the EGFR pathway and upregulation of Bim by Notch inhibition inhibited Bcl-2.
GSI is not specific for Notch and is known to mediate the proteolysis of several transmembrane proteins, including amyloid precursor protein, E-cadherin, CD44, and ErbB4 [27–29]. We have already shown that the antitumor effect of GSI, as MRK-003 (Merck), was relatively Notch3 dependent, using Notch3 siRNA stable clones and transient transfection [9]. Moreover, we have previously demonstrated that the combined antitumor effect of GSI and radiation was effective only in Notch-expressing cell lines, because the Notch-negative cell line, H1395, showed no combined antitumor effect [29]. In this study, we found that H1395 displayed resistance to GSI XX and no synergistic antitumor effect, following treatment with the combination of GSI XX and ABT-737, indicating that the combined antitumor effect was dependent on the Notch pathway.
ABT-737 binds to Bcl-2 and Bcl-xL, thereby functioning as an antagonist, and disrupts Bcl-2/BAX association [16–19]. In this study, we showed that ABT-737 induced Bim and that the combination treatment enhanced Bim expression, which suggested that ABT-737 not only participated in the apoptotic signaling pathway through inhibition of the function of Bcl-2/Bcl-xL, but that it could also enhance apoptosis induced by Bim. It was reported that ABT-737 upregulates Bim expression through the activation of JNK/c-Jun signaling pathways in erythroid cells and HeLa cells [30, 31]. Although we have not investigated the association between Bim and the JNK pathway and there have been no reports that Bim induced by ABT-737 was dependent on the JNK pathway in NSCLC, the JNK pathway might be involved in the enhancement of apoptosis observed in this study. Further study is warranted to establish this point.
Although ABT-737 shows high affinity for Bcl-2 and Bcl-xL, it exhibits low affinity for Mcl-1 [16]. Consistent with the low affinity of ABT-737 for Mcl-1, tumor cells that overexpress Mcl-1 are resistant to ABT-737, and neutralization of Mcl-1 increases drug sensitivity [32, 33]. In multiple myeloma cells and breast cancer cells, addition of GSI upregulates the expression of the BH3-only protein, Noxa, which is a potent regulator of Mcl-1 and enhances sensitivity of the cells to ABT-737 [20, 21]. However, the mechanism of GSI-induced Noxa upregulation is unclear. We previously showed that Notch inhibition results in downregulation of the MAPK pathway, which modulates Bim, and that following GSI administration, Bim induction was observed but Noxa expression was not affected [15]. Consistent with our former data, in this study the addition of GSI XX had no impact on the expression of Noxa and Mcl-1 and increased apoptosis through modulation of Bcl2/BclxL and Bim. The function of Notch has been reported to be context dependent and complicated. For example, although Notch3 is activated and promotes the proliferation of NSCLC [9, 15], Notch1 suppresses the growth of SCLC cell lines [34, 35]. Thus, the origin of the tumor and the various types of Notch pathway regulation affected by GSI might account for the difference in activation of Bcl-2 subfamily proteins between different types of malignant diseases.
The current study is the first to clarify the combined antitumor effect of GSI and ABT-737 in Notch-expressing NSCLC. The combination therapy of GSI XX and ABT-737 showed greater antitumor effect than single drug therapy against Notch-expressing NSCLC both in vitro and in vivo, indicating that this therapy might be a new strategy for lung cancer therapy.
