Abstract
Background
The objective was to evaluate the incidence of second primary malignancies (SPMs) in thyroid cancer patients compared to age- and gender-matched controls without thyroid cancer from the general population of the same region.
Methods
Tampere and Oulu University Hospitals treated 910 patients with well-differentiated thyroid cancer during 1981–2002. The Finnish cancer registry provided follow-up data for patients and controls (n = 4542) for an average of 16 years. The incidence of invasive malignancies per 10 000 person-years was calculated and compared between patients and controls. The follow-up period ended December 31st, 2011.
Results
Young patients <40 years [Rate Ratio (RR) 1.73, p = 0.037] and patients diagnosed since 1996 (RR 1.51, p = 0.029) had an increased incidence of SPMs. Patients had an increased risk of sarcomas and soft tissue tumours (RR 4.37, p = 0.004) and haematological and lymphatic malignancies (RR 1.87, p = 0.035), especially non-Hodgkin lymphomas (RR 2.78, p = 0.035). The overall incidence of SPMs was not statistically higher in patients (109 SPMs/910 patients vs. 500 SPMs/4542 controls, RR 1.12, p = 0.269). Most patients were radioiodine-treated (81 %). The risk of SPMs with low cumulative radioiodine doses was RR 0.94 (≤3.7 GBq, p = 0.650) and with high doses RR 1.37 (>3.7 GBq, p = 0.143). Cumulative radioiodine dose increased during the study period.
Conclusions
The overall incidence of SPMs was not higher in patients than in controls. The incidence of SPMs in thyroid carcinoma patients was higher in patients <40 years old and patients diagnosed since 1996. The incidence of sarcomas and lymphomas was higher in patients than in controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common endocrine malignancy and accounts for 1–2 % of all cancer cases. The incidence of thyroid cancer has increased in western countries during the past few decades, especially among young people [1–3]. In Finnish patients less than 40 years old, thyroid cancer is the 4th most common malignancy in females and 7th most common malignancy in males [4]. Differentiated thyroid cancers (DTCs) arise from the follicular cells of the thyroid gland. Further, DTCs are classified as papillary thyroid cancers (80–90 %) and follicular thyroid cancers (10–20 %). The prognosis of DTC is favourable [5, 6].
The treatment of DTC consists of total thyroidectomy, followed by radioactive iodine (RAI) ablation, which destroys residual thyroid tissue after surgery [7]. In addition, RAI eradicates and destroys possible DTC metastasis in local lymph nodes and distant sites. Most frequent sites of distant metastasis are the lungs and bone [8]. Serum thyroglobulin acts as a tumour marker after RAI ablation. RAI ablation may cause irritation of the salivary glands and mucous membranes of the gastrointestinal and respiratory tract acutely or subacutely [9]. There is also concern about radiation-induced second primary malignancies (SPMs) because DTC affects relatively young patients [3, 10].
In previous studies, DTC patients have been associated with 9–33 % greater risk of SPMs than general population [10–16]. The aim of this study was to evaluate the risk of SPMs in a cohort of Finnish DTC patients compared to age- and gender-matched controls of the same region. Furthermore, we wanted to investigate which subgroups of patients had the highest risk of SPMs.
Materials and methods
Study population and the follow-up
The study included 920 consecutive patients treated for DTC at two Finnish university hospitals between 1981 and 2002. Tampere University Hospital treated 493 patients and Oulu University Hospital 427 patients [2, 17]. Follow-up data were collected from the hospitals’ medical records and included the date of birth, date of cancer diagnosis, date and extent of surgery, cumulative dose of RAI treatments and the latest date of follow-up. Each patient was assigned an index date, which was the date of RAI ablation for patients treated with RAI and the date of diagnosis of DTC for those who did not receive RAI treatment. Missing information in the registry database, errors in identification numbers or data release limitations caused exclusion of 10 patients. A total of 910 patients were available for analysis.
For each patient, five control subjects from the general population were selected from the databases of Population Register Center of Finland and matched for age, gender and place of residence. The follow-up period of the control subjects started at the index date of the corresponding patients. The Finnish Cancer Registry provided statistics for the patients and controls since 1960 [4]. The overall cancer incidence included only invasive cancers. In situ-cancers, suspicions of cancer and benign tumours were excluded from the Finnish Cancer Registry data. Basal cell carcinomas, considered as indolent skin cancers, were also excluded. We evaluated the incidence of benign CNS tumours separately.
