Abstract
Background
To explore arterial stiffness during the administration of androgen deprivation therapy (ADT) in patients with prostate cancer (PCa), a new indicator, the cardio-ankle vascular index (CAVI), and serum lipid profile changes were monitored.
Methods
A prospective study assessed the changes in arterial stiffness using the CAVI and clinical laboratory variables among 58 men with prostate cancer treated with ADT for 6 months. Furthermore, patients who had a high risk of developing arterial stiffness after ADT were investigated.
Results
The whole cohort had no significant increase in arterial stiffness within 6 months after ADT, but 55.2 % of patients had an increased CAVI. Serum levels of total cholesterol, high-density-lipoprotein cholesterol (HDL-C), and low-density-lipoprotein cholesterol (LDL-C) increased significantly at 1 month after the start of ADT and maintained high values thereafter. At baseline, HDL-C was lower and LDL-C and LDL-C/HDL-C were higher in the group with than without an increased CAVI after 6 months of ADT administration.
Conclusions
Although the whole cohort did not show a significant change in arterial stiffness with ADT, some patients showed an increased arterial stiffness monitored with the CAVI. The balance between LDL-C and HDL-C, or LDL-C/HDL-C, might have an impact on the development of arterial stiffness after ADT administration. Thus, clinicians might be able to monitor PCa patients who have a high risk of development of arterial stiffness after ADT administration by referring to LDL-C/HDL-C levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is one of the most common cancers affecting men in Western countries, and its incidence in Japan is rapidly increasing [1, 2]. The overall 5-year survival of all patients with PCa is very favorable [3, 4]. Because of the high survival rate of patients with this cancer, consideration of mortality related to chronic treatment has become important. In recent years, androgen deprivation therapy (ADT) has been increasingly used in PCa patients [5]. The role of ADT in the treatment of PCa has been extended from being used predominantly in symptomatic metastatic disease to asymptomatic metastatic disease, primary therapy in localized disease when men are considered unfit for surgery or radiotherapy, adjunct treatment in high-risk disease treated with radiotherapy, and salvage therapy following a biochemical relapse after surgery or radiotherapy for presumed localized disease [6, 7].
ADT can be accomplished with a gonadotropin-releasing hormone (GnRH) agonist, a GnRH antagonist, or bilateral orchiectomy. Anti-androgen drugs can also be used with GnRH agonists to produce combined androgen blockade. However, as a result of ADT, the profound testosterone deficiency is associated with a range of potential adverse short- and long-term consequences affecting physical and psychological health, including hot flushes, sexual dysfunction, obesity, sarcopenia, dyslipidemia, hyperinsulinemia, osteoporosis, type 2 diabetes mellitus (DM), and cardiovascular disease (CVD) [5–7]. In particular, CVD and the many adverse cardio-metabolic effects caused by ADT may negatively impact the overall health of PCa survivors [6].
Arterial stiffness is a useful surrogate marker for CVD. It is based on the structural changes occurring prior to plaque or thrombus formation in muscular and elastic vessels [8, 9]. Although pulse wave velocity (PWV) has been designed to assess arterial stiffness as a general method, it is known to depend on blood pressure (BP) at the time of measurement. Hence, the cardio-ankle vascular index (CAVI) was subsequently developed to obtain a more accurate arterial stiffness index that is not affected by BP at the time of measurement [8, 9]. The CAVI showed high values in aging, CVD, chronic kidney disease, and cerebral infarction [10]. Furthermore, patients with CVD risk factors, such as hypertension, DM, dyslipidemia, and obesity, also showed high values [11].
The strong relationship between the serum lipid profile and the incidence of CVD is well established. Low-density-lipoprotein cholesterol (LDL-C) is the main parameter for some risk assessment tools of CVD [12]. Another alternative parameter is the ratio of LDL-C to high-density-lipoprotein cholesterol (HDL-C) (LDL-C/HDL-C), which has been reported to be strongly associated with CVD and is thought to be a better predictor of future CVD than LDL-C [12].
Given the close association between arterial stiffness and CVD, it is crucial to determine whether ADT increases the likelihood of arterial stiffness. Therefore, the effects of short-term ADT on arterial stiffness changes were investigated using the CAVI in patients with PCa. In addition, the effects of ADT on the serum lipid profile, including LDL-C/HDL-C, were investigated, because of the close relationships between arterial stiffness, CVD, and dyslipidemia.
