Abstract
Background
Long-term outcomes of dose-escalated intensity-modulated radiation therapy (IMRT) combined with neoadjuvant (NA) androgen deprivation therapy (ADT) under an early salvage policy in patients with locally advanced prostate cancer (LAPC) were evaluated.
Methods
Data from 120 patients with T3-T4N0M0 adenocarcinoma of the prostate treated with IMRT were analyzed. NA-ADT with a median duration of 6 months was provided in all cases. Seventy-eight Gy, at 2 Gy per fraction, was delivered to the prostate and seminal vesicles. Adjuvant ADT (A-ADT) was not provided for any patient following the completion of IMRT. Salvage ADT (S-ADT) commenced when PSA values >4 ng/ml.
Results
The median follow-up period was 97 months. S-ADT was initiated in 39 patients. The median PSA value at the initiation of S-ADT was 5.7 ng/ml. The 8-year biochemical relapse-free survival, prostate cancer-specific survival, overall survival and S-ADT-free rates were 53.2 % [95 % confidence interval (CI) 43.4, 62.1], 96.6 % (95 % CI 91.2, 98.7), 89.1 % (95 % CI 81.5, 93.7) and 66.6 % (95 % CI 60, 74.6), respectively. The estimated 8-year cumulative incidence rates of grade 2–3 late gastrointestinal, and grade 2–3 genitourinary toxicity were 7.6 and 10.7 %, respectively. No grade 4 toxicity was observed.
Conclusions
High-dose IMRT, combined with NA-ADT for LAPC, was associated with favorable long-term disease-specific and overall survival outcomes, despite non-provision of A-ADT under the early S-ADT provision policy. This approach may represent a viable alternative to uniform provision of long-term A-ADT, because two-thirds of the patients maintained ADT-free status over an 8-year period after IMRT. Prospective trials will be required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
External-beam radiation therapy (EBRT) represents one of the major treatment modalities for locally advanced (T3-T4N0M0) prostate cancer (LAPC), although there has been conjecture regarding the appropriate standard approach for LAPC [1]. However, treatment outcomes, with the standard dose of EBRT and using conventional or three-dimensional conformal radiotherapy (3D-CRT) techniques, are unsatisfactory [2, 3]. In addition, it is widely recognized that local dose escalation significantly improves the likelihood of biochemical recurrence-free outcomes, not only in patients with localized diseases but also in those with LAPC [4–7]. Therefore, dose escalation is considered one of the key tenets of EBRT for LAPC.
However, dose escalation resulted in an increased incidence of severe late rectal toxicity, because the rectal dose increased commensurate with dose escalation at the prostate [6, 7]. Intensity-modulated radiation therapy (IMRT) solved this problem by balancing dose escalation at the targets, and sparing a significant volume of the rectum from high-dose radiation. IMRT significantly reduces the incidence of late rectal toxicities compared with 3D-CRT [8]. IMRT has principally been applied to localized prostate cancer, because the majority of prostate cancer cases in Western countries are characterized by localized disease [9]. In contrast, there are few reports describing the long-term clinical outcomes of LAPC patients treated with high-dose IMRT.
Combination long-term adjuvant androgen deprivation therapy (A-ADT) is now considered the standard approach for treating LAPC by EBRT because of its significant survival benefit [10, 11], based on the findings of clinical trials using a conventional dose of EBRT (66–70 Gy) [3, 12, 13]. However, the optimal duration and timing for combination androgen deprivation therapy (ADT) remains unclear [14, 15]. To date, only long-term clinical outcomes for high-dose IMRT not routinely combined with A-ADT have been reported [16, 17].
In addition, early initiation of salvage ADT (S-ADT) may improve the survival rate in patients with prostate-specific antigen (PSA) recurrence following EBRT [18–20]. Unfortunately, the timing of S-ADT initiation was not pre-defined or reported in the protocol, for any previously conducted randomized trials seeking to justify long-term A-ADT following EBRT for locally advanced cases.
Therefore, the aim of the present study was to report the long-term outcomes of high-dose IMRT combined with neoadjuvant ADT (NA-ADT) under a uniform salvage policy at a single institution. To our knowledge, this report utilized the largest cohort of T3-T4N0M0 prostate cancer patients treated using high-dose IMRT under uniform and pre-determined salvage conditions, and with the longest follow-up period.
