Abstract
Background
To treat local recurrence of brain metastases after gamma knife radiosurgery (GKS), we have used fractionated stereotactic radiotherapy (SRT). The purpose of this study was to evaluate the efficacy and toxicity of SRT in these patients.
Methods
Fifty locally recurrent metastatic brain tumors in 47 patients were treated with SRT. The median prescribed dose of GKS was 20 Gy at the periphery. The median interval between the GKS (the last session in cases in which multiple GKS procedures were performed) and recurrence was 7.5 (range 1–33) months. Several dose-fractionation protocols were used for SRT, depending on the size and location of the tumor and previous GKS dose. The median prescribed dose of the SRT at the isocenter was 30 Gy with a median of ten fractions.
Results
Among the 50 lesions treated with SRT, 26 did not recur locally before the patient’s death or the last follow-up examination, and 24 recurred locally. The median follow-up period for the surviving patients was 24 months after the first GKS procedure, and the overall survival rate was 80% at 1 year and 57% at 2 years. The median time to local re-recurrence after the SRT (16 months) was significantly longer than the median interval between the last GKS and recurrence (7.5 months; P < 0.001). Only two patients developed ≥grade 2 radiation necrosis.
Conclusions
Stereotactic radiotherapy appeared to be an effective treatment for recurrent metastatic brain tumors and yielded relatively good local control. The associated adverse events were generally acceptable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gamma knife radiosurgery (GKS) is an established primary treatment for solitary or oligometastatic brain tumors [1–4], allowing multiple metastatic brain tumors to be treated in a single session. However, local recurrence of the tumor(s) after GKS is not uncommon, and the management of local recurrence after GKS poses a problem due to the normal tissue tolerance limits of the brain to radiation [5]. A second GKS is occasionally employed for short-term palliation, and although whole-brain radiotherapy may be indicated, especially when recurrent tumors are multiple, attending physicians often fear late adverse effects, such as dementia and brain atrophy [6, 7]. Consequently, the attending physicians may be hesitant to initiate whole-brain radiation therapy when there is no other tumor besides local recurrence. On the other hand, the recently published results from a phase III randomized trial [Japan Clinical Oncology Group (JCOG) 0504] demonstrate that there were no significant differences in the 1-year Mini-Mental State Examination (MMSE) scores and performance status (PS) between postoperative patients treated by stereotactic radiosurgery and those treated by whole-brain radiotherapy and that intracranial control rates were higher in the whole-brain-treated group [8]. Thus, although the optimal salvage therapy for recurrent tumors after initial radiosurgical treatment may remain open to discussion, we postulated that fractionated stereotactic radiotherapy (SRT) might be an option for metastatic brain tumors that recur after a single high-dose GKS.
Our institution treats more than 500 patients with brain metastases using GKS each year, and we encounter local recurrence after GKS relatively frequently. Since the installation of a modern linear accelerator at our institution in 2008, we have used SRT rather than GKS to treat patients who fulfill the criteria outlined in the Materials and methods. We chose to use SRT as a second-line treatment because we considered that it would cause fewer and less severe toxicities and have a greater biological effect than single-fraction GKS and because such tumors are often large and/or located in close proximity to critical structures. The purpose of this study was to evaluate the efficacy and toxicity of SRT for locally recurrent brain metastases that had previously been treated with GKS.
Materials and methods
Patient characteristics
This retrospective study was approved by the institutional review board of Fujieda Heisei Memorial Hospital (No. 26-1). Informed consent had been obtained from all patients prior to initiation of SRT. From October 2008 to December 2013, 1238 patients were treated with GKS for brain metastases at our institution. Although about 30% of the patients were not followed, local recurrence of brain metastases was identified in 156 patients. Among these, 27 were not re-irradiated, and 82 were treated with a second GKS for local recurrence. The remaining 47 patients with a total of 50 recurrent metastatic brain tumors that had developed after the GKS were treated with SRT, and it is this patient cohort that is the focus of present study. The choice of GKS or SRT for re-irradiation was mainly at the discretion of the attending neurosurgeons, but tumors existing near critical structures, such as the brain stem and optic apparatus, and larger tumors were more likely be treated with SRT. There appeared to be no difference in the patient characteristics, such as age, gender, and PS, between the GKS- and SRT-treated patients, but patients with extensive extracranial metastases tended to be treated with GKS.