Patients and methods
Study participants and design
This was a prospective observational study approved by the institutional review board of Toho University Sakura Medical Center (No. 2012-008). Patients’ personal data were treated with strict confidentiality. All patients gave their written, informed consent.
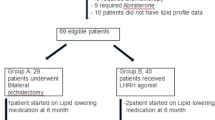
A total of 65 patients with pathologically confirmed PCa scheduled to receive ADT for more than 6 months were seen in our institution between March 2012 and December 2014. Seven patients were excluded from the present study because of other cancer deaths, lost to follow-up, or missing values at 6 months after the start of ADT. Finally, the data of 58 patients were statistically analyzed in the present study. All patients had Eastern Cooperative Oncology Group performance status of 0–1. Table 1 shows the demographic and baseline characteristics of the patients included in the final analysis. Indications for ADT in this study were the following: 35 patients as primary treatment, 18 patients as combination with radiotherapy, and 5 patients due to biochemical recurrence after radical prostatectomy. The following agents were used as ADT at the discretion of each urologist. Thirty-two patients received goserelin subcutaneously in combination with oral bicalutamide 80 mg daily. Twenty-one patients received leuprorelin subcutaneously in combination with oral bicalutamide 80 mg daily. Five patients received only degarelix subcutaneously.
The following clinical data were assessed at baseline (Table 1): age, body mass index, systolic and diastolic BP, pulse pressure, HbA1c, and comorbidities. Moreover, the following clinical data were assessed and compared at baseline and after 1, 3, and 6 months of follow-up (Fig. 1): prostate-specific antigen (PSA), testosterone, estimated glomerular filtration rate (eGFR), triglycerides, total cholesterol (TC), HDL-C, LDL-C, LDL-C/HDL-C, C-reactive protein (CRP), diacron-reactive oxygen metabolites (d-ROMs), and the CAVI.
Comparisons of clinical laboratory variables between baseline and after 1, 3, and 6 months of follow-up. Androgen deprivation therapy significantly lowers PSA and testosterone. After 1 month of follow-up, TC, HDL-C, and LDL-C are significantly increased, while the other clinical laboratory variables do not show significant changes. *P < 0.001. PSA prostate-specific antigen, eGFR estimated glomerular filtration rate, TC total cholesterol, HDL-C high-density-lipoprotein cholesterol, LDL-C low-density-lipoprotein cholesterol, CRP C-reactive protein, d-ROMs diacron-reactive oxygen metabolites, CAVI cardio-ankle vascular index
Measurement of the CAVI
The CAVI was measured with a VaSera CAVI instrument (Fukuda Denshi Co. Ltd., Tokyo, Japan) by the method described previously [8]. Briefly, cuffs were applied to bilateral upper arms and ankles, with the subject lying supine and the head held in the midline position. After resting for 10 min, the examinations were performed. To detect the brachial and ankle pulse waves with cuffs, a low cuff pressure from 30 to 50 mmHg was used to minimize the effect of cuff pressure on hemodynamics. BP was then measured. Finally, to compare with PWV results, scale conversion was performed. The CAVI was calculated using the following formula: CAVI = a{(2ρ/ΔP) × ln(Ps/Pd) × PWV2} + b, where Ps is systolic BP, Pd is diastolic BP, PWV is pulse wave velocity, ΔP is Ps − Pd, ρ is blood density, and a and b are constants. BP values were obtained using the cuff applied to the upper arm. PWV was obtained by dividing the length of the blood vessel by the time taken for the pulse wave to propagate from the aortic valve to the ankle, and it was measured using the cuffs attached to the upper arms and ankles. To be compatible with the aortic PWV method established by Hasegawa and coworkers [13], scale conversion constants (a, b) were determined so as to match the CAVI with the aortic PWV method. Using the scale conversion constants, the CAVI data obtained can be compared with the massive amount of previous data of PWV. All the measurement and calculation functions are integrated in the VaSera CAVI instrument that automatically calculates and generates the final data. The average coefficient of variation of the CAVI is less than 5 %, which is small enough for clinical usage and indicates that the CAVI has good reproducibility [14]. Furthermore, the CAVI is a patient-friendly method of assessing arterial stiffness in daily clinical practice, because it can show age-reference intervals in healthy persons.