Patients and methods
Data from 120 LAPC patients who were consecutively treated with IMRT between October 2002 and December 2006 were analyzed. The IMRT application was approved by the Local Ethics Committee (approval number 281) and written informed consent was provided by all patients. Data accumulation pertaining to toxicities and clinical outcomes was prospectively planned using a follow-up datasheet at every visit. This data analysis proposal was also approved by the Local Ethics Committee (approval number E1806).
Patient characteristics
Between October 2002 and December 2006, 147 consecutive patients with T3-T4N0M0 adenocarcinoma of the prostate, were treated using EBRT. Twenty-seven cases were treated with 3D-CRT due to the limited capability of IMRT; the remaining 120 patients, who were treated with high-dose IMRT, were evaluated in the present study. The median age of these 120 patients at the initiation of IMRT was 71 years (range 51–80 years). Pre-treatment prostate-specific antigen (iPSA) values ranged between 4 and 179 ng/ml (mean 37 ng/ml; median 25 ng/ml). Approximately half of the patients (n = 56) had a Gleason score (GS) of ≥8, whereas only 8 cases had a GS of 6, based on random biopsies of ≥6 cores. T stage was determined based on digital rectal examination (DRE), transrectal ultrasound (TRUS), computed tomography (CT) and magnetic resonance imaging (MRI). DRE, TRUS and CT were conducted in all cases, and MRI was performed in most cases. A bone scan was also conducted in all cases. Patient characteristics are summarized in Table 1.
Androgen deprivation therapy
NA-ADT consisted of combined androgen blockade (CAB) for 6 months, which was planned prior to IMRT initiation in our IMRT protocol for LAPC. However, there were variations in the durations of NA-ADT because a considerable number of patients were referred from outside of our institution, having commenced ADT several months previously. In addition, the IMRT capability of our institution was limited at that time, which resulted in relatively long waiting times to initiate IMRT following the initial consultation. CAB was planned for NA-ADT; however, patients with liver dysfunction were treated using luteinizing hormone-releasing hormone (LH-RH) agonists alone. A-ADT was not provided for any patient following the completion of IMRT. S-ADT was initiated if the PSA value >4 ng/ml, in a monotonically increasing manner, or if any clinical failure was detected.
Intensity-modulated radiation therapy
The details of our procedures for CT simulation and IMRT treatment planning have been reported elsewhere [21]. Briefly, patients were immobilized in the prone position using a thermoplastic shell in combination with a vacuum pillow and an original leg support. Patients were instructed to void the bladder and rectum approximately 1–1.5 h prior to CT simulation, according to their individual urinary conditions. Target delineations and treatment planning, including inverse optimization of IMRT, were performed using the CadPlan (ver. 6.2.7) or Eclipse software packages (ver. 7.1.35) (Varian Medical Systems, Palo Alto, CA, USA). Photon beams (15 MV) of Clinac 2100C or 2300 C/D (Varian Medical Systems) were used to deliver IMRT. The clinical target volume (CTV) was defined as the prostate plus two-thirds of the proximal seminal vesicles for non-T3b cases, and as the prostate plus whole seminal vesicles for T3b cases. To create the planning target volume (PTV) the following margins were added to the CTV in a three-dimensional setting—9 mm margins universally, except for a 6 mm margin posteriorly (to the rectum side).
A five-field dynamic multileaf collimator technique was used for IMRT beam delivery. Inverse optimizations were performed with the aim of fulfilling the planning goals established in our planning protocol [21]. The prescribed dose was 78 Gy, at 2 Gy per fraction in the PTV, although the dose was reduced to 70 or 74 Gy if patients presented with unfavorable risk factors for high-dose radiation such as anticoagulant therapy or severe diabetes mellitus (glycosylated hemoglobin ≥8. 0 %).
Setup errors were evaluated based on the pelvic bony structure using film-based portal imaging (PI). Off-line systematic setup error correction was performed based on sequential PIs obtained on the first of 3–5 consecutive treatment days, followed by weekly acquisition of the PI for setup verification and correction if necessary.