We calculated the time interval for the development of SPM from the index date to the date of SPM. Antecedent malignancies, cancers occurring before the index date, were analysed separately. If the time between the index date and the date of SPM was ≤12 months, SPM was considered synchronous. Antecedent or synchronous malignancies were not exclusion criteria for patients or controls, with one exception, as prior thyroid cancer was an exclusion criterion for controls. The incidence of SPMs did not include antecedent or synchronous malignancies. The follow-up period ended either at the date of the first SPM, the emigration date from Finland, the date of death or on December 31, 2011, whichever came first. Total number of person-years at risk was 14104 for the patients and 72716 for the controls.
Second cancers and categories
The classification system used by the Finnish Cancer Registry is the International Classification of Diseases for Oncology (ICD-O-3). ICD-O-3 identifies the anatomical region of the cancer (topography) and the cancer histology (morphology) [18]. Topographical classification of ICD-O-3 follows closely the clinical classification (ICD-10) with a few exceptions. The majority of observed SPMs were categorised using the topography. However, morphology was used in some cancers occurring in multiple anatomical sites. These cancers included haematological and lymphatic malignancies [morphology (M)9590–M9989] and mesenchyme-derived malignancies (bone and soft tissue cancers, M8800–M9262). The subgroup of latter, sarcomas and soft tissue cancers was grouped according to separate ICD-O-3 guidelines [18]. Furthermore, a combination of malignant and benign CNS tumours (M9380–M9571) and benign meningiomas (M9530–M9539) were analysed according to morphology.
Ethics
The Ethics Committee of the Pirkanmaa Hospital District approved the study protocol. In addition, the National Research and Development Center for Welfare and Health gave permission to use data from the Population Register Center and the Finnish Cancer Registry. The Head of Science Centre at Tampere University Hospital and Medical Director of Oulu University Hospital gave permissions to use medical records. We conducted the study in accordance with the Declaration of Helsinki.
Statistical analysis
Our study group performed statistical analyses with SPSS software version 20 (IBM Corporation, New York, NY, USA). In Table 1, we represent continuous variables as the means with standard deviation and categorical variables with percentages. We compared continuous variables with unpaired t test and categorical variables with the Chi-square test. RAI doses with skewed distributions were compared with the Mann–Whitney U test between the time periods. In Fig. 1, Kaplan–Meier analysis and the log-rank test were used when comparing various subgroups of patients. Significances are two-tailed, and p values ≤0.05 were considered statistically significant.
The top left figure illustrates all patients and corresponding controls, followed by a comparison of subgroups of women and men with controls. In the second row, patients are compared with controls in the age groups >60, 40–60 and <40 years. In the third row, patients with no radioiodine treatment, with a cumulative radioiodine dose of ≤3.7 GBq and with a cumulative radioiodine dose of >3.7 GBq are compared with controls. In the fourth row, patients diagnosed before and after January 1996 are compared with controls. The last image on the fourth row illustrates a subgroup of women <60 years old diagnosed after January 1996 with the corresponding controls. In this figure, the log-rank test was used for testing statistical significance
Stata software (StataCorp, College Station, TX, USA) and R software version 3.2.1 (R Development Core Team) were used to calculate post-index date cancer cases, person-years-at-risk and cancer incidences per 10,000 persons-years. The considered rate ratios have been estimated by using Poisson regression models with patient-years defined as the offset variable, see more about Poisson regression analysis for incidence rates, e.g., Frome and Checkoway [19]. In Table 2, the estimates of rate ratios based on two-way interaction Poisson regression models are presented. The overall significance of case–control factor was tested with the likelihood ratio test by comparing the fit of interaction model to the fit of Poisson regression model with the case–control factor completely excluded. Also, the p-values for the significance tests of interaction effect are reported, and the Cochran-Mantel–Haenszel type of estimates of rate ratios based the main effects Poisson regression models are presented in Table 2.
Results
Table 1 presents the information on the 910 DTC patients and 4542 controls. The mean age of patients and controls at the beginning of follow-up was 49 years, and 82 % were female. Mean follow-up time, mean time to SPM and mortality were equal between the groups. Only few patients and controls emigrated (0.7 vs. 0.6 %, p = 0.736). Follow-up continued for the majority of patients and controls (68.8 vs. 70.5 %) at the study end-date of the 31st of December 2011.
The surgical treatment of DTC did not differ significantly between the two hospitals participating in the study. Most patients, 710 (78 %) had a total thyroidectomy and in 142 patients (16 %) had a subtotal thyroidectomy. DTC type was papillary in 715 patients (79 %), a follicular variant of papillary carcinoma in 96 patients (10 %) and follicular carcinoma in 99 patients (11 %). The majority, 480 (53 %) of tumours were ≤20 mm (T1) and 158 (17 %) were 21–40 mm in size (T2). Hundred-fifty (16 %) patients had lymph node metastasis in the cervical region; 61 patients (6.7 %) had a distant metastatic disease.