Statistical analysis
The results are reported as means (±standard deviation). First, the CAVI was evaluated at baseline using t tests or Mann–Whitney U tests for the categorical variables and correlation analyses for the continuous variables. Second, the changes in the clinical laboratory variables were compared in all patients using the paired t test or the Wilcoxon signed-rank test between baseline and 1, 3, and 6 months of follow-up. Third, the patients were divided into two groups according to whether the CAVI increased at 6 months of follow-up. In each group of patients, the changes in the clinical laboratory variables were compared using the paired t test or the Wilcoxon signed-rank test between baseline and 6 months of follow-up. Last, univariate analyses were performed to evaluate the association between increased CAVI and baseline variables using the t test or Mann–Whitney U test for continuous variables and the χ 2 test for categorical variables. Statistical significance was defined as P < 0.050. All statistical analyses were performed using IPSS Statistics 22 (IBM, Chicago, IL, USA).
Results
At baseline, the CAVI was significantly higher in patients with hypertension [9.3 (±0.9) versus 8.6 (±1.0), P = 0.005] and DM [9.5 (±0.7) versus 8.8 (±1.1), P = 0.009]. It was also higher in patients with dyslipidemia [9.1 (±0.9) versus 8.9 (±1.0), P = 0.262] and CVD [9.3 (±0.9) versus 8.9 (±1.0), P = 0.112]. Moreover, the CAVI was significantly correlated with age (r = 0.373, P = 0.004), pulse pressure (r = 0.367, P = 0.005), and HbA1c (r = 0.297, P = 0.026). The following clinical variables at baseline were not significantly correlated with CAVI: BMI (r = 0.103, P = 0.443), systolic BP (r = 0.233, P = 0.079), diastolic BP (r = −0.019, P = 0.890), PSA (r = −0.115, P = 0.391), testosterone (r = −0.137, P = 0.307), eGFR (r = −0.073, P = 0.588), triglycerides (r = 0.102, P = 0.444), total cholesterol (r = −0.042, P = 0.753), HDL-C (r = 0.116, P = 0.385), LDL-C (r = −0.133, P = 0.321), LDL-C/HDL-C (r = −0.124, P = 0.307), d-ROMs (r = −0.023, P = 0.869), and CRP (r = 0.131, P = 0326).
Figure 1 shows the changes as a whole in the following clinical laboratory variables at baseline and 1, 3, and 6 months of follow-up: PSA, testosterone, eGFR, triglycerides, TC, HDL-C, LDL-C, LDL-C/HDL-C, CRP, d-ROMs, and the CAVI. ADT significantly lowered serum testosterone levels to castration level in all patients (P < 0.001). It also significantly lowered PSA (P < 0.001) (Fig. 1a, b). After 1 month of follow-up, TC, HDL-C, and LDL-C increased significantly from 185.5 (±34.1) to 206.2 (±33.6) mg/dL (P < 0.001), from 51.2 (±11.3) to 59.0 (±15.0) mg/dL (P < 0.001), and from 108.1 (±31.3) to 119.2 (±32.9) mg/dL (P < 0.001), respectively (Fig. 1e, f, g). On the other hand, after ADT administration, no significant changes were observed in the following variables: eGFR [from 67.3 (±14.3) to 66.2 (±13.9) mL/min/1.73 m2, P = 0.230), triglycerides [from 147.7 (±77.5) to 160.7 (±113.9) mg/dL, P = 0.521], LDL-C/HDL-C [from 2.2 (±0.8) to 2.2 (±0.9), P = 0.294), CRP [from 0.13 (±0.28) to 0.09 (±0.21) mg/dL, P = 0.301), d-ROMs [from 341.1 (±63.8) U.CARR to 336.9 (±73.3) U.CARR, P = 0.564], and the CAVI [from 9.0 (±1.0) to 9.0 (±1.2), P = 0.585] (Fig. 1c, d, h, i, j, k). Moreover, TC, HDL-C, and LDL-C were significantly higher at 3 and 6 months of follow-up compared to their baseline values. CRP gradually decreased and was significantly lower at 6 months of follow-up compared to the baseline value. The other variables did not show significant changes at 3 and 6 months of follow-up compared with their baseline values.
