Abstract
Background
A retrospective, multi-institutional collaborative study was conducted to evaluate the impact of second transurethral resection (TUR) on the clinical outcome of non-muscle invasive high-grade bladder cancer and to identify predictors of invasion to the lamina propria (pT1) or deeper and residual tumor at the second TUR.
Methods
The clinical and pathological features of 198 patients with non-muscle invasive high-grade bladder cancer treated in five medical institutions from April 1990 to March 2013 were reviewed retrospectively. All patients underwent a second TUR within a mean of 1.5 months after the first resection. Clinicopathological findings of the first and second TURs were compared. Cancer-specific survival and recurrence-free survival were evaluated. Univariate and multivariate analyses for predictors of residual cancer at the second TUR were performed using a logistic regression model.
Results
At the second TUR, no tumor was found in 111 (56 %) patients, and 87 (44 %) had residual cancer. At the first TUR, five pT1 patients (3 %) were upstaged to pT2, one pTa patient (1 %) was upstaged to pT1, and 12 G2 patients (6 %) had their tumor upgraded to G3. Patients the group with less than stage pT1 cancer at the second TUR had significantly better survival than those in the group with stage pT1 or deeper cancer. Tumor multiplicity at the first resection was an independent risk factor for pT1 or deeper tumor at the second TUR.
Conclusion
A second TUR is a valuable diagnostic procedure for accurate staging of non-muscle invasive high-grade bladder cancer. Tumor multiplicity at the first TUR was a significant independent predictor of pT1 or deeper tumor at the second TUR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is the seventh most common cancer in men and the 17th most common in women worldwide [1–3]. Non-muscle invasive disease confined to the mucosa [TNM stage Ta or carcinoma in situ (CIS)] or lamina propria (T1) is seen in approximately 75 % of patients with bladder cancer at presentation [4]. Among patients with non-muscle invasive bladder cancer (NMIBC) at the time of presentation, approximately 70 and 20 % of the bladder cancers involve TNM stage Ta and T1 tumors, respectively, and 10 % have CIS lesions [5]. TUR of bladder tumors (TUR-BT) is performed on patiens with Ta and T1 bladder cancers and CIS, and all endoscopically visible lesions are removed. However, approximately 50 % of patients show residual tumors at the second TUR performed 2–6 weeks after the first TUR in patients diagnosed with T1 bladder cancer [6–9], and 10–25 % of these patients show MIBC at the second TUR [6–8]. A meta-analysis of TUR-BT in non-muscle invasive urothelial bladder cancers noted that residual tumors were observed in 47 % of patients and that 24 % had up-staging at the second TUR [9]. Therefore, it has been strongly recommended by various guidelines that a second TUR should be performed for patients with newly diagnosed high-grade T1 bladder cancer within 6 weeks after the first TUR [4, 10–13].
A long-term study of high-risk NMIBC cases showed progression in 53 % and cancer death in 34 % of cases [14]. Most T1 bladder cancers are high grade and have the potential to progress to muscle invasion and extravesical dissemination, leading to metastasis and death [15, 16]. Progression of NMIBC has been defined by the International Bladder Cancer Group as an increase in the T stage, not only as the development of T2 or greater, but also as an increase in the T stage from CIS or Ta to T1 [15].
Several substaging systems have been proposed in an attempt to stratify risk among T1 bladder cancers, and many studies have reported predictors for progression and/or survival, but these substaging systems are not in wide use in clinical practice [17–19]. One of the main reasons for this lack of widespread use is that consistent, accurate assessment of TUR-BT tissue for the actual depth of invasion is difficult due to orientation and artificial changes [20, 21]. A new substaging system developed to discern T1m and T1e bladder cancers is showing promise as a means to provide accurate and reliable information on progression and survival; however, it still needs to be validated [22].
Endoscopic resection of all visible tumors, possibly followed by adjuvant intravesical therapy, is the standard treatment for non-muscle invasive (Ta, T1) urothelial carcinoma of the bladder. However, the progression and prognosis of T1 bladder cancers after the second TUR are heterogeneous. Some tumors may progress to muscle invasive or metastatic disease, a progression which needs to be taken into account when the treating physician is determining whether aggressive treatment, such as radical cystectomy, is warranted. Alternatively, some tumors are completely resected at the first TUR, implying that aggressive post-TUR treatment could be considered excessive. Intravesical bacillus Calmette–Guérin (BCG) therapy is the current standard treatment for patients with high-grade T1 bladder cancer who have pT0 histology at the second TUR, but evidence on whether these patients require additional intravesical therapy is lacking [23]. Thus, for patients with T1 bladder cancer, the pathological findings of the second TUR specimen should be taken into account when determining optimal management and treatment.