Patient follow-up and salvage androgen deprivation therapy
Following IMRT completion, patients were followed-up without adjuvant therapy, including A-ADT. PSA values were monitored at 1- to 3-month intervals during the first 2 years and at 4- to 6-month intervals thereafter. S-ADT was initiated when the PSA value was >4 ng/ml, in a monotonically increasing manner, or when any clinical recurrence was detected. Before initiating S-ADT, CT and bone scans were conducted to check the existence of clinical failures. Contents of the S-ADT were individually selected based on the status of each patient at the initiation of S-ADT.
Outcome evaluation and statistical analyses
The overall survival (OS), prostate cancer-specific survival (PCSS), PSA failure-free survival (PFFS), and S-ADT-free rates were calculated using the Kaplan–Meier estimation from the initiation date of IMRT. Cumulative incidences of late radiation toxicities were also estimated using the Kaplan–Meier method. Acute toxicities were evaluated based on the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0. Late rectal and urinary toxicities were graded using the definitions of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment (EORTC) [22]. PFFS rates were evaluated based on the Phoenix definition [23]. Survival curve differences were estimated using the log-rank test. Patients who were lost-to-follow-up with castration-resistant diseases were categorized as ‘dead from prostate cancer’ at the point of the last visit. Univariate and multivariate (i.e., Cox model) regression models were used to determine independent prognostic factors for PFFS. A value of p < 0.05 was taken to indicate statistical significance. Statistical analyses were performed using the GraphPad Prism (ver. 5.04, GraphPad Software Inc., La Jolla, CA, USA) and StatView (ver. 5.0, SAS Institute Inc. Cary, NC, USA) software packages.
Results
Treatments
The duration of NA-ADT ranged between 3 and 15 months, with a median of 6 months. Of the 120 patients included in this study, 111 received CAB which consisted of an LH-RH agonist (goserelin acetate or leuprorelin acetate) plus an anti-androgen (flutamide or bicalutamide). Nine patients were treated using the LH-RH agonist alone due to liver dysfunction. The prescribed dose of 78 Gy was delivered to 111 patients, while the dose was reduced to 70 or 74 Gy in 9 patients with unfavorable risk profiles for high-dose radiation. The treatment components are summarized in Table 1.
Oncological and survival outcomes
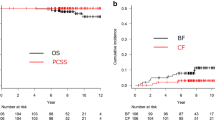
The median follow-up period was 97 months (range 21–120 months). Two patients were lost-to-follow-up at 81 and 86 months after the initiation of IMRT, respectively. The 8-year PFFS and S-ADT-free rates were 53.2 % (95 % CI 43.4, 62.1) and 66.6 % (95 % CI 60, 74.6), respectively (Fig. 1a, b). The PCSS and OS rates at 8 years were 96.6 % (95 % CI 91.2, 98.7) and 89.1 % (95 % CI 81.5, 93.7), respectively (Fig. 1c, d).
S-ADT was initiated in 39 patients; the PSA values at initiation ranged between 2.7 and 32.2 ng/ml with a median value of 5.7 ng/ml (Table 1). The median interval between the last date of IMRT and the initiation date of S-ADT was 37 months (range 6–97 months). Of the 39 patients who commenced with S-ADT, clinical failure was observed in 14 cases (35.9 %) according to CT or bone scans (bone metastases n = 11, lung metastases n = 2, and both bone and pelvic lymph node metastases n = 1). All of the remaining patients (n = 25; 64.1 %) developed PSA failure.
T-stage (T3a vs T3b-T4), iPSA and Gleason scores were significant prognostic factors for PFFS, according to both univariate and multivariate analyses; age, total dose and NA-ADT duration were not significant prognostic factors (Table 2).
Toxicities
A summary of adverse events is reported in Table 3. Acute urinary toxicities were mostly consisted of urinary frequency, urgency and retention. Grade 3 dysuria, which required self-catheterization, was observed in one patient when 6 Gy was delivered. Therefore, the total dose was reduced to 70 Gy with this particular patient; however, the symptom was completely resolved 31 months after IMRT. Acute rectal symptoms were mostly pain and bleeding with defecation, and resolved in 1–2 months after IMRT. No grade 3 or 4 rectal adverse events were observed.