Although the CAVI in the whole cohort did not show a significant change after the start of ADT, the CAVI increased in 32 patients at 6 months of follow-up. Table 2 shows the changes in clinical laboratory variables from baseline to 6 months of follow-up, after patients were divided into two groups according to whether the CAVI increased at 6 months of follow-up. HDL-C and LDL-C increased significantly in both groups, while LDL-C/HDL-C did not increase significantly in either group (Table 2). Table 3 shows univariate analyses of the clinical variables at baseline for increased arterial stiffness after ADT. HDL-C was lower and LDL-C/HDL-C was higher in the group with than without an increased CAVI, although it did not reach statistical significance (Table 3).
Discussion
The CAVI reflects the stiffness of the whole arterial segment composed of the aorta, femoral artery, and tibial artery, which is not affected by BP at the time of measurement [8–11, 13, 14]. Thus, the CAVI can be used to compare the degree of arteriosclerosis. The CAVI is reported to increase in the elderly and in patients with hypertension, DM, dyslipidemia, and CVD [8–11]. In the present study, it was also confirmed that the CAVI at baseline was higher in patients with hypertension, DM, dyslipidemia, and CVD, as described in previous studies [8–11]. Moreover, the CAVI at baseline was significantly correlated with age, pulse pressure, and HbA1c. Medical treatment regimens for hypertension, DM, and dyslipidemia did not change within 6 months after ADT administration, although drugs, such as statins and angiotensin II receptor blockers, were reported to be able to improve the CAVI [8]. Thus, medical treatment regimens for hypertension, DM, and dyslipidemia were not thought to affect the change in the CAVI of patients who received ADT during this short-term study.
This study was the first to assess arterial stiffness induced by ADT in PCa patients using the CAVI. It demonstrated that 32 of 58 (55.2 %) patients showed an increase in arterial stiffness at 6 months of follow-up, although the whole cohort did not show a significant increase. Previous studies suggested that patients who received GnRH agonist monotherapy, such as goserelin or leuprorelin, developed a significant increase in arterial stiffness within 3–6 months after ADT initiation [15–17]. It was not possible to simply compare the present results with the results of previous studies, because previous studies included smaller numbers of enrolled patients, patients with different backgrounds, and different methods to assess arterial stiffness, such as PWV [15–17]. Some patients actually had a risk of developing arterial stiffness after ADT administration, although no patients had new-onset cardiovascular events in the present study during the observation period.
Direct vasodilatory effects of testosterone on calcium channels, potassium channels, or both of vascular smooth muscle have also been recognized [16–19]. Endogenous testosterone may exert a continuous beneficial effect on the vessel wall [16, 17]. Therefore, testosterone suppression under ADT might have an adverse effect on the general vasculature in PCa patients. However, the whole cohort did not show increased arterial stiffness, despite complete suppression of testosterone in all patients (Fig. 1b). Thus, we could not explain the pathogenesis of arterial stiffness in patients who received ADT by the direct effect of testosterone only.
To date, many researchers have reported that ADT promotes the development of metabolic disturbances, particularly insulin resistance and lipid profile changes [6, 7, 15–17]. The relationship between ADT and insulin resistance appears to be a continuum, with short-term therapy leading to reduced insulin sensitivity and long-term ADT resulting in hyperglycemia, increased risk of metabolic syndrome, and, finally, overt type 2 DM [6, 20–23]. On the other hand, the reported effects of ADT on the lipid profile are variable. TC increased significantly in the first 3–12 months of ADT [7, 16, 24]. Triglycerides increased significantly in the first 3–12 months of ADT [7, 25], while Dockery et al. and Brada-Basaria et al. reported that triglycerides did not increase significantly after ADT administration [16, 26]. HDL-C increased significantly in the first 3–12 months of ADT [7, 16, 25], while Smith et al. reported that HDL-C decreased up to 5 % after 12 months of ADT [27]. LDL-C increased significantly in the first 6–12 months of ADT [17, 25, 27–29], while Dockery et al. and Smith et al. reported that LDL-C did not increase significantly [16, 27]. In the present study, TC, HLD-C, and LDL-C increased significantly at 1 month after the start of ADT and maintained high values thereafter. Additionally, the patients were divided into two groups according to whether they were taking lipid-lowering drugs. Subsequently, lipid profile changes were compared in each group between baseline and 1, 3, and 6 months of follow-up. In both groups, TC, HDL-C, and LDL-C increased significantly at 1 month of follow-up and maintained high values thereafter (data not shown). If serum levels of LDL-C or LDL-C/HDL-C worsen in patients during ADT, clinicians should consider a medical treatment regimen for dyslipidemia.