The patients with non-muscle invasive high-grade bladder cancer who were enrolled in the retrospective, multi-institutional collaborative study reported here routinely underwent a second TUR, regardless of the pathological grade of the cancer, multiplicity, or recurrence factor. The presence and location of previously undetected residual disease, changes to the histopathological staging/grading, and therapeutic changes that were determined from this second TUR were evaluated. Furthermore, predictors of pT1 or deeper and residual tumor at the second TUR were identified.
Patients and methods
Study population
The clinical and pathological features of 198 patients diagnosed with T1 or Ta high-grade bladder cancer in five medical institutions from April 1990 to March 2013 were reviewed retrospectively. All resections were performed by experienced urologists. After the first resection, the surgeon documented the location, size, and number of tumors on a specially designed bladder map. A second TUR was performed for any residual tumor that was unexpected and/or scar of the first resection within a mean of 1.5 months after the first TUR. The TURs followed a fully standardized protocol, and the bladder was thoroughly examined endoscopically with a 30° lens. The location, number, and size of bladder tumors were recorded on a cystoscopy diagram. Hot-loop TUR of all visible tumors was then performed, including the muscle layer, and several deep muscle samples were also taken from the tumor base. During the second TUR, all visible tumors and previous resection scar or edematous areas from initial biopsy sites were resected, with the aim of completely resecting all tumors. Deep muscle specimens were taken, with an emphasis on the previous tumor areas. No intravesical therapy was given following the first TUR, regardless of the histological results of this TUR. Patients who underwent narrow-band imaging and blue-light cystoscopy were excluded. When the results of the histopathological evaluation of these specimens were available, the final treatment strategy was discussed with the patients. Pathological staging was determined according to the 2009 TNM staging system [24], with invasion to the lamina propria classified as pT1. Grading was determined according to the 1973 World Health Organization histological classification of tumors [25].
Statistical analysis
Statistical significance was determined using the Mann–Whitney U test, Student’s t test, Kruskal–Wallis test, log-rank test, and simple regression analysis, as appropriate. Survival curves were created using the Kaplan–Meier method with the log-rank test used to test differences. Multivariate stepwise logistic regression analysis with a Cox proportional hazards model was used to identify significant independent predictors for residual tumor at the second TUR. All statistical tests were two-sided, and a p value of <0.05 was considered to be significant. Relative risks and 95 % confidence intervals were also derived. All statistical analyses were performed using SPSS version 11.0 (IBM Corp., Armonk, NY).
Results
A total of 198 patients [167 (84 %) male, 31 (16 %) female; mean age 67.3 years, range 41–87 years] were included in the study, with a median follow-up period of 23.8 ± 16.0 (range 0–81.6) months. Table 1 shows the clinical characteristics of the enrolled patients at the first TUR. There was a previous history of bladder cancer in 36 patients (18 %). Pathological stage Tis or a, and T1 tumors were present in 26 (13 %) and 172 (87 %) patients, respectively, with concomitant CIS in 36 patients (18 %). Tumor grades G2, G3, and unknown were present in 72 (36 %), 121 (61 %), and 5 (3 %) patients, respectively. Residual tumors were detected at the second TUR in 87 of the 198 (44 %) patients. In five pT1 patients at the first TUR (3 %), the disease was upstaged to pT2; in one of the pTa patients at the first TUR (1 %), the disease was upstaged to pT1; in 12 G2 patients at the first TUR (6 %), tumor grade was upgraded to G3. Table 2 shows the treatment episodes following the second TUR according to tumor stage. Immediate postoperative treatments (after second TUR) consisted of intravesical BCG therapy (156 patients, 79 %), intravesical chemotherapy with mitomycin C (2 patients, 2 %), and radical cystectomy (9 patients, 4.5 %). Of the nine patients who underwent radical cystectomy, three received cisplatin-based systemic chemotherapy before the surgery, and one patient received cisplatin-based systemic chemotherapy immediately thereafter. Five patients (2.5 %) underwent radical cystectomy after intravesical BCG therapy, and one patient received radiation therapy after intravesical BCG therapy. Twenty-four patients received with no additional treatment after the second TUR.