The estimated cumulative incidence rates of grade ≥2 late gastrointestinal, and grade ≥2 genitourinary toxicity, based on the RTOG/EORTC criteria, were 7.6 % (95 % CI 0.7, 26.2) and 10.7 % (95 % CI 1.8, 29.8), respectively, at 8 years (Fig. 2a, b). The majority of the late toxicities with bleeding were transient, improved over time, and were judged as grade 0 or 1 at the last follow-up. Grade 3 urinary bleeding due to radiation cystitis was observed in 4 cases. Timings of the appearance of bleeding were 102, 26, 62 and 69 months after IMRT. Treatment for bleeding consisted of conservative therapy for the former two cases and coagulation therapy for the latter two cases. Grade 3 bleeding was finally resolved (became grade 0 or 1) in the first three cases after 1 week, 2 months and 1 month, respectively. In the last case, the bleeding intermittently persisted until the last follow-up date (85 months after IMRT). Grade 3 late rectal bleeding was observed in 3 cases; two of them had been administered anticoagulant agents due to the history of cerebral infarction or coronary stenting. Argon plasma coagulation was conducted in all the three cases. Timings of the appearance of the symptom were 37, 64 and 10 months, respectively. The bleedings resolved to grade 0 or 1 after 6 months, 1 month and 9 months, respectively. No grade 4 late toxicity was observed.
Discussion
It is widely accepted that combined ADT significantly improves both biochemical control and survival rates compared with EBRT alone, in patients with intermediate or high risk prostate cancer. Meta-analyses of randomized control trials comparing ADT plus EBRT with EBRT alone, clearly demonstrate that combined ADT significantly improves not only biochemical but also survival outcomes [24, 25]. These benefits are observed regardless of the duration of the combined ADT (i.e., short- or long-term). In addition, Bolla et al. reported that a 6-month course of A-ADT, combined with a standard dose of EBRT, was associated with inferior survival rates compared with a 3–year course of A-ADT with EBRT in patients with LAPC (81 vs 84.5 % at 5 years; hazard ratio = 1.45, p = 0.65 for non-inferiority) [12]. Therefore, combining long-term ADT with EBRT for patients with LAPC is currently recommended as the standard of care [10, 11].
However, the doses used in trials that assessed the impact of ADT on EBRT ranged between 65 and 70 Gy, which is substantially lower than the standard of care applied in the current IMRT era. It has been well-established that dose escalation significantly improves PFFS outcomes in patients with prostate cancer, including LAPC treated with EBRT.
Because we designed the currently reported IMRT approach for LAPC before long-term A-ADT became standard care, we only applied NA-ADT for relatively short periods (median 6 months). However, approximately two-thirds of our cohort remained ADT-free 8 years after high-dose IMRT, despite markedly unfavorable conditions (T3–T4; mean PSA = 37 ng/ml). In addition, our approach to LAPC was associated with favorable survival outcomes (8-year PCSS and OS rates were 96.6 and 89.1 %, respectively) despite non-application of A-ADT. Although somewhat speculative, both dose escalation and the early initiation of S-ADT might have contributed to these favorable results.
Bolla et al. demonstrated the significant survival benefit of long-term (3 years) A-ADT over a 6-month period when ADT was combined with an EBRT of 70 Gy (prostate cancer-specific mortality 3.2 vs 4.7 % at 5 years, p = 0.002) [12]. However, the difference in prostate cancer-specific mortality was far smaller in comparison to the difference observed in a previously reported study comparing EBRT alone versus EBRT in conjunction with 3-year A-ADT (30.4 vs 10.3 % at 10 years, p < 0.0001) [3]. Similarly, in the RTOG 92-02 study which compared the impact of short-term (4 months) and long-term (24 months) ADT combined with standard-dose EBRT, the difference in prostate cancer-specific mortality was also smaller, although the difference was statistically significant (16.1 vs 11.3 % at 10 years, p = 0.0042) [26]. In the 92-02 study, the OS rate was not statistically significant between the arms (51.6 vs 53.9 % at 10 years, p = 0.36). In addition, the TROG 0304 study which compared the impact of short-term (6 months) and intermediate-term (18 months) ADT combined with standard-dose EBRT ± zoledronic acid, failed to show any significant difference in both prostate cancer-specific mortality and all-cause mortality [27].