Table 2 shows that HDL-C and LDL-C increased significantly at 6 months of follow-up in the groups with and without an increased CAVI. Table 3 shows univariate analyses of the clinical variables at baseline for increased arterial stiffness after ADT administration. At baseline, HDL-C was lower and LDL-C and LDL-C/HDL-C were higher in the group with than without an increased arterial stiffness after ADT administration. Although both ‘good cholesterol’ HDL-C and ‘bad cholesterol’ LDL-C increased at 6 months of follow-up, the present study suggested that the balance between HDL-C and HDL-C, or LDL-C/HDL-C, might have an impact on the development of arterial stiffness after ADT administration. LDL-C/HDL-C is a routine variable that should be readily available to clinicians [12]. Thus, clinicians might be able to monitor PCa patients who have a high risk of developing arterial stiffness after ADT administration by referring to baseline LDL-C/HDL-C levels prior to ADT.
There are some limitations in the present study. First, the duration of follow-up after ADT administration was short. Further investigation is necessary to confirm patients’ arterial stiffness with long-term ADT. Second, the sample size was small, although the number of enrolled patients in this study was larger than in previous studies. Third, it was not possible to assess the effects of insulin resistance or hyperinsulinemia, because insulin levels were not measured during the present study. However, in the present study, no patients were confirmed to have new-onset DM or drug therapy within 6 months of follow-up. Fourth, this study included three kinds of ADT, such as goserelin or leuprorelin in combination with bicalutamide, or degarelix. However, the CAVI and lipid profile changes were not significantly different depending on the type of ADT (data not shown).
The results of the present study demonstrated that testosterone suppression leads to increased arterial stiffness in some patients who received ADT, although patients as a whole did not show a significant change with short-term ADT. The present findings suggest that higher baseline LDL-C/HDL-C levels may be able to predict patients who have a high risk of developing arterial stiffness after ADT administration.
References
Suzuki H, Kamiya N, Imamoto T et al (2008) Current topics and perspectives relating to hormone therapy for prostate cancer. Int J Clin Oncol 13:401–410
Kamiya N, Suzuki H, Nishimura K et al (2014) Development of nomogram to non-steroidal antiandrogen sequential alternation in prostate cancer for predictive model. Jpn J Clin Oncol 44:263–269
Imamoto T, Suzuki H, Utsumi T et al (2009) Association between serum sex hormone levels and prostate cancer: effect of prostate cancer on serum testosterone levels. Future Oncol 5:1005–1013
Kamiya N, Suzuki H, Ueda T et al (2014) Clinical outcomes by relative docetaxel dose and dose intensity as chemotherapy for Japanese patients with castration-resistant prostate cancer: a retrospective multi-institutional collaborative study. Int J Clin Oncol 19:157–164
Shahinian VB, Kuo YF, Freeman JL et al (2005) Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 103:1615–1624
Allan CA, Collins VR, Frydenberg M et al (2014) Androgen deprivation therapy complicaions. Endocr Relat Cancer 21:T119–T129
Smith MR, Finkelstein JS, McGovern FJ et al (2002) Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 87:599–603
Shirai K, Hiruta N, Song M et al (2011) Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb 18:924–938
Nagayama D, Ohira M, Saiki A et al (2014) Sarpogrelate hydrochloride decreases cardio-ankle vascular index accompanied by increased serum lipidprotein lipase mass in type 2 diabetic patients. Int Heart J 55:337–341
Shirai K, Song M, Suzuki J et al (2011) Contradictory effects of β1- and α1-adrenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)—The independency of CAVI from blood pressure. J Atheroscler Thromb 18:49–55