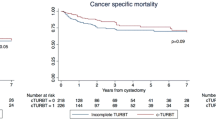
During follow-up, disease progression occurred in 15 patients (8 %), and five patients (3 %) died of bladder cancer. Cancer-specific survival (CSS) and recurrence-free survival (RFS) were estimated using the Kaplan–Meier method. CSS at 5 years was 92.6 %. In a subsequent analysis the patients were divided into two groups according to cancer stage at the second TUR: stage pT1 or deeper, or less than stage pT1. Patients in the group with less than stage pT1 tumors at the second TUR (n = 168) had a significantly better survival rate than those in group with stage pT1 or deeper tumors (n = 30) (p < 0.05) (Fig. 1a). On the other hand, there was no significant difference in CSS according to the presence or absence of immediate intravesical BCG therapy after the second TUR (data not shown). The period of RFS was defined as the time from the second TUR to intravesical recurrence. Thirty-six patients (18 %) developed intravesical recurrence after the second TUR. Pathological stage pT0, pTis or a, and T1 tumors were present in 16 (45 %), eight (22 %), and 12 (33 %) patients at the second TUR, respectively, and three (8 %) patients had concomitant CIS. Intravesical BCG therapy was performed in 25 patients (69 %) with intravesical recurrence after the second TUR. A comparison of patients who received and did not receive intravesical BCG therapy immediately after the second TUR revealed that the group of patients who received immediate intravesical BCG therapy (n = 162) had a significantly better recurrence-free rate than the group who did not (n = 31) (p = 0.0153) (data for 5 patients were not available for analysis).
We used both univariate and multivariate analyses to identify parameters predicting a pT1 or deeper tumor at the second TUR among the 198 patients (Table 3). On univariate analyses, tumor multiplicity, tumor grade, and concomitant CIS at the first TUR were significant predictors of a stage pT1 or deeper tumor at the second TUR (p < 0.05). On multivariate analysis, tumor multiplicity at the first TUR was a significant independent predictor of a stage pT1 or deeper tumor at the second TUR (p < 0.05). We also used both univariate and multivariate analyses to identify parameters predicting residual tumor at the second TUR among the 198 patients (data not shown). On univariate analyses, tumor multiplicity, tumor grade, and concomitant CIS at the first TUR were significant predictors of residual tumor at the second TUR (p < 0.05). On multivariate analysis, tumor multiplicity and concomitant CIS at the first TUR were significant independent predictors of residual tumor at the second TUR (p < 0.05). Concomitant CIS at the first TUR was a particularly significant independent predictor of residual tumor at the second TUR (p < 0.005).
Discussion
A second TUR is performed when a high-grade, T1, or possibly Ta tumor is detected at the first TUR. Divrik et al. reported that a second TUR was very beneficial in preventing progression, but it is unclear whether these authors conducted an appropriate evaluation of the muscle layer [8]. Overall, 33–55 % of patients show persistent disease after resection of T1 tumors, with about 40 % showing persistent disease after resection of TaG3 tumors [26, 27]. These cases are especially critical because the resection included no muscularis propria [6]. Moreover, the tumor is frequently understaged at the first TUR. The rate of muscle invasive disease at the second TUR of a first T1 tumor ranges from 4 to 25 %, increasing to 45 % if there was no muscle in the first TUR [28]. In our study, there was muscle invasive disease in approximately 3 % of cases at the second TUR. The benefit of the second TUR may derive from resection of a definite muscle layer, leading to correct staging and treatment [29].
