Abstract
The second edition of the Japan Society of Gynecologic Oncology guidelines for the treatment of uterine cervical cancer was published in 2011. The guidelines comprise eight chapters and five algorithms. They were prepared by consensus among the members of the Japan Society of Gynecologic Oncology Guidelines Formulation Committee and Evaluation Committee and are based on a careful review of the evidence obtained from the literature, health insurance system, and actual clinical settings in Japan. The highlights of the 2011 revision are (1) the recommended grades have been changed to five stages—A, B, C1, C2, and D; (2) the revisions are consistent with the new International Federation of Gynecology and Obstetrics staging system; (3) the roles are shared between the ‘Japanese classification of cervical cancer’ and the new guidelines; (4) clinical questions related to adenocarcinoma have been revised; and (5) a clinical question regarding cervical cancer in pregnant patients has been added. Each chapter includes a clinical question, recommendations, background, objectives, explanations, and references. Each recommendation is accompanied by a classification of recommendation categories. The objective of these guidelines is to update the standard treatment strategies for cervical cancer, thus eliminating unnecessary and insufficient treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An estimated 6,000 new cases of invasive cervical cancer were diagnosed in Japan in 2011 [1], and 2,737 women died of the disease [2]. The mortality rate associated with cervical cancer in Japan decreased from the 1960s until 1995; however, the incidence of cervical cancer has slightly increased [2].
The first edition of the Japan Society of Gynecologic Oncology (JSGO) guidelines for the treatment of uterine cervical cancer was published in 2007 [3]; however, some clinical questions (CQs) in the first edition remained unanswered. The second edition, published in 2011, was intended to represent an aggregation of domestic evidence while collecting up-to-date international evidence without providing a new section. For the first time, we accepted specialist physicians engaged in clinical practice in cancer centers or university hospitals as candidates for the committee. Radiation oncologists and pathologists were also members of the guideline committee.
The highlights of the 2011 revision are indicated below.
-
1.
The recommended grades have been changed to five stages—A, B, C1, C2, and D.
-
2.
The revisions are consistent with the new International Federation of Gynecology and Obstetrics (FIGO) staging system. The new FIGO staging system was revised during the creation of these updated guidelines. The new FIGO classification excludes stage 0 carcinoma in situ; however, stage 0 still has high importance in the guidelines because many people, especially young people, have stage 0 disease. Therefore, stage 0 is present in the guidelines. Additionally, stage IIA has been reclassified to stage IIA1 and stage IIA2 in the new FIGO classification. This revision from the Japan Society of Obstetrics and Gynecology ‘Japanese classification of cervical cancer’ has been adopted, and the reclassification to stage IIA1 and IIA2 is present in the new guidelines.
-
3.
Roles are shared between the ‘Japanese classification of cervical cancer’ and the new guidelines. A specific radiotherapy technique is detailed in the guidelines.
-
4.
CQs related to adenocarcinoma have been revised. Few clinical trials on adenocarcinoma alone have been conducted; thus, the chapter on adenocarcinoma was deleted and a CQ related to adenocarcinoma is described in each chapter.
-
5.
A CQ regarding cervical cancer in pregnant patients has been added. Because of the increasing incidence of cervical cancer in younger patients and of pregnancy in older patients, the treatment of cervical cancer and its complications owing to pregnancy should be addressed. Therefore, these treatment guidelines are described in detail by increasing the CQs relevant to this topic.
Treatment guidelines for cervical cancer
Chapter 1: Overview of guidelines
1. How to use these guidelines
These guidelines are intended for doctors (general practitioners and specialists) who provide medical care for patients with cervical cancer. The guidelines aim to provide useful treatment methods by integrating previous evidence of treatment benefits. However, the guidelines are not intended to be limited to the therapies listed. Their main purposes are (1) to indicate the current cervical cancer treatments that are considered appropriate, (2) to reduce differences in therapy among various institutions, (3) to improve the prognosis and safety of treatments, (4) to reduce the economic and psychosomatic burden on patients by performing appropriate treatment, and (5) to promote mutual understanding between healthcare professionals and patients.
The JSGO bears the responsibility for the content and descriptions of these guidelines. However, the final decision to use these guidelines should be made by the individual user. Thus, the physicians in charge of treatment are responsible for the outcome of treatment.
2. Method used to prepare these guidelines
To create these guidelines, the Guidelines Formulation Committee and Evaluation Committee were established independently from the Committee for the Treatment Guidelines for Cervical Cancer. The initial draft was created by thoroughly evaluating the various opinions from within and outside the JSGO prior to incorporating them into the final draft. The guidelines were published after approval by the JSGO.
(1) Classification of evidence
-
1.
The guidelines were created in accordance with the international standard procedures of evidence-based medicine used for the creation of clinical practice guidelines.
-
2.
In principle, searches of data and published literature were performed prior to December 2009 in Japan and overseas, and evidence was collected.
-
3.
This collected evidence was evaluated for quality using the criteria of the Japan Society of Clinical Oncology and its Formulation Committee on clinical practice guidelines for the use of anticancer agents [4, 5]; however, it was modified to allow some of it to fit into the guidelines (Table 1).
Table 1 Classification of evaluation criteria for evidence quality
(2) Clinical questions and classification of recommendation categories
As a result of the discussions held by the Guideline Committee, controversial issues were selected as CQs and associated recommendations were made. Each recommendation in response to a CQ is accompanied by a classification of the evidence and a classification of the recommendation categories based on the consensus reached by the Guideline Committee members.
The strengths of the recommendations in our guidelines were also determined by the recommendation criteria of the Japan Society of Clinical Oncology and its Formulation Committee of Clinical Practice Guidelines for the Use of Anticancer Agents [6]. These were modified while referring to the ‘Guide 2007 Minds practice guidelines’ (Tables 2, 3).
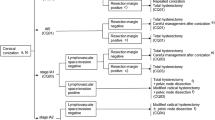
Chapter 2: Primary treatment for stage 0 to IA cervical cancer (Fig. 1)
CQ01. What treatments are recommended for carcinoma in situ?
Recommendations A cervical cone biopsy is recommended (grade B).
Primary treatment for stage 0 to IA cervical cancer. a If cervical conization is difficult because of atrophy of the cervix, such as in older patients, omission of the conization may be considered. However, prior to surgery, it is necessary to carefully review the cytology, colposcopy, and biopsy tissue findings; this allows for the performance of a hysterectomy suitable for the estimated lesion. b Cervical canal curettage should be performed at the time of cervical conization. If cervical curettage is positive, the patient should be treated as if they have positive margins. c Hysterectomy may be considered if the patient does not wish to preserve her fertility. d Residual lesions are reportedly found in about 20 % of cases involving negative margins. Careful inspection is required to preserve the uterus. e In the NCCN clinical practice guidelines in oncology, radiation therapy is also an option for patients with cervical cancer. f Operative procedures should be individualized according to the histopathological findings of the conization specimens, namely the extent of invasion and the presence or absence of lymphovascular infiltration
CQ02. What treatments are recommended for recurrence following conservative treatment?
Recommendations (1) For recurrence following laser cone biopsy or the loop electrosurgical excision procedure, the same procedure should be repeated or a total hysterectomy considered, depending on the patient (grade B). (2) For recurrence following laser ablation or cryotherapy, either a cone biopsy or total hysterectomy is recommended (grade B).
CQ03. What treatments are recommended for stage IA1 disease?
Recommendations (1) It is possible to preserve the uterus by performing a cervical cone biopsy in patients who strongly desire fertility preservation; however, these patients must have no vascular or lymphatic infiltration, negative resection margins, and negative histological results from endocervical curettage (grade B). (2) A total hysterectomy without pelvic lymphadenectomy is recommended for patients with no evidence of vascular or lymphatic infiltration (grade B). (3) Both a modified radical hysterectomy and pelvic lymphadenectomy are sometimes performed for patients with vascular or lymphatic infiltration (grade C1).
CQ04. What treatments are recommended for stage IA2 disease?
Recommendations (1) A modified radical hysterectomy or a more extensive procedure with lymphadenectomy should be considered for stage IA2 disease (grade C1). (2) After thorough histopathological examination of a specimen obtained by diagnostic conization, omission of lymphadenectomy in patients with no vascular or lymphatic infiltration can be considered (grade C1).
CQ05. What treatments are recommended if the disease is upstaged to stage IB or higher following total hysterectomy?
Recommendations Adjuvant radiotherapy or concurrent chemoradiotherapy (CCRT) should be considered (grade C1).
CQ06. What treatments are recommended for adenocarcinoma in situ?
Recommendations (1) A total hysterectomy is recommended (grade B). (2) Uterus preservation can be considered with cervical cone biopsy in patients who strongly desire fertility preservation. However, careful management is required (grade C1).
CQ07. What treatments are recommended for stage IA adenocarcinoma?
Recommendations (1) In cases involving deep invasion, a radical hysterectomy or modified radical hysterectomy with pelvic lymphadenectomy should be considered (grade C1). (2) In cases involving shallow invasion, a hysterectomy without pelvic lymphadenectomy (total hysterectomy or modified radical hysterectomy) can also be considered (grade C1). (3) If the patient strongly desires fertility preservation, a cervical cone biopsy can be performed to preserve the uterus. Careful case selection is required (grade C1).
Chapter 3: Primary treatment for stage IB to II cervical cancer (Fig. 2)
CQ08. What treatments are recommended for stage IB1 and IIA1 squamous cell carcinoma?
Recommendations A radical hysterectomy or radiation therapy is recommended (grade B).
Primary treatment for stage IB to II cervical cancer (including squamous cell carcinoma and adenocarcinoma). a Primary treatment for stage IB to II cervical cancer should be performed with caution because the tolerability of concurrent chemoradiation therapy among Japanese women has not been sufficiently tested
CQ09. What treatments are recommended for stage IB2 and IIA2 squamous cell carcinoma?
Recommendations A radical hysterectomy (+ adjuvant therapy) or CCRT is recommended (grade B).
CQ10. What treatments are recommended for stage IIB squamous cell carcinoma?
Recommendations A radical hysterectomy (+adjuvant therapy) or CCRT is recommended (grade B).
CQ11. Is neoadjuvant chemotherapy recommended for stage IB and II squamous cell carcinoma?
Recommendations Neoadjuvant chemotherapy can be considered depending on the extent and size of the tumor (grade C1).
CQ12. Is pelvic nerve preservation recommended in radical hysterectomy?
Recommendations Pelvic nerve preservation can be considered when curability is not impaired (grade C1).
CQ13. Is ovary preservation possible in radical hysterectomy?
Recommendations (1) Ovary preservation is possible without compromising curability if appropriate case selection is performed by considering the patient’s histological type or stage (grade B). (2) If the ovaries are to be preserved, ovarian transposition and fixation outside of the pelvic radiation field can be considered (grade C1).
CQ14. Is para-aortic lymphadenectomy recommended in radical hysterectomy?
Recommendations If diagnostically useful, para-aortic lymphadenectomy can be considered to search for metastasis or determine the irradiation field (grade C1).
CQ15. What treatments are recommended for stage IB and II adenocarcinoma?
Recommendations In principle, surgery should be considered for stage IB and II disease (grade C1).
Chapter 4: Postoperative therapy for stage IB to II cervical cancer (Fig. 3)
CQ16. What is the recommended postoperative adjuvant therapy?
Recommendations (1) CCRT is recommended for patients at high risk of recurrence (grade B). (2) Radiation therapy is recommended for patients at intermediate risk of recurrence. However, CCRT can be considered depending on the number and extent of risk factors (grade C1).
CQ17. What irradiation methods are recommended when performing postoperative adjuvant radiotherapy for a patient at high risk of relapse?
Recommendations (1) Whole-pelvis irradiation is recommended (grade B). (2) Three-dimensional treatment planning is recommended (grade B). (3) The addition of intracavitary irradiation is not recommended with the exception of cases involving positive margins (grade C2).
CQ18. For whom is prophylactic para-aortic irradiation indicated?
Recommendations Para-aortic irradiation can be considered for patients with a high risk of recurrence in the para-aortic lymph nodes (grade C1).
CQ19. Are oral anticancer drugs and immunotherapy recommended as maintenance therapies?
Recommendations (1) Oral anticancer agents are not recommended because their usefulness is unclear (grade C2). (2) Immunotherapy is not recommended because its usefulness has not been fully verified (grade C2).
Chapter 5: Primary therapy for stage III to IV cervical cancer (Fig. 4)
CQ20. Which is the recommended radiotherapy for stage III and IVA disease: definitive radiotherapy or CCRT?
Recommendations CCRT is recommended rather than radiation monotherapy (grade B).
Primary treatment for stage III to IV cervical cancer (including squamous cell carcinoma and adenocarcinoma). a Primary treatment for stage III to IV cervical cancer should be performed with caution because the tolerability of concurrent chemoradiation therapy among Japanese women has not been sufficiently tested
CQ21. What CCRT regimens are recommended for stage III and IVA disease?
Recommendations Regimens that include cisplatin are recommended (grade A).
CQ22. Is chemotherapy recommended prior to principal treatment for stage III and IVA disease?
Recommendations (1) Chemotherapy is not recommended before radiotherapy (grade D). (2) Chemotherapy is not recommended before surgery (grade C2). (3) For adenocarcinoma, chemotherapy is not recommended before primary treatment (grade C2).
CQ23. Is surgery recommended for stage III and IVA disease?
Recommendations Surgery is not recommended (grade C2).
CQ24. What treatments are recommended for stage IVB disease?
Recommendations (1) Systemic chemotherapy can be considered for patients with a good performance status and preserved organ function (grade C1). (2) Surgery, radiotherapy, chemotherapy, or a combination of these treatments can be selected for patients with distant metastatic lesions, such as resectable lung metastases, or with lymph node metastases only (grade C1). (3) If the patient has severe symptoms accompanying oncological complications, palliative radiotherapy of the causal lesion is recommended (grade B).
CQ25. What treatments are recommended for stage III and IV adenocarcinoma?
Recommendations CCRT involving external irradiation and intracavitary irradiation is recommended for stage III or VIA adenocarcinoma (grade B). (2) A platinum-based agent other than cisplatin, either as monotherapy or as part of combination chemotherapy, can also be considered for patients with stage IVB adenocarcinoma with preserved organ function (grade C1).
Chapter 6: Therapies for relapsed cervical cancer (Fig. 5)
CQ26. What treatment methods are recommended for recurrence confined to the pelvis if radiotherapy has not been previously performed?
Recommendations (1) Radiotherapy is recommended (grade B). (2) CCRT can also be considered (grade C1).
CQ27. What treatments are recommended for recurrence within the radiation field?
Recommendations (1) Palliative treatment for symptomatic relief is the general rule for treatment (grade C1). (2) Chemotherapy can also be considered, keeping in mind that the response rate is low for recurrence within the radiation field (grade C1). (3) Localized radiotherapy or pelvic exenteration can also be considered for central recurrence in the vaginal stump after a thorough preoperative evaluation (grade C1). (4) Re-irradiation is not recommended (grade C2).
CQ28. What treatments are recommended for recurrence outside the radiation field or for extrapelvic recurrence if radiotherapy has not been previously performed?
Recommendations (1) Para-aortic metastasis: radiation therapy or CCRT can be considered for solitary metastasis (grade C1). (2) Brain metastasis: (a) stereotaxic radiosurgery along with whole-brain radiation therapy (WBRT) or WBRT alone is recommended for metastases of up to three sites (grade B). (b) WBRT is recommended for more than four metastases (grade B). (3) Bone metastasis: (a) single-fraction or multi-fraction radiotherapy is recommended for pain relief (grade B). (b) Bisphosphonates are recommended for symptom relief (grade B). (c) Strontium chloride can be considered for multiple bone metastases if medical therapy is ineffective (grade C1). (4) Lung metastasis: resection or stereotactic body radiotherapy can be considered for one to three localized metastases (grade C1).
CQ29. Is systemic chemotherapy recommended for recurrence?
Recommendations Systemic chemotherapy is recommended for patients with disease that is difficult to control by surgery or radiotherapy as well as for patients with a good performance status and preserved organ function (grade B).
CQ30. What systemic chemotherapy regimens are recommended to treat recurrent disease?
Recommendations (1) Cisplatin as either monotherapy or part of two-drug combination chemotherapy is recommended (grade B). (2) A platinum-based agent other than cisplatin, as either monotherapy or part of two-drug combination chemotherapy, can also be recommended (grade B). (3) Cisplatin as either monