Abstract
Background
We investigated whether scintigraphy was superior to radiography for detecting migrated seeds after brachytherapy for prostate cancer.
Methods
We studied 57 patients with early prostate cancer who were treated with free 125I transperineal brachytherapy. Scintigraphy was used to detect seed migration on postoperative day (POD) 1, radiography was used on POD 2, and both tests were used on POD 30.
Results
The total number of implanted seeds in this study was 3,753 in 57 patients. Overall, there were 19 seed migrations in 12 patients. On POD 1, there were 4 seed migrations in 4 patients that were detected by scintigraphy. On POD 2, there were 10 seed migrations in 9 patients that were detected by radiography. On POD 30, 17 seed migrations were detected in 10 patients by radiography and 18 seeds migrations were detected by scintigraphy. However, 1 seed migration which was located outside of the detectable range of radiography was detected only by scintigraphy.
Conclusions
Both scintigraphy and radiography have similar abilities to detect migrated seeds 1 month after 125I brachytherapy for prostate cancer. While both tests have advantages and disadvantages, it is reasonable to only use radiography if scintigraphy is not available.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
125I brachytherapy has become a standard treatment for early prostate cancer in Japan over the past 10 years. Although urinary obstructive symptoms often develop after brachytherapy, severe symptoms and other complications are rare, making brachytherapy the preferred minimally invasive treatment for prostate cancer. However, seed migration outside of the prostate gland after brachytherapy is not uncommon [1–3]. Although seed migration rarely causes clinical problems or reduces radiotherapy efficacy, it is still important to confirm the presence or absence of seed migration.
We have used chest and abdominal radiography to detect seed migration in patients at our institution since 2007, when we began using brachytherapy. However, Kono et al. previously reported that scintigraphy was superior to radiography for detecting migration and recommended scintigraphy as the standard method [4]. In the present study, we evaluated scintigraphy versus radiography as methods to detect seed migration after brachytherapy.
Materials and methods
This retrospective study was conducted according to the ethical guidelines for clinical studies of the Ministry of Health, Labor and Welfare of Japan, and was approved by the ethics committee of our institution (approval number 1621). Fifty-seven patients who were treated in consecutive order at Tokyo Medical University from March 2012 to April 2013 were included in the present study. All patients were treated with transperineal interstitial brachytherapy with 125I seeds. Patient characteristics are shown in Table 1. Initially, only patients with low-risk prostate cancer were included, but eventually patients with intermediate- or high-risk prostate cancer were included in the study. Twenty-four patients (42 %) received neoadjuvant hormonal therapy with luteinizing hormone-releasing hormone agonists and/or anti-androgens.
One month prior to seed implantation, treatment planning was performed using transrectal ultrasound transverse images at 5-mm intervals. The minimum target dose for treatment was set at 144 Gy using a peripheral approach. All patients were placed in the high lithotomy position under spinal anesthesia. Seeds were inserted using a Mick applicator (Mick Radio-Nuclear Instruments, Bronx, NY, USA) under the guidance of transrectal ultrasonography. Two kinds of I−125 seeds were used during the study period (BARD STM1251:Bard Inc. and Oncoseed 6711:GE Healthcare).
The following protocol was utilized to assess seed migration. Scintigraphy was performed on postoperative day (POD) 1, radiography (chest and abdomen) was performed on POD 2, and radiography and scintigraphy were both performed on POD 30. For scintigraphy, a gamma camera was used to detect seed migration. A very small amount of 99mTC pertechnetate (approximately 1.53 MBq/2 drops) was placed on the parietal area and bilateral iliac lining as a marker. The scintigraphy results were prospectively interpreted by a radiologist with no knowledge of the radiography results. The radiography results were interpreted by a urologist with no knowledge of the scintigraphy results.
Thirty days after implantation, patients underwent pelvic computed tomography for a post-procedure dosimetry study. A 14-Fr Foley catheter was inserted to identify the urethra. All pre- and post-implant plans were calculated using the Variseed 7.1 planning system (Varian Medical Systems, Palo Alto, CA, USA). All patients provided informed consent to participate in the examination of seed migration using both radiography and scintigraphy.