Intravesical BCG therapy reduces the risk of intravesical recurrence of NMIBC. In our study, about 80 % of patients received immediate postoperative intravesical BCG therapy after the second TUR. However, depending on the depth of invasion and tumor grade, intravesical therapy may be recommended based on the estimated probability of recurrence and progression to a more advanced, usually muscle invasive stage, and progression should be considered independently [10]. For patients with stage Ta and T1 bladder cancer who do not undergo a second TUR or receive intravesical BCG therapy, risk factors for intravesical recurrence include multiplicity, size of tumor, prior recurrence rate, and presence of concomitant CIS [30]. Furthermore, Segal et al. reported that tumor location was the major risk factor for recurrence in patients with high-grade T1 bladder cancer who did not undergo a routine second TUR [31]. In our study, the presence of concomitant CIS and number of tumors at the first TUR were risk factors for residual tumors at the second TUR. The same tendency was shown in a previous study that included patients who underwent a second TUR [30]. These data indicate that, in patients with risk factors, the first TUR might be insufficient for complete resection of bladder tumors. As previously discussed, even when urologists believe they have achieved complete resection by TUR, no muscle layer is found in some specimens. After an apparently complete first TUR, a second TUR is recommended in patients with high-risk NMIBC to improve staging accuracy and resection of residual tumor [4, 8]. Furthermore, the second TUR can increase recurrence-free survival [8, 26].
Additional treatments following the detection of pT1 disease again at the second TUR are controversial, particularly the timing of cystectomy. In our study, pT1 disease remained in the cystectomy specimens of only one patient who underwent immediate cystectomy. Five patients who were upstaged to pT2 from pT1 underwent radical cystectomy. In addition, progressive disease developed in six patients receiving intravesical BCG therapy intended to preserve the bladder, and they later underwent radical cystectomy. Dalbagni et al. reported that the 2-year probability of deferred cystectomy in patients having pT1 disease at the restaging TUR is as high as 28 % [32]. Based on these observations, these authors suggested that early cystectomy can offer a high potential for cure because patients with pT1 at the second TUR are at high risk for progression. On the other hand, they also reported that survival rates were not significantly different for patients who underwent immediate cystectomy compared to those who were maintained on surveillance with cystectomy deferred until required [32]. However, adequate intravesical BCG therapy still remains a reasonable option for selected patients, given the complications associated with radical cystectomy, chemotherapy, and radiation therapy. Our study provides the important clinical information that patients in the group with less than stage pT1 cancer at the second TUR had significantly better survival rates than those in the group with stage pT1 or deeper cancer, and that the number of tumor lesions at the first TUR was a significant independent predictor of pT1 or deeper tumor at the second TUR. In the future, we will develop a nomogram to predict pT1 or deeper tumor at the second TUR in cases of NMIBC.
In conclusion, high-grade T1 bladder cancer is considered to be invasive and have the potential to progress to muscle invasive or metastatic disease. A second TUR is recommended in patients with high-risk NMIBC to improve staging accuracy and resection of residual tumor. Therefore, high-quality TUR (for both the first and second TURs) is required, and it may result in a good prognosis. In particular, tumor multiplicity at the first TUR was found to be a significant independent predictor of stage pT1 or deeper tumor at the second TUR. If residual T1 cancer is found at the second TUR, aggressive treatment based on the pathological findings should be considered. Further investigation is necessary to identify appropriate treatment for high-grade T1 NMIBC patients.
References
Kakehi Y, Hirao Y, Kim WJ et al (2010) Bladder Cancer Working Group report. Jpn J Clin Oncol 40:57–64
Kitamura H, Kakehi Y (2015) Treatment and management of high-grade T1 bladder cancer: what should we do after second TUR? Jpn J Clin Oncol 45:315–322
Burger M, Catto JW, Dalbagni G et al (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63:234–241
Babjuk M, Burger M, Zigeuner R et al (2013) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 64:639–653
Kirkali Z, Chan T, Manoharan M et al (2005) Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 66:4–34
Herr HW (1999) The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 162:74–76
Schips L, Augustin H, Zigeuner RE et al (2002) Is repeated transurethral resection justified in patients with newly diagnosed superficial bladder cancer? Urology 59:220–223