Of the 47 patients, 20 were male and 27 were female. At the time of the SRT, the median age of the patient cohort was 61 (range 40–85) years. Tumor diameter ranged from 14 to 63 (median 32) mm. Tumor recurrence was first suspected based on the patients’ magnetic resonance imaging (MRI) findings [9, 10]. Since functional imaging techniques, such as positron emission tomography (PET), are considered to be useful diagnostic tools for distinguishing metastatic lesions from necrosis [11], we used 18F-fluorodeoxyglucose (FDG)-PET or 11C-methionine-PET (methionine-PET) to diagnose local recurrence in all suspected cases, with positive findings observed on all of the images. Figure 1 shows typical images of FDG-PET and methionine-PET; apparent uptake of the isotopes was observed in the contrast-enhanced regions on MRI. Of the 50 tumors which were imaged, 13 had been treated twice with GKS, and one had been treated 3 times with GKS. None of the patients had received whole-brain radiotherapy before the SRT. The characteristics of the patients and tumors are summarized in Table 1.
Gamma knife treatment
Gamma knife surgery has been described previously [12, 13]. Briefly, each patient underwent a contrast-enhanced MRI examination for treatment planning (SIGNA EXCITE XL 1.5 T scanner; GE Healthcare, Milwaukee, WI) and a Leksell frame attached to the head under local anesthesia. The treatment planning was performed using Leksell GammaPlan (Elekta AB, Stockholm, Sweden). The planning target volume (PTV) was defined as the contrast-enhanced tumor region plus a 1-mm margin. The GKS was performed with a Leksell gamma knife unit (Model C; Elekta AB). The median prescribed dose was 20 Gy at the periphery (the 50% isodose line). Of the 50 tumors, seven were subjected to two GKS sessions, which were performed 2 months apart. The median PTV was 10.3 (range 0.4–72.5) cm3, and the median interval between the GKS (the last session in the cases involving multiple GKS procedures) and recurrence was 7.5 (range 1–33) months.
Stereotactic radiotherapy method
The SRT was performed with a linear accelerator (Synergy S, Elekta AB) equipped with 4-mm multileaf collimators. The treatment planning was carried out using the Pinnacle3 Treatment Planning System (Philips NV, Amsterdam, The Netherlands). The PTV was defined as the gross tumor volume plus 2-mm margins in the lateral and anteroposterior directions and a 3-mm margin in the craniocaudal direction. Several dose-fractionation protocols were used for the SRT, depending on the size and location of the tumor and previous GKS dose. The median prescribed dose for SRT at the isocenter was 30 (range 20–36) Gy, and a median of ten fractions were administered (range 6–12). The median D95 (dose that covers 95% of the PTV) was 94.2% (range 71.0–97.5%) of the isocenter dose. The median V90 (PTV volume that received ≥90% of the prescribed dose) was 98.6% (range 69.1–100%). The median PTV for the SRT was 28.8 (range 7.1–103) cm3, which was significantly larger than the median PTV for the GKS (median 10.4 cm3; range 0.4–72.5; P < 0.0001). Detailed information about the GKS and SRT is summarized in Table 2. Biological effective doses (BED) for α/β = 10 Gy (BED10) are shown for convenience. However, it has been reported that the standard BED10 values for single-fraction treatment are incorrect [14] and very recently suggested that the BED10 values for GKS would reflect the true biological effects of the procedure more accurately if they were reduced by ≥20% [15].