Furthermore, the timing of S-ADT initiation was neither clearly defined nor reported in those studies. Logically, if we shifted a PSA value corresponding to the threshold for S-ADT commencement such that it became progressively lower, it would eventually reach an almost-identical value to that of A-ADT, at which point comparable survival outcomes would obviously be expected. Therefore, we hypothesize that the inferior survival outcomes that characterize shorter ADT arms are, at least partially, attributable to the delayed initiation of S-ADT.
In fact, it has been suggested that delayed initiation of salvage ADT results in inferior survival outcomes compared with that started in an earlier phase [18–20]. Shipley et al. reported a significant increase in PCSS rates among patients who developed PSA recurrence following treatment with EBRT alone, or combined with short-term NA-ADT in the RTOG 86-10 trial, when patients with PSAs of <20 ng/ml were compared to those with PSAs ≥20 ng/ml at the time of S-ADT commencement (p = 0.03) [18]. Mydin et al. also reported that OS rates differ significantly (p < 0.005) between recurred patients without distant metastases with PSA levels of ≤10 ng/ml at the time of S-ADT initiation, (early S-ADT), and patients with PSAs levels of >10 ng/ml (delayed S-ADT) (78 vs 42 % at 10 years, respectively), based on a secondary analysis of the Irish Clinical Oncology Research Group trial 97-01 [19]. Similarly, Souhami et al. reported that OS rates were significantly higher in an early (PSAs of <10 ng/ml) versus late S-ADT (PSAs of ≥10 ng/ml) group (hazard ratio 1.5; p = 0.01), based on a secondary analysis of the RTOG trial 85-31 [20].
A protocol for definitive 3D-CRT combined with short-term NA-ADT for patients with T1-T4N0M0 prostate cancer was established at Kyoto University in 1997. In this protocol, A-ADT was not included because the benefit of adding long-term A-ADT for patients with LAPC treated with EBRT had not been reported. In addition, a trigger PSA value of >4 ng/ml for initiating S-ADT was set with the intention to observe the true effect of EBRT on prostate cancer [28, 29]. In the subsequently established IMRT protocol in 2001 (reported previously), the same approach was maintained, because the intermediate-term outcomes of the 3D-CRT protocol appeared promising.
In Japan, a randomized trial [30] comparing 6-month A-ADT followed by intermittent ADT versus long-term (5 years) A-ADT in patients with LAPC treated by 6-month NA-ADT plus EBRT of 72 Gy has been conducted, and currently the study is in the follow-up period. The trigger PSA value for restarting ADT in the intermittent arm was set at 5 ng/ml. Therefore, the approach of the intermittent arm is similar to our approach, although there are some differences in the radiation dose (72 vs 78 Gy), the period of A-ADT (6 months vs 0 months), and the trigger PSA value of re-initiating ADT (5 vs 4 ng/ml). It was reported that A-ADT was in the rest condition in 98.3 % of the total period for intermittent cases [31]. This suggests, in an indirect manner, that the survival outcome may comparable between the two arms, although we need to wait for the final results of the study.
Although the guidelines recommend the addition of long-term A-ADT to LAPC cases [10, 11], we call into question the validity of uniformly providing long-term A-ADT. First, approximately two-thirds of our cases still enjoy ADT-free status at 8 years following IMRT, as well as favorable PCSS and OS outcomes. Second, the adverse effects of providing long-term ADT are not negligible [32, 33]. The use of long-term ADT might lead to numerous side-effects, including osteoporosis, obesity, sarcopenia, lipid alterations, insulin resistance, and increased risk for diabetes and cardiovascular morbidity [33], as well as significant deterioration of self-reported, health-related quality of life [32].