Divrik RT, Sahin AF, Yildirim U et al (2010) Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomized clinical trial. Eur Urol 58:185–190
Vianello A, Costantini E, Del Zingaro M et al (2011) Repeated white light transurethral resection of the bladder in nonmuscle-invasive urothelial bladder cancers: systematic review and meta-analysis. J Endourol 25:1703–1712
National Comprehensive Cancer Network (NCCN) (2015) Clinical practice guidelines in oncology (NCCN Guidelines®). Bladder Cancer Version2. http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 2015
European Association of Urology (EAU) (2015) Oncology guidelines (EAU Guidelines®). Non-muscle-invasive Bladder Cancer (Ta, T1 and CIS) http://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-muscle-invasive-Bladder-Cancer-2015-v1.pdf. Accessed Mar 2015
Hall MC, Chang SS, Dalbagni G et al (2007) Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 178:2314–2330
Nieder AM, Brausi M, Lamm D et al (2005) Management of stage T1 tumors of the bladder: International Consensus Panel. Urology 66:108–125
Cookson MS, Herr HW, Zhang ZF et al (1997) The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 158:62–67
Kulkarni GS, Hakenberg OW, Gschwend JE et al (2010) An updated critical analysis of the treatment strategy for newly diagnosed high-grade T1 (previously T1G3) bladder cancer. Eur Urol 57:60–70
van Rhijn BW, Burger M, Lotan Y et al (2009) Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol 56:430–442
Bertz S, Denzinger S, Otto W et al (2011) Substaging by estimating the size of invasive tumour can improve risk stratification in pT1 urothelial bladder cancer-evaluation of a large hospital-based single-centre series. Histopathology 59:722–732
Roupret M, Seisen T, Comperat E et al (2013) Prognostic interest in discriminating muscularis mucosa invasion (T1a vs T1b) in nonmuscle invasive bladder carcinoma: French national multicenter study with central pathology review. J Urol 189:2069–2076
Andius P, Johansson SL, Holmang S (2007) Prognostic factors in stage T1 bladder cancer: tumor pattern (solid or papillary) and vascular invasion more important than depth of invasion. Urology 70:758–762
Lamm D, Persad R, Brausi M et al (2014) Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. J Urol 191:20–27
Pasin E, Josephson DY, Mitra AP et al (2008) Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol 10:31–43
van Rhijn BW, van der Kwast TH, Alkhateeb SS et al (2012) A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol 61:378–384
Kunieda F, Kitamura H, Niwakawa M et al (2012) Watchful waiting versus intravesical BCG therapy for high-grade pT1 bladder cancer with pT0 histology after second transurethral resection: Japan Clinical Oncology Group Study JCOG1019. Jpn J Clin Oncol 42:1094–1098
Edge SB, Byrd DR, Compton CC et al (eds) (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Mostofi FK, Sobin LH, Torloni H (1973). Histological typing of urinary bladder tumors. World Health Organization, Geneva 1973
Grimm M-O, Steinhoff CH, Simon X et al (2003) Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study. J Urol 170:433–437
Lazica DA, Roth S, Brandt AS et al (2014) Second transurethral resection after Ta high-grade bladder tumor: a 4.5-year period at a single university center. Urol Int 92:131–135
Herr HW, Donat SM (2008) Quality control in transurethral resection of bladder tumours. BJU Int 102:1242–1246
Shindo T, Masumori N, Kitamura H et al (2014) Clinical significance of definite muscle layer in TUR specimen for evaluating progression rate in T1G3 bladder cancer: multicenter retrospective study by the Sapporo Medical University Urologic Oncology Consortium (SUOC). World J Urol 32:1281–1285
Takaoka E, Matsui Y, Inoue T et al (2013) Risk factors for intravesical recurrence in patients with high-grade T1 bladder cancer in the second TUR era. Jpn J Clin Oncol 43:404–409
Segal R, Yafi FA, Brimo F et al (2012) Prognostic factors and outcome in patients with T1 high-grade bladder cancer: can we identify patients for early cystectomy? BJU Int 109:1026–1030
Dalbagni G, Vora K, Kaag M et al (2009) Clinical outcome in a contemporary series of restaged patients with clinical T1 bladder cancer. Eur Urol 56:903–910
Acknowledgments
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (Contract Grant Nos. 25462503, 15k06882, 25860401).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyoshi Suzuki received a research Grant from Astellas, Takeda, Novartis, Pfizer, Daiichi Sankyo, Taiho, Kissei, and Nihon Kayaku. Hiroyoshi Suzuki received lecture fees from Astra Zeneca, Astellas, Takeda, Sanofi, Daiichi Sankyo, and Janssen. The other authors have no conflicts of interest to declare.
About this article
Cite this article
Kamiya, N., Suzuki, H., Suyama, T. et al. Clinical outcomes of second transurethral resection in non-muscle invasive high-grade bladder cancer: a retrospective, multi-institutional, collaborative study. Int J Clin Oncol 22, 353–358 (2017). https://doi.org/10.1007/s10147-016-1048-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1048-z