Statistical analysis
A paired t-test and chi-squared test were used where appropriate. Statistical analyses were performed using STATA IC 13 (Stata Corp, College Station, TX, USA). Statistical significance was defined as a two-sided p value <0.05.
Results
Clinical and pathological features are shown in Table 1. Patient ages ranged from 52−77 years (average 68.1 years). Patient prostate specific antigen (PSA) levels ranged from 4.25−16.8 ng/mL (average 7.89 ng/mL). The total number of implanted seeds ranged from 35−95 (average of 65). The mean amount of total radioactivity at the time of the post-plan was 844 ± 139 MBq (range 454−1195 MBq).
The total number of implanted seeds in this study was 3,753 in 57 patients. Overall, there were 19 seed migrations in 12 patients from POD 1 to POD 30. On POD 1, there were 4 four seed migrations in 4 four patients that were detected by a scintigraphy (Table 2). On POD 2, there were 10 seed migrations in 9 patients that were detected by radiography. On POD 30, there were 17 seeds migrations in 10 patients that were detected by both scintigraphy and radiography. One seed migration in one patient was detected only by scintigraphy and not by radiography; in this case, the seed was located outside the detectable radiography range. In one case, one seed migration to the pelvis was detected with both scintigraphy and radiography on POD 1 and 2, but was unable to be detected by either scintigraphy or radiography on POD 30.
Of the 19 migrated seeds, six seeds migrated to the chest, and 13 seeds migrated to the abdomen and/or pelvis (Table 2 and Fig. 1). None of the 12 patients had symptoms related to the migrated seeds. Of the 13 seeds in the abdomen and/or pelvis, six were found on POD 1 or POD 2, and seven were found on POD 30. Of the six seeds in the lung, four were found on POD 1 or POD 2, and two were found on POD 30. Only one seed moved from the pelvis on POD 2 to the lung on POD 30. Figure 2 shows four migrated seeds that were identified by both radiography and scintigraphy on POD 30. While the two seed types were of different size, the frequency of migration was not statistically different between these types (16.7 % for Oncoseed™ and 25.9 % for Brachysource).
Imaging studies (a chest X-ray, b abdominal X-ray, c scintigraphy) at 30 days after brachytherapy. a Chest X-ray showing migrated seeds in both lungs, b KUB (kidney ureter bladder) X-ray showing a migrated seed in the pelvis and below the prostate and c scintigraphy showing 2 migrated seeds in the lung (1 in the pelvis and 1 below the prostate). All 4 migrated seeds detected by scintigraphy corresponded to radiography results (a and b)
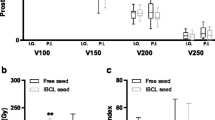
A post-procedure dosimetry study showed that the V100 (volume receiving >100 % of prescribed dose) and D90 (dose received by 90 % of prostate volume) values for the 45 patients with no seed migration were 96.3 % (93.3–99.9) and 173.3 Gy (145–198.2), respectively, compared to 97.3 % (95.1–99.6) and 175.8 Gy (157.7–195.4f) for 12 patients with seed migration, respectively (P = 0.49 and P = 0.26, respectively).
Discussion
While seed migration is commonly observed after brachytherapy, it rarely causes a clinical problem [2, 3]. However, such rare cases cannot be neglected since there are previous reports of seed migration to the coronary artery, kidney, vertebral venous plexus, and other sites [5–8]. Nguyen et al. reported three cases of seed migration to the right kidney that were found at 4 months, 7 months, and 3 years after brachytherapy [8]; these patients presented gross hematuria and/or flank pain that mimicked the symptoms of renal lithiasis. Zhu et al. first reported seed migration to the right coronary artery that caused an acute myocardial infarction [6]. Although clinical problems due to seed migration after brachytherapy are extremely rare, their occurrence necessitates the effective identification of seed migration.