DTC patients (n = 910) had 43 (4.7 %) antecedent cancers and 4542 controls had 115 antecedent cancers (2.5 %). Therefore, more malignancies were detected in patients than controls before the diagnosis of DTC (p < 0.001). Number of synchronous malignancies was 6 (0.7 %) in patients and 17 (0.4 %) in controls (p = 0.226).
Second primary malignancies
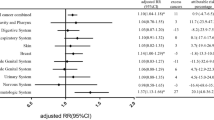
Table 2 illustrates the risk for SPMs in various subgroups of patients. SPMs were observed in 109 patients (12.0 %) and in 500 controls (11.0 %) [Rate ratio (RR) 1.12, p = 0.269). Multiple second cancers were observed in 3 (0.3 %) patients and in 35 (0.8 %) controls (p = 0.144). Patients <40 years (RR 1.73, p = 0.037) had an increased risk of SPMs when compared to controls. Patients 40–60 or >60 years did not have a significantly higher risk for SPMs than the corresponding controls (RR 1.12 and 1.01, respectively). If the patient was treated 1996 onwards, the risk for SPMs was significantly higher than in the controls (RR 1.51, p = 0.029). The risk of SPMs in the patients diagnosed during 1981–1989 and 1990–1995 was not significantly higher than in the controls (RR 0.95, p = 0.773 and RR 1.07, p = 0.773 respectively). There were no differences in the incidence of SPMs in patients with a papillary or a follicular thyroid cancer compared to the controls. In multivariable analysis no significant effects were found. Figure 1 illustrates the observed differences between various groups of patients and controls.
Specific tumour sites
Table 3 shows incidences of SPMs in specific tumour sites in patients and corresponding controls. The risk of sarcomas and soft tissue tumours was higher in patients than in controls (RR 4.37, p = 0.004). More lymphatic and hematologic malignancies were observed in patients when compared to controls (RR 1.87, p = 0.035). The risk for non-Hodgkin lymphoma was higher in patients (RR 2.78, p = 0.035). The incidence of thyroid cancer as an SPM was higher in the patients than in controls (RR 2.99, p = 0.016). The combined incidence of benign and malignant CNS tumours was higher in patients than in controls (RR 2.56, p = 0.017), and benign meningioma was a more common finding in patients (RR 3.15, p = 0.007).
RAI treated patients
The majority, 740 patients (81 %) had a RAI ablation postoperatively. Patients with persistent or recurrent disease (n = 211, 23 %) had multiple RAI treatments. The median dose of RAI was 3.7 GBq (100 mCi), and the mean was 5.3 GBq (±3.7 GBq). The cumulative dose of RAI was ≤3.7 GBq (≤100 mCi) in 526 7patients (71 %) and >3.7 GBq in 214 patients (29 %). The mean cumulative dose of RAI rose during the study period. During 1981–1989 the RAI dose was 4.4 GBq (±3.1 GBq), during 1990–1995 5.6 GBq (±4.1 GBq) and 1996 onwards 5.8 GBq (±3.5 GBq) (p < 0.001). Patients with a good prognosis (n = 170, 19 %) did not receive RAI treatment. This group included patients with small-sized intra-thyroidal cancers with no lymph node metastasis. Twenty-eight patients (3 %) had external radiotherapy.
Table 4 shows the incidences of SPMs in RAI-treated patients and controls. The overall cancer incidence was equal between patients and controls (RR 1.04, p = 0.721). The incidence of sarcomas and soft tissue tumours was higher in RAI-treated patients than in controls (RR 6.37, p = 0.002). RAI treated patients had one case of salivary gland cancer. The incidence of hepato-biliary-pancreatic cancers was lower in the RAI-treated patients than in controls (RR 0.00, p = 0.024), but not in RAI-non-treated patients (RR 2.54, p = 0.114). The incidence of lung and respiratory tract cancers was lower in the RAI-treated patients than in controls (RR 0.26, p = 0.041), while patients without RAI treatment had a tendency to the opposite direction (RR 5.09, p = 0.072). When compared to the controls, the RR of SPMs in patients with ≤3.7 GBq RAI dose was 0.94 (p = 0.650) and with >3.7 GBq RAI dose 1.37 (p = 0.143).