Our study had several limitations, such as the limited number of cases, and the everyday clinical practice-based design employed. However, to our knowledge, this is the first report pertaining to long-term outcomes in a substantial cohort of T3-T4 prostate cancer patients, treated using high-dose IMRT combined with NA-ADT under a predefined early S-ADT policy. In addition, we believe that our approach merits prospective comparison with the current standard approach, of uniformly providing long-term A-ADT, because our data suggests the potential benefit of liberating a considerable proportion of LAPC cases from long-term A-ADT. The validity of our approach should be tested in a prospective randomized trial, in which PCSS or OS was set as the primary endpoint.
Conclusions
Survival outcomes of LAPC patients treated with high-dose IMRT combined with NA-ADT under an early salvage policy were excellent despite non-provision of A-ADT; two-thirds of the patients maintained ADT-free status over the following 8 years. The outcomes of the current study appear promising, and should confer advantages to patients with LAPC in terms of both quality of life and cost. Therefore, this approach might be a viable alternative to routine provision of long-term A-ADT following high-dose EBRT for patients with LAPC, and merits further validation in prospective trials.
References
Ciezki JP, Hsu IC, Abdel-Wahab M et al (2012) American College of Radiology Appropriateness Criteria®—locally advanced (high–risk) prostate cancer. Clin Oncol (R Coll Radiol) 24:43–51. doi:10.1016/j.clon.2011.08.003
Zelefsky MJ, Yamada Y, Kollmeier MA et al (2008) Long-term outcome following three-dimensional conformal/intensity-modulated external-beam radiotherapy for clinical stage T3 prostate cancer. Eur Urol 53:1172–1179. doi:10.1016/j.eururo.2007.12.030
Bolla M, Van Tienhoven G, Warde P et al (2010) External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10 year results of an EORTC randomised study. Lancet Oncol 11:1066–1073. doi:10.1016/s1470-2045(10)70223-0
Viani GA, Stefano EJ, Afonso SL (2009) Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 74:1405–1418. doi:10.1016/j.ijrobp.2008.10.091
Shipley WU, Verhey LJ, Munzenrider JE et al (1995) Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 32:3–12. doi:10.1016/0360-3016(95)00063-5
Kuban DA, Tucker SL, Dong L et al (2008) Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70:67–74. doi:10.1016/j.ijrobp.2007.06.054
Al-Mamgani A, van Putten WL, Heemsbergen WD et al (2008) Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 72:980–988. doi:10.1016/j.ijrobp.2008.02.073
Ohri N, Dicker AP, Showalter TN (2012) Late toxicity rates following definitive radiotherapy for prostate cancer. Can J Urol 19:6373–6380
Cooperberg M, Lubeck DP, Penson D et al (2003) Sociodemographic and clinical risk characteristics of patients with prostate cancer within the Veterans Affairs health care system: data from CaPSURE. J Urol 170:905–908. doi:10.1097/01.ju.0000081200.63275.0b
(2015) NCCN.org. NCCN clinical practice guidlines in Oncology: Prostate cancer Version 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed June 2015
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137. doi:10.1016/j.eururo.2013.09.046
Bolla M, de Reijke TM, Van Tienhoven G et al (2009) Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 360:2516–2527. doi:10.1056/NEJMoa0810095
Denham JW, Steigler A, Lamb DS et al (2011) Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10 year data from the TROG 96.01 randomised trial. Lancet Oncol 12:451–459. doi:10.1016/S1470-2045(11)70063-8
Zhou ZR, Zhu XD, Xia J et al (2013) Short-term versus long-term hormone therapy plus radiotherapy or prostatectomy for prostate cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 139:783–796. doi:10.1007/s00432-013-1383-7
Ciezki JP (2013) High-risk prostate cancer in the modern era: does a single standard of care exist? Int J Radiat Oncol Biol Phys 87:440–442. doi:10.1016/j.ijrobp.2013.06.006
Zelefsky MJ, Kollmeier M, Cox B et al (2012) Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 84:125–129. doi:10.1016/j.ijrobp.2011.11.047
Alicikus ZA, Yamada Y, Zhang Z et al (2011) Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer 117:1429–1437. doi:10.1002/cncr.25467