References
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Allenspach EJ, Maillard I, Aster JC et al (2002) Notch signaling in cancer. Cancer Biol Ther 1:466–476
Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194:237–255
Das I, Craig C, Funahashi Y et al (2004) Notch oncoproteins depend on gamma-secretase/presenilin activity for processing and function. J Biol Chem 279:30771–33080
Curry CL, Reed LL, Broude E et al (2007) Notch inhibition in Kaposi’s sarcoma tumor cells leads to mitotic catastrophe through nuclear factor-kappaB signaling. Mol Cancer Ther 6:1983–1992
Duechler M, Shehata M, Schwarzmeier JD et al (2005) Induction of apoptosis by proteasome inhibitors in B-CLL cells is associated with downregulation of CD23 and inactivation of Notch2. Leukemia 19:260–267
Reedijk M, Odorcic S, Chang L et al (2005) High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 65:8530–8537
Konishi J, Kawaguchi KS, Vo H et al (2007) Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res 67:8051–8057
Hengatner MO (2000) The biochemistry of apoptosis. Nature (Lond) 407:770–776
Cory S, Huang DC, Adamas JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590–8607
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337
Petros AM, Olejniczak ET, Fesik SW (2004) Structural biology of the Bcl-2 family of proteins. Biochem Biophys Acta 1644:83–94
Willis SN, Fletcher JI, Kaufmann T et al (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315:856–859
Konishi J, Yi F, Chen X et al (2010) Notch3 cooperates with the EGFR pathway to modulate apoptosis through the induction of Bim. Oncogene 29:589–596
Oltersdorf T, Elmore SW, Shoemaker AR et al (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature (Lond) 435:677–681
Chauhan D, Velankar M, Brahmandam M et al (2007) A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene 26:2374–2380
Tahir SK, Yang X, Anderson MG et al (2007) Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res 67:1176–1183
Tagscherer KE, Fassl A, Campos B et al (2008) Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene 27:6646–6656
Li M, Chen F, Clifton N et al (2010) Combined inhibition of Notch signaling and Bcl-2/Bcl-xL results in synergistic antimyeloma effect. Mol Cancer Ther 9:3200–3209
Séveno C, Loussouarn D, Bréchet S et al (2012) γ-Secretase inhibition promotes cell death, Noxa upregulation, and sensitization to BH3 mimetic ABT-737 in human breast cancer cells. Breast Cancer Res 14:R96
Dang TP, Gazdar AF, Virmani AK et al (2000) Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst 92:1355–1357
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res 70:440–446
Dalrymple S, Antony L, Xu Y et al (2005) Role of Notch-1 and E-cadherin in the differential response to calcium in culturing normal versus malignant prostate cells. Cancer Res 65:9269–9279
Costa DB, Halmos B, Kumar A et al (2007) BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PloS Med 10:1669–1679
Yoo AS, Bais C, Greenwald I (2004) Crosstalk between the EGFR and LIN-12/Notch pathways in C. elegans vulval development. Science 303:663–666
Lammich S, Okochi M, Takeda M et al (2002) Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J Biol Chem 277:44754–44759
Ni CY, Murphy MP, Golde TE et al (2001) Gamma-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294:2179–2181
Mizugaki H, Sakakibara-Konishi J, Ikezawa Y et al (2012) γ-Secretase inhibitor enhances antitumour effect of radiation in Notch-expressing lung cancer. Br J Cancer 106:1953–1959
Will B, Siddiqi T, Jordà MA et al (2010) Apoptosis induced by JAK2 inhibition is mediated by Bim and enhanced by the BH3 mimetic ABT-737 in JAK2 mutant human erythroid cells. Blood 115:2901–2919
Wang H, Yang YB, Shen HM et al (2012) ABT-737 induces Bim expression via JNK signaling pathway and its effect on the radiation sensitivity of HeLa cells. PLoS One 7:e52483
Goldsmith KC, Gross M, Peirce S et al (2012) Mitochondrial Bcl-2 family dynamics define therapy response and resistance in neuroblastoma. Cancer Res 72:2565–2577
Lucas KM, Mohana-Kumaran N, Lau D et al (2012) Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res 18:783–795
Sriuranpong V, Borges MW, Ravi RK et al (2001) Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 61:3200–3205
Hassan WA, Yoshida R, Kudoh S et al (2014) Notch1 controls cell invasion and metastasis in small cell lung carcinoma cell lines. Lung Cancer 86:304–310
Acknowledgements
We thank M. Maeda (First Department of Medicine, Hokkaido University School of Medicine) for providing special support and experimental assistance during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2016_1060_MOESM1_ESM.pptx
Supplementary Fig. S1: The expression of BH3-only proteins in control or drug-treated NSCLC cell lines was evaluated by Western blotting. No significant change in expression was observed between control and treated cells. The expression of Bim and Noxa is presented in Fig. 3C. (PPTX 266 kb)
About this article
Cite this article
Sakakibara-Konishi, J., Ikezawa, Y., Oizumi, S. et al. Combined antitumor effect of γ-secretase inhibitor and ABT-737 in Notch-expressing non-small cell lung cancer. Int J Clin Oncol 22, 257–268 (2017). https://doi.org/10.1007/s10147-016-1060-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1060-3