Evaluation
All of the patients were scheduled to undergo contrast-enhanced MRI or computed tomography before the SRT and every 2–4 months after the SRT, although regular follow-up was occasionally not possible in patients with poor health. Local re-recurrence was suspected when enlargement of the contrast enhancement of the irradiated region (>20% in the longest diameter compared with that before SRT) was detected on MRI or CT images. Shrinkage of the tumors was observed in most cases after the initial GKS. Slight enlargement of the shrunken contrast-enhanced mass was followed for a short period to time because radiation-induced changes are more likely to develop due to repeat irradiation, and when the contrast-enhanced mass became larger than the initial size, further imaging evaluation was performed. Suspected re-recurrent lesions were identified using the same MRI criteria as were used to detect the initial recurrent lesions [11]. Re-recurrence was definitively diagnosed based on strong uptake of radioactivity on FDG- or methionine-PET (standardized uptake value: >5) or the findings of surgery or biopsy. Time to local re-recurrence was evaluated based on the imaging follow-up. Toxicities were evaluated using the Common Terminology Criteria for Adverse Events Version 4.0 (National Institutes of Health/National Cancer Institute, Bethesda, MD).
Statistical analysis
Differences in patient or tumor characteristics between the groups were examined using the Chi-square test or t test. Overall survival was calculated from the start of the first GKS and also from the start of SRT using the Kaplan–Meier method. The log-rank test was used to compare survival curves. The time to local recurrence was calculated from the last GKS or SRT. Fine and Gray’s competing risks regression model was used to estimate and compare cumulating incidences of local recurrence, thereby considering patient death as a competing risk. All statistical analyses were carried out using R version 2.13.0 for Windows (The R Foundation for Statistical Computing, Vienna, Austria). P values of <0.05 were defined as significant.
Results
Of the 47 patients included in this retrospective analysis, 12 were alive and 35 were dead at the time of the study. In total, 14 patients died of systemic disease, 15 patients died due to the progression of brain lesions, two patients died of other diseases, and the cause of death was unclear in four patients. Among the 15 patients who died of brain lesions, nine died as a result of re-recurrence after the SRT, three died of other new brain metastases, and three patients died of both causes. After the first GKS, the median follow-up period for all patients was 24 (range 3–83) months, and the median survival time was 27 months. The overall survival rates after the first GKS were 80% at 1 year and 57% at 2 years (Fig. 2). After the SRT, the median follow-up period for all patients was 10 (range 1–40) months, the median survival time was 12 months, and the 1-year overall survival rate was 50%. The 36 tumors in 34 patients were primarily salvaged by SRT. In this group, the median survival time was 27 months after the first GKS, and the median survival time was 13 months after the SRT. The other 14 tumors in 13 patients had been first treated by GKS for local recurrence. This group of patients had a median survival time of 35.5 months after the initial GKS and 11 months after the SRT. There were no differences between these two groups.
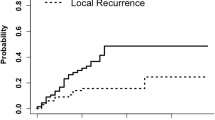
The median imaging follow-up period using MRI or CT after the SRT was 7 months for all patients (range 1–28 months). Of the 50 lesions that were treated with SRT, 26 did not recur prior to the patient’s death or the last follow-up examination, and the remaining 24 recurred. Local re-recurrence was confirmed by surgery or biopsy in seven patients. The cumulative incidence of local recurrence after SRT was 15% at 6 months and 37% at 1 year, and the median time to local recurrence was 16 months. On the other hand, the local recurrence rate after the last GKS was 44% at 6 months and 78% at 1 year, and the median time to local recurrence was 7.5 months. The median time to re-recurrence after the SRT (16 months) was significantly longer than the median interval between the last GKS and recurrence (P < 0.001) (Fig. 3). In the group primarily salvaged by SRT, the median time to local re-recurrence was 6.5 months after GKS and 16 months after SRT. In the group primarily salvaged by GKS, the median time to local re-recurrence was 10 months after the last GKS and 14 months after SRT. There were no differences between the two groups.
Cumulative incidences of local recurrence after GKS and SRT. GKS indicates the last session of GKS in the cases in which multiple GKS procedures were performed. The median time to local re-recurrence after SRT was significantly longer than the median interval between the last GKS and recurrence (P < 0.001)
Performance status scores were maintained or became higher than the pretreatment values until recurrence or last follow-up in 21 of the 47 patients (45%). Symptomatic radiation-induced edema was noted in 24 patients (49%) at 1–12 (median 4) months after the SRT. Corticosteroids were used temporarily by 22 patients and for >4 months by 2 patients; four patients died during the period of corticosteroid administration. Eight patients developed grade ≥2 radiation necrosis at 3–17 (median 5) months, and two patients underwent surgery for necrosis. These patients had no apparent risk factor with respect to the tumor size and radiation dose for developing brain necrosis. One patient undergoing surgery for necrosis was administered corticosteroid therapy for >4 months.