Previously, Kono et al. studied 16 patients who were treated with 125I [4]. They detected 20 migrated seeds by scintigraphy and 7 by radiography, and seed migration was detected in 11 of 16 patients (69 %) by scintigraphy compared to 4 of 16 patients (35 %) by radiography (P = 0.016). Therefore, they concluded that scintigraphy was superior to radiography for identifying seed migration. However, they examined both radiography and scintigraphy 1 day after brachytherapy in 14 out of 16 patients. They did not show the timing of the tests, but it is likely that radiography was performed earlier than scintigraphy. In the current study, scintigraphy performed on POD 1 detected four seeds compared to 10 seeds detected by radiography on POD 2, and both tests on POD 30 showed similar results. Kono et al. examined two patients at 8 and 29 days after brachytherapy, and these patients showed the same results by both scintigraphy and radiography. Additionally, the location of the seeds that were missed by radiography were in the bladder (three patients), pelvis (two patients), and lung (one patient). However, based on the results in the present study, it may not be difficult to detect migration to those locations by radiography.
Sugawara et al. reported three cases with seed migration to the pelvic area, lung, or liver [9]. All 3 cases were detected by radiography; however, one case with migration in the pelvic area was missed by scintigraphy. The seed missed by scintigraphy was located in the right ischial bone and the authors suggested that this may be due to the fact that 125I photons emitted from the migrated seed might have been largely attenuated by bone tissue [10, 11]. While there were only three cases with seed migration in their series, the authors suggested limiting the use of scintigraphy as a standard method to detect seed migration. We agree that radiography provides important basic information for detecting seed migration.
In recent years, linked or stranded seeds have been utilized for prostate seed implants in some countries [3, 12, 13]. Seeds embedded in absorbable suture material are suggested to increase seed stabilization and greatly inhibit seed migration. Tapen et al. analyzed 289 patients treated with 125I and 103Pd seed implants and showed that the seed embolization rate was 11 % (16/146) versus 0.7 % (1/143) for free seed implants versus linked seed implants (P = 0.0002), respectively [3]. Reed et al. performed a prospective randomized comparison of stranded versus loose 125I seeds and showed a strong trend toward a decrease in post-implant seed loss with stranded seeds [13]. Battermann compared the use of loose and stranded seeds in 249 patients and reported a decrease in seed migration from 10 to 1–3 % of patients using stranded seeds [14]. These studies suggest that linked seeds should be used to avoid seed migration.
Theoretically, the outcomes of post-implant dosimetric treatment should be improved by using stranded seeds; however, mixed results have been reported [15, 16]. Lee et al. and Fuller et al. showed clear improvement in the dosimetric parameters for stranded seeds compared to loose seeds [15]. Gao et al. reported that greater numbers of displaced seeds resulted in lower values of D90 and V150 (volume receiving >150 % of the prescribed dose) [16]. Fagundes et al. reported an improvement in V100 from 88.4 to 92.5 % (P < 0.005) using stranded seeds [17]. On the other hand, Heysek et al. reported that stranded seeds yielded essentially the same dosimetry as loose seeds, with an average D90 of 101.9 % (stranded seeds) versus 99.3 % (loose seeds) [18]. Ngyuen reported that seed migration does not cause significant changes in radiation planning for the gland because of the relatively small occurrence of this event [5]. Sugawara et al. found no statistically significant difference in the post-implant prostate D90 value between patients with and without seed migration treated with loose seeds (P = 0.992) [1]. They suggested that in most patients with seed migration, only one or two seeds will have migrated, which will have a minimal effect on the dosimetry of the prostate. The mixed results of these studies indicate that continued efforts to avoid seed migration are needed.
There are several possible advantages and disadvantages for both radiography and scintigraphy. The disadvantages of radiography include limited shooting range, difficulty in identifying seed migration in the lung base and bone, and exposure to radiation. The advantage of radiography is that it is typically available anywhere that brachytherapy is performed. The disadvantages of scintigraphy include limited access at some institutions, limited availability of radiologists capable of interpreting scintigraphy images, and the inability of scintigraphy to detect seed migration several months after brachytherapy. The advantages of scintigraphy include absence of radiation exposure and whole-body evaluation, including the lower limb and neck/head area.