Shipley WU, Desilvio M, Pilepich MV et al (2006) Early initiation of salvage hormone therapy influences survival in patients who failed initial radiation for locally advanced prostate cancer: a secondary analysis of RTOG protocol 86-10. Int J Radiat Oncol Biol Phys 64:1162–1167. doi:10.1016/j.ijrobp.2005.09.039
Mydin AR, Dunne MT, Finn MA et al (2013) Early salvage hormonal therapy for biochemical failure improved survival in prostate cancer patients after neoadjuvant hormonal therapy plus radiation therapy—a secondary analysis of irish clinical oncology research group 97-01. Int J Radiat Oncol Biol Phys 85:101–108. doi:10.1016/j.ijrobp.2012.03.001
Souhami L, Bae K, Pilepich M et al (2010) Timing of salvage hormonal therapy in prostate cancer patients with unfavorable prognosis treated with radiotherapy: a secondary analysis of Radiation Therapy Oncology Group 85-31. Int J Radiat Oncol Biol Phys 78:1301–1306. doi:10.1016/j.ijrobp.2009.10.007
Norihisa Y, Mizowaki T, Takayama K et al (2012) Detailed dosimetric evaluation of intensity-modulated radiation therapy plans created for stage C prostate cancer based on a planning protocol. Int J Clin Oncol 17:505–511. doi:10.1007/s10147-011-0324-1
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346. doi:10.1016/0360-3016(95)00060-C
Roach M 3rd, Hanks G, Thames H Jr et al (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65:965–974
Bria E, Cuppone F, Giannarelli D et al (2009) Does hormone treatment added to radiotherapy improve outcome in locally advanced prostate cancer?: meta-analysis of randomized trials. Cancer 115:3446–3456. doi:10.1002/cncr.24392
Sasse AD, Sasse E, Carvalho AM et al (2012) Androgenic suppression combined with radiotherapy for the treatment of prostate adenocarcinoma: a systematic review. BMC Cancer 12:54. doi:10.1186/1471-2407-12-54
Horwitz EM, Bae K, Hanks GE et al (2008) Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26:2497–2504. doi:10.1200/JCO.2007.14.9021
Denham JW, Joseph D, Lamb DS et al (2014) Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): an open-label, randomised, phase 3 factorial trial. Lancet Oncol 15:1076–1089. doi:10.1016/S1470-2045(14)70328-6
Sakamoto M, Mizowaki T, Mitsumori M et al (2010) Long-term outcomes of three-dimensional conformal radiation therapy combined with neoadjuvant hormonal therapy in Japanese patients with locally advanced prostate cancer. Int J Clin Oncol 15:571–577. doi:10.1007/s10147-010-0109-y
Mizowaki T, Takayama K, Norihisa Y et al (2012) Long-term outcomes of three-dimensional conformal radiation therapy combined with neoadjuvant hormonal therapy for Japanese patients with T1c-T2N0M0 prostate cancer. Int J Clin Oncol 17:562–568. doi:10.1007/s10147-011-0326-z
Yamanaka H, Ito K, Naito S et al (2005) Effectiveness of adjuvant intermittent endocrine therapy following neoadjuvant endocrine therapy and external beam radiation therapy in men with locally advanced prostate cancer. Prostate 63:56–64. doi:10.1002/pros.20171
Ito K et al (2007) Abstract of the 95th Annual Meeting of the Japanese Urological Association. (in Japanese). Nihon Hinyoukikagakkai-zasshi 98:87
Berg A, Dahl AA, Bruland OS et al (2009) Definitive radiotherapy with adjuvant long-term antiandrogen treatment for locally advanced prostate cancer: health-related quality of life and hormonal changes. Prostate cancer and prostatic diseases 12:269–276. doi:10.1038/pcan.2009.8
Isbarn H, Boccon-Gibod L, Carroll PR et al (2009) Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol 55:62–75. doi:10.1016/j.eururo.2008.10.008
Acknowledgments
This work was supported in part by Grants-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (24591838) Japan, and New Energy and Industrial Technology Development Organization (NEDO).
Conflict of interest
Authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mizowaki, T., Norihisa, Y., Takayama, K. et al. Long-term outcomes of intensity-modulated radiation therapy combined with neoadjuvant androgen deprivation therapy under an early salvage policy for patients with T3-T4N0M0 prostate cancer. Int J Clin Oncol 21, 148–155 (2016). https://doi.org/10.1007/s10147-015-0867-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0867-7