Discussion
In the present study, the risk of SPMs in DTC patients did not differ when compared with age, gender and place of residence-matched controls of the general population. Young patients <40 years and patients treated since 1996 had an increased incidence of SPMs when compared to controls.
The strengths of this study include the excellent quality of the register data, the good matching of the patients and controls. The follow-up time was long, with the median follow-up of 16 years, and the maximum of over 30 years. Clinical follow-up was carried out at the same two university hospitals. The coverage of the study was good. Less than 1 % of patients immigrated. Therefore, the registry follow-up was rather comprehensive. Another strength of this study is the availability of detailed information on RAI treatments. In most registry-based studies, information on RAI treatments is imprecise or lacking [10].
Patient’s age affected the risk of SPM. Patients <40 years old had 73 % higher risk for SPM than controls, whereas in patients ≥40 years the risk was not significantly higher. Ronckers et al. [20] reported that in DTC patients <40 years, the risk of SPM was 39 % higher than in the general population. In older patients, the risk was only 6 % higher. In a study by Lu et al. [15], the risk of SPMs was highest in patients less than 50 years old. It is possible that the young DTC patients have genetic, environmental or lifestyle-related factors, which make them more susceptible to carcinogenesis than their peers in the general population. A higher incidence of antecedent malignancies in DTC patients suggests that the general carcinogenic susceptibility of DTC patients is higher than in general population. Previous studies have discovered that there is a bidirectional association between the incidence of DTC and non-thyroidal cancers [20, 21]. Hence, patients with DTC have more SPMs than the general population, and patients with other malignancies have more thyroid cancers than the general population. Young patients probably have the most noticeable difference in incidence, as the overall cancer incidence is lower than in older patients.
Patients treated since 1996 had an increased risk of SPMs in this study (RR 1.51), whereas patients treated before 1996 had an equal risk of SPM when compared to controls. Kim et al. [22] also made a similar observation. The risk for SPMs in DTC patients was higher in patients diagnosed after 2003 when compared to controls (RR 1.45), and the risk was lower for patients diagnosed earlier (RR 1.03–1.21). A higher incidence of SPMs in DTC patients in recent decades is an interesting, yet poorly understood, observation. Changes in environmental risk factors may affect the incidence of DTC [23]. The proportion of small-size papillary carcinomas with favourable prognosis has rapidly increased during recent decades [23, 24]. Good prognosis and longer survival of DTC patients may contribute to the increased risk of SPMs. On the other hand, disease-specific mortality has not changed significantly during the past decades [2, 25]. In our study, RAI dose has increased during past decades, which may have affected the incidence of SPMs.
In some cancer sites and types, an increased incidence of SPMs in patients was observed. Sarcomas and soft tissue tumours were more common in DTC patients than in controls (RR 4.37). This finding is consistent with previous studies. Sandeep et al. [10] reported SIRs of 3.63 for soft tissue sarcoma and 3.62 for bone malignancies, whereas Rubino et al. [12] observed an RR of 4.0 for bone and soft tissue cancers. The risk of lymphatic and hematologic malignancies was elevated, especially the risk of non-Hodgkin lymphoma (RR 2.78). Sandeep et al., Brown et al. and Lu et al. [10, 11, 15] each also reported an increased risk of non-Hodgkin lymphoma (SIR 1.68, 1.75 and 2.66, respectively). We observed increased incidence of meningiomas in patients when compared to controls. In other studies, high incidence of CNS cancers (RR 2.2-4.0) has been reported in DTC patients [12, 15].
RAI-treated patients did not have a higher overall incidence of SPMs when compared to controls or patients without the RAI treatment. In subgroups, RAI-treated patients had an increased incidence of sarcomas and soft tissue cancers. Patients had a lower incidence of hepato-biliary-pancreatic cancers and lung and respiratory organ cancers than controls. Alcohol and tobacco consumption increase the risk of these malignancies, and the decrease in incidence may be due to lifestyle changes among the patients [26]. In other studies, RAI treatment has been associated with 12–19 % higher incidence of SPMs when compared to DTC patients without radiation therapy [27, 28]. In previous studies, DTC survivors have had a decreased incidence of lung cancers [11, 20].
Due to limited number of patients and observed SPMs in this study, the results of subgroup analyses must be interpreted with caution. The risk of false positive results exists due to the limited number of events and multiple testing. Increased incidence of thyroid carcinomas as SPMs is explained by the date inconsistencies between the clinical and registry databases during the 1980′s. The use of morphological classification in some cancer types may differ from some previous studies, which rely solely on topographical classification. Due to space limitations, we did not discuss antecedent or synchronous cancers in detail.