Dobsak P, Soska V, Sochor O et al (2015) Increased cardio-ankle vascular index in hyperlipidemic patients without diabetes or hypertension. J Atheroscler Thromb 22:272–283
Mizuno K, Nakaya N, Teramoto T et al (2012) Usefulness of LDL-C-related parameters to predict cardiovascular risk and effect of pravastatin in mild-to-moderate hypercholesterolemia. J Atheroscler Thromb 19:176–185
Hasegawa M, Rodbard S (1979) Effect of posture on arterial pressure, timing of the arterial sounds and pulse wave velocities in the extremities. Cardiology 64:122–132
Yamabe T, Yoshizawa M, Saijo Y et al (2004) Brachio-ankle pulse wave velocity and cardio-ankle vascular index (CAVI). Biomed Pharmacother 58(Suppl 1):S95–S98
Smith JC, Bennett S, Evans LM et al (2001) The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86:4261–4267
Dockery F, Bulpitt CJ, Agarwal S et al (2003) Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 104:195–201
Dockery F, Bulpitt CJ, Agarwal S et al (2009) Effects of androgen suppression compared with androgen receptor blockade on arterial stiffness in men with prostate cancer. J Androl 30:410–415
Deenadayalu VP, White RE, Stallone JN et al (2001) Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281:H1720–H1727
Jones RD, English KM, Pugh PJ et al (2002) Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol 39:814–823
Shahani S, Braga-Basaria M, Basaria S (2008) Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab 93:2042–2049
Sağlam, HS, Köse O, Kumsar S et al (2012) Fasting blood glucose and lipid profile alterations following twelve-month androgen deprivation therapy in men with prostate cancer. Sci World J 2012:696329. doi:10.1100/2012/696329
McGrowder DA, Jackson LA, Crawford TV (2012) Prostate cancer and metabolic syndrome: is there a link? Asian Pac J Cancer Prev 13:1–13
Mohamedali HZ, Breunis H, Timilshina N et al (2011) Changes in blood glucose and cholesterol levels due to androgen deprivation therapy in men with non-metastatic prostate cancer. Can Urol Assoc J 5:28–32
Saylor PJ, Smith MR (2009) Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol 181:1998–2008
Morote J, Gŏmez-Caamaňo A, Alvarez-Ossorio JL et al (2015) The metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapy. J Urol 193:1963–1969
Braga-Basaria M, Muller DC, Carducci MA et al (2006) Lipoprotein profile in men with prostate cancer undergoing androgen deprivation therapy. Int J Impot Res 18:494–498
Smith MR, Malkowicz SB, Chu F et al (2008) Toremifene improves lipid profiles in men receiving androgen-deprivation therapy for prostate cancer: interim analysis of a multicenter phase III study. J Clin Oncol 26:1824–1829
Torimoto K, Samma S, Kagebayashi Y et al (2011) The effects of androgen deprivation therapy on lipid metabolism and body composition in Japanese patients with prostate cancer. Jpn J Clin Oncol 41:577–581
Salvador C, Planas J, Agreda F et al (2013) Analysis of the lipid profile and atherogenic risk during androgen deprivation therapy in prostate cancer patients. Urol Int 90:41–44
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyoshi Suzuki has received honoraria and/or research grants from Astellas Pharma Inc., GlaxoSmithKline K., Takeda Pharmaceutical Company Ltd., Astra Zeneca, Novartis Pharma, Daiichi-Sankyo, Sanofi KK., and Janssen.
The following authors have no conflict of interest: Ryo Oka, Takanobu Utsumi, Takumi Endo, Masashi Yano, Shuichi Kamijima, Naoto Kamiya, and Kohji Shirai.
Funding statement
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports and Culture of Japan (No. 25462503) to Hiroyoshi Suzuki.
About this article
Cite this article
Oka, R., Utsumi, T., Endo, T. et al. Effect of androgen deprivation therapy on arterial stiffness and serum lipid profile changes in patients with prostate cancer: a prospective study of initial 6-month follow-up. Int J Clin Oncol 21, 389–396 (2016). https://doi.org/10.1007/s10147-015-0891-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0891-7