Discussion
Re-irradiation of central nervous system (CNS) tumors has long been considered to be contraindicated based on the belief that normal brain tissue would likely not be able to recover from the tissue damage caused by the radiation. More recently, however, it has been shown that re-irradiation of previously irradiated regions does not necessarily result in an excessive risk of late radiation damage, leading to re-irradiation protocols being used with increasing frequency [16]. Nevertheless, radiation oncologists remain reluctant to re-irradiate the CNS using conventional radiotherapy techniques. Localized irradiation, to the contrary, appears to be an acceptable alternative as it seems to exhibit an acceptable toxicity profile. One approach to localized irradiation is intraoperative radiotherapy. Shibamoto et al. [17] obtained durable remission after tumor resection and intraoperative radiation in patients with ependymoma, anaplastic ependymoma, or anaplastic oligodendroglioma. Stereotactic radiosurgery and SRT are increasingly being used as treatments for recurrent CNS tumors [16]. The accumulation of clinical data is expected to prove the safety and efficacy of such approaches.
A second GKS is occasionally performed to treat locally recurrent brain metastases after the initial GKS. The median survival time of patients undergoing a second GKS has been reported to range from 15 to 22.4 months from the first GKS [18–20]. The median overall survival time after the first GKS of the patients in our study was 27 months, which compares favorably with that reported in previous studies in which second GKS was performed for locally recurrent brain metastases. We divided our patients into two groups, one treated primarily by SRT for local recurrence and the other treated first by GKS and then by SRT, and we found no differences between the two groups in terms of prognosis after SRT. This result may suggest the usefulness of SRT even after repeat GKS for local recurrence. The relatively favorable survival outcomes in our patients may partly derive from our exclusion of patients with extensive extracranial metastases. Of the 35 patients who died in our study, 15 (42%) died due to uncontrolled brain metastases. Although this rate is similar to that previously reported by Penny et al. [11], the frequency of neurogenic death after first-line GKS was reported to be 6–10% in a recent large prospective study [21]. This difference indicates that our patients may have been selected for secondary treatment for local recurrence after GKS because their extracranial metastases were not extensive. Nevertheless, the favorable survival outcomes obtained in our study were derived, at least in part, from the use of SRT for re-treatment.
Although this study focused on second-line treatment for tumor recurrence, the median time to local re-recurrence after the SRT (16 months) was significantly longer than the median interval between the last GKS and recurrence (7.5 months). The 1-year local recurrence rate was 37% whereas it was 78% after the GKS. Since the second-line treatment yielded better results than the first-line treatment, one may question whether the diagnosis of local recurrence was correct (none of the recurrent masses were subjected to biopsy examinations before the SRT). We carefully followed all of the patients in this study, and a review of their follow-up data suggested that the diagnosis of recurrence was correct in all cases. Namely, contrast-enhanced masses shrunk in response to the SRT in the majority of cases, and the shrunken masses remained stable or enlarged thereafter. One of the possible reasons for the longer interval to local re-recurrence may be our policy of diagnosing re-recurrence. As stated in the Materials and methods, we did not diagnose re-recurrence even when the contrast-enhanced image of the shrunken mass showed enlargement because it was difficult to distinguish between re-recurrence and radiation-induced changes. We performed a further evaluation only after the contrast-enhanced image became larger than the size of pre-SRT mass. Because of this, the time to local re-recurrence might have been elongated by a few months.