In conclusion, our results show that certain groups of DTC patients had an elevated incidence of SPMs when compared to age, gender and region-matched controls of the general population. DTC patients <40 years old, as well as patients diagnosed since 1996, had a higher risk of second primary malignancy when compared to controls. DTC patients had increased the incidence of sarcomas and soft tissue cancers and hematologic and lymphatic malignancies. RAI treated patients had markedly increased the incidence of sarcomas and soft tissue cancers. On the contrary, the risk of hepato-biliary-pancreatic cancers and respiratory organ cancers was decreased in radioiodine treated patients. However, the latter results must be interpreted cautiously due to limited patient and event count. The overall incidence of second primary malignancies was not significantly higher in DTC patients.
References
Elisei R, Molinaro E, Agate L et al (2010) Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab 95:1516–1527
Hakala T, Kellokumpu-Lehtinen P, Kholova I et al (2012) Rising incidence of small size papillary thyroid cancers with no change in disease-specific survival in Finnish thyroid cancer patients. Scand J Surg 101:301–306
Hay ID, Gonzalez-Losada T, Reinalda MS et al (2010) Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg 34:1192–1202
Engholm G, Ferlay J, Christensen N et al (2014) NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Association of the Nordic Cancer Registries. Acta Oncol 49:725–736
Sipos JA, Mazzaferri EL (2010) Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol) 22:395–404
Verburg FA, Stokkel MP, Duren C et al (2010) No survival difference after successful (131)I ablation between patients with initially low-risk and high-risk differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 37:276–283
Cooper DS, Doherty GM, Haugen BR et al (2009) Revised american thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
Haq M, Harmer C (2005) Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol 63:87–93
Van Nostrand D (2009) The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid 19:1381–1391
Sandeep TC, Strachan MW, Reynolds RM et al (2006) Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab 91:1819–1825
Brown AP, Chen J, Hitchcock YJ et al (2008) The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 93:504–515
Rubino C, De Vathaire F, Dottorini M et al (2003) Second primary malignancies in thyroid cancer patients. Br J Cancer 89:1638–1644
Berthe E, Henry-Amar M, Michels J et al (2004) Risk of second primary cancer following differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 31:685–691
Verkooijen RB, Smit JW, Romijn JA et al (2006) The incidence of second primary tumors in thyroid cancer patients is increased, but not related to treatment of thyroid cancer. Eur J Endocrinol 155:801–806
Lu CH, Lee KD, Chen PT et al (2013) Second primary malignancies following thyroid cancer: a population-based study in Taiwan. Eur J Endocrinol 169:577–585
Iyer NG, Morris LG, Tuttle RM et al (2011) Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer 117:4439–4446
Jukkola A, Bloigu R, Ebeling T et al (2004) Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocr Relat Cancer 11:571–579
Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin M et al (2000) International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), vol 2014. WHO, Geneva
Frome EL, Checkoway H (1985) Epidemiologic programs for computers and calculators. Use of Poisson regression models in estimating incidence rates and ratios. Am J Epidemiol 121:309–323
Ronckers CM, McCarron P, Ron E (2005) Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer 117:281–288
Van Fossen VL, Wilhelm SM, Eaton JL et al (2013) Association of thyroid, breast and renal cell cancer: a population-based study of the prevalence of second malignancies. Ann Surg Oncol 20:1341–1347
Kim C, Bi X, Pan D et al (2013) The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid 23:575–582
Pellegriti G, Frasca F, Regalbuto C et al (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013:1–10
Cramer JD, Fu P, Harth KC et al (2010) Analysis of the rising incidence of thyroid cancer using the surveillance, epidemiology and end results national cancer data registry. Surgery 148:1147–1153
Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167
Blanchard CM, Denniston MM, Baker F (2003) Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav 27:246–256
de Gonzalez AB, Curtis RE, Kry SF et al (2011) Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol 12:353–360
Sawka AM, Thabane L, Parlea L et al (2009) Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid 19:451–457
Acknowledgments
We would like to thank Adjunct Professor Jarkko Isotalo (University of Helsinki) and Ph.D. Maarit Leinonen (the Finnish Cancer Registry) for their contributions. This study was financially supported by Elna Kaarina Savolainen’s fund allocated for the development of cancer treatment and by the research funding of the Pirkanmaa Hospital District.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Hakala, T.T., Sand, J.A., Jukkola, A. et al. Increased risk of certain second primary malignancies in patients treated for well-differentiated thyroid cancer. Int J Clin Oncol 21, 231–239 (2016). https://doi.org/10.1007/s10147-015-0904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0904-6