Ideally, both radiography and scintigraphy can be used to confirm seed migrations 1 month after brachytherapy, followed periodically by radiography. However, it is reasonable to use only radiography if scintigraphy is not available. Importantly, this study of seed migration and previous studies, including a study by Kono et al. [4], suggest that the assessment of seed migration within a few days of brachytherapy seems inappropriate.
References
Sugawara A, Nakashima J, Kunieda E et al (2011) Incidence of seed migration to the chest, abdomen, and pelvis after transperineal interstitial prostate brachytherapy with loose (125)I seeds. Radiat Oncol 6:130
Older RA, Synder B, Krupski TL et al (2001) Radioactive implant migration in patients treated for localized prostate cancer with interstitial brachytherapy. J Urol 165:1590–1592
Tapen EM, Blasko JC, Grimm PD et al (1998) Reduction of radioactive seed embolization to the lung following prostate brachytherapy. Int J Radiat Oncol Biol Phys 42:1063–1067
Kono Y, Kubota K, Mitsumoto T et al (2008) Scintigraphic detection of 125I seeds after permanent brachytherapy for prostate cancer. J Nucl Med 49:541–545
Nguyen BD (2006) Cardiac and hepatic seed implant embolization after prostate brachytherapy. Urology 68(673):e17–e19
Zhu AX, Walner KE, Frivold GP et al (2006) Prostate brachytherapy seed migration to the right coronary artery associated with an acute myocardial infarction. Brachytherapy 5:262–265
Nakano M, Uno H, Gotoh T et al (2006) Migration of prostate brachytherapy seeds to the vertebral venous plexus. Brachytherapy 5:127–130
Nguyen BD, Schild SE, Wong WW et al (2009) Prostate brachytherapy seed embolization to the right renal artery. Brachytherapy 8:309–312
Sugawara A, Kunieda E, Nakashima J et al (2010) Planar scintigraphy does not always detect migrated seeds after transperineal interstitial permanent prostate brachytherapy with 125I radioactive seeds. Internet J Urol 8:10
Mandell L, Nori D, Anderson L et al (1985) The effects of permanent I-125 interstitial implantation on cortical bone. Int J Radiat Oncol Biol Phys 11:1029–1034
Huang DY, Schell MC, Weaver KA et al (1990) Dose distribution of 125I sources in different tissues. Med Phys 17:826–832
Merrick GS, Butler WM, Dorsey AT et al (2000) Seed fixity in the prostate/periprostatic region following brachtherapy. Int J Radiat Oncol Biol Phys 46:215–220
Reed DR, Wallner KE, Merrick GS et al (2007) A prospective randomized comparison of stranded versus loose 125I seeds for prostate brachytherapy. Brachytherapy 6:129–134
Battermann JJ (2000) I-125 implantation for localized prostate cancer: the Utrecht University experience. Radiother Oncol 57:269–272
Lee WR, deGuzman AF, Tomlinson SK et al (2002) Radioactive sources embedded in suture are associated with improved postimplant dosimetry in men treated with prostate brachytherapy. Radiother Oncol 65:123–127
Gao M, Wang JZ, Nag S et al (2007) Effects of seed migration on post-implant dosimetry of prostate brachytherapy. Med Phys 34:471–480
Fagundes HM, Keys RJ, Wojcik MF et al (2004) Transperineal TRUS-guided prostate brachytherapy using loose seeds versus RAPIDStrand: a dosimetric analysis. Brachytherapy 3:136–140
Heysek RV, Gwede CK, Torres-Roca J et al (2006) A dosimetric analysis of unstranded seeds versus customized stranded seeds in transperineal interstitial permanent prostate seed brachytherapy. Brachytherapy 5:244–250
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Odagaki, Y., Ohori, M., Yoshimura, M. et al. Is scintigraphy necessary to detect migration of 125I seeds after brachytherapy for early prostate cancer?. Int J Clin Oncol 21, 397–401 (2016). https://doi.org/10.1007/s10147-015-0901-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0901-9