Several authors have obtained encouraging clinical results when using SRT for the treatment of large brain tumors [22]. Investigators have recently also used fractionated GKS to treat large brain metastases [23, 24]. In our study, the median PTV for the SRT was 28.8 cm3, which was significantly larger than the median PTV for the GKS (10.4 cm3), and we used SRT. Large tumors may contain more hypoxic cells, which are resistant to radiation [25, 26]; consequently, the reoxygenation of such cells may be important during the treatment of recurrent metastatic brain tumors [27, 28]. SRT has radiobiological advantages over GKS because the reoxygenation of hypoxic tumor cells and the redistribution of the cell cycle to a more sensitive phase are expected to occur between fractions in SRT [29]. Since the backgrounds of the patients in our study who underwent SRT for local recurrence did not seem to differ from those of the patients undergoing GKS for local recurrence, the radiobiological aspect may be important in explaining the better outcome in our study. Thus, the re-treatment of recurrent tumors with SRT was effective and yielded relatively good local control. GKS may be suitable to treat small local recurrences owing to the high conformity of such recurrences, and SRT should be considered especially for cases involving large locally recurrent lesions.
The results of this study suggest the potential usefulness of fractionated radiotherapy for recurrent brain metastases. Since the doses we used are similar to those employed in whole-brain radiotherapy, the relative merits of SRT compared with whole-brain radiation remain controversial and should be investigated in future studies. It is possible that whole-brain radiotherapy may achieve comparable results. In a Chubu Radiation Oncology Group study, brain atrophy developed after whole-brain radiation in up to 30% of patients, but it was not necessarily accompanied by a decline in the MMSE score, and dementia after whole-brain radiotherapy unaccompanied by tumor recurrence was infrequent [7]. Coupled with the findings obtained in the JCOG 0504 study [8], the neurocognitive decline after whole-brain radiation may develop after 2 years. So, the possibility of developing clinically significant decline in cognitive function is considered to be small in the re-irradiation setting compared with the initial treatment because of the relatively short expected survival period. The risk of cognitive dysfunction may not be a major problem in most of the current cohort of patients with poor life expectancy. On the other hand, incidences of grade 2–4 adverse events were higher in the whole-brain-treated group than in the radiosurgery group in the JCOG 0504 study [8], whereas intracranial disease control was better with whole-brain radiotherapy, and a new brain lesion was the cause of death in six of 15 patients who died of brain lesions. To summarize, each approach has both merits and demerits.
The adverse events experienced by the patients in our study were generally acceptable. Symptomatic radiation-induced edema was noted in 24 patients, but in most cases it was controllable with corticosteroids. GKS for large brain metastases (maximum diameter ≥3 cm) can lead to severe neurotoxicity, and it is recommended that the marginal dose should be reduced [2, 30]. However, SRT allows the delivery of a higher radiation dose and causes fewer adverse events than GKS [6].
Conclusion
The results of our study suggest the potential usefulness of salvage SRT in the treatment of locally recurrent brain metastases after GKS. However, the question of whether the salvage SRT approach is superior to the whole-brain radiotherapy approach remains open to discussion. At present, both approaches may be possible options, and further studies are necessary.
References
Flickinger JC, Kondziolka D, Lunsford LD et al (1994) A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys 28:797–802
Kida Y, Kobayashi T, Tanaka T (1995) Radiosurgery of the metastatic brain tumours with gamma-knife. Acta Neurochir Suppl 63:89–94
Shibamoto Y, Sugie C, Iwata H (2009) Radiotherapy for metastatic brain tumors. Int J Clin Oncol 14:281–288
Serizawa T (2009) Radiosurgery for metastatic brain tumors. Int J Clin Oncol 14:289–298
Hazuka MB, Kinzie JJ (1988) Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys 15:433–437
Gaspar LE, Mehta MP, Patchell RA et al (2010) The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:17–32
Shibamoto Y, Baba F, Oda K et al (2008) Incidence of brain atrophy and decline in mini-mental state examination score after whole-brain radiotherapy in patients with brain metastases: a prospective study. Int J Radiat Oncol Biol Phys 72:1168–1173
Kayama T, Sato S, Sakurada K et al (2016) JCOG0504: A phase III randomized trial of surgery with whole brain radiation therapy versus surgery with salvage stereotactic radiosurgery in patients with 1 to 4 brain metastases. J Clin Oncol 34 suppl; abstr 2003
Raimbault A, Cazals X, Lauvin MA et al (2014) Radionecrosis of malignant glioma and cerebral metastasis: a diagnostic challenge in MRI Diagn Interv. Imaging 95:985–1000
Reddy K, Westerly D, Chen C (2013) MRI patterns of T1 enhancing radiation necrosis versus tumour recurrence in high-grade gliomas. J Med Imaging Radiat Oncol 57:349–355
Penny KS, Norbert K, James LR (2014) Brain metastases and neoplastic meningitis. In: Niederhuber JE (ed) Abeloff’s clinical oncology, 5th edn. Elsevier, Amsterdam, pp 725–738.e4
Mori Y, Kobayashi T, Shibamoto Y (2006) Stereotactic radiosurgery for metastatic tumors in the pituitary gland and the cavernous sinus. J Neurosurg 105:37–42
Serizawa T, Higuchi Y, Ono J et al (2006) Gamma knife surgery for metastatic brain tumors without prophylactic whole-brain radiotherapy: results in 1000 consecutive cases. J Neurosurg 105:86–90
Miyakawa A, Shibamoto Y, Otsuka S et al (2014) Applicability of the linear-quadratic model to single and fractionated radiotherapy schedules: an experimental study. J Radiat Res 55:451–454
Shibamoto Y, Miyakawa A, Otsuka S et al (2016) Radiobiology of hypofractionated stereotactic radiotherapy: what are the optimal fractionation schedules? J Radiat Res 57(Suppl 1):i76–i82
Mario A, Charles SC, Mark EL et al (2010) The role of retreatment in the management of recurrent/progressive brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:85–96
Shibamoto Y, Yamashita J, Takahashi M et al (1994) Intraoperative radiation therapy for brain tumors with emphasis on retreatment for recurrence following full-dose external beam irradiation. Am J Clin Oncol 17:396–399
Kwon KY, Kong DS, Lee JI et al (2007) Outcome of repeated radiosurgery for recurrent metastatic brain tumors. Clin Nerol Neurosurg 109:132–137
Shuto T, Fujino H, Inomori S et al (2004) Repeated gamma knife radiosurgery for multiple metastatic brain tumours. Acta Neurochir (Wein) 146:989–993
Yamanaka K, Iwai Y, Yasui T et al (1999) Gamma Knife radiosurgery for metastatic brain tumor: the usefulness of repeated Gamma Knife radiosurgery for recurrent cases. Stereotact Funct Neurosurg 72[Suppl 1]:73–80
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395
Murai T, Ogino H, Manabe Y et al (2014) Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol (R Coll Radiol) 26:151–158
Higuchi Y, Serizawa T, Nagano O et al (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74:1543–1548
Yomo S, Hayashi M, Nicholson C (2012) A prospective pilot study of two-session Gamma Knife surgery for large metastatic brain tumors. J Neurooncol 109:159–165
Rampling R, Cruickshank G, Lewis AD et al (1994) Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys 29:427–431
Shibamoto Y, Yukawa Y, Tsutsui K et al (1986) Variation in the hypoxic fraction among mouse tumors of different types, sizes, and sites. Jpn J Cancer Res 77:908–915
Murata R, Shibamoto Y, Sasai K et al (1996) Reoxygenation after single irradiation in rodent tumors of different types and sizes. Int J Radiat Oncol Biol Phys 34:859–865
Chang WS, Mi-Sook K, Chinsoo Cho L et al (2014) Radiobiological basis of SBRT and SRS. Int J Clin Oncol 19:570–578
Shibamoto Y, Hashizume C, Baba F et al (2012) Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer. A multicenter study. Cancer 118:2078–2084
Engenhart R, Kimmig BN, Höver KH et al (1993) Long-term follow-up for brain metastases treated by percutaneous stereotactic single high-dose irradiation. Cancer 71:1353–1356
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Miyakawa, A., Shibamoto, Y., Takemoto, S. et al. Fractionated stereotactic radiotherapy for metastatic brain tumors that recurred after gamma knife radiosurgery results in acceptable toxicity and favorable local control. Int J Clin Oncol 22, 250–256 (2017). https://doi.org/10.1007/s10147-016-1058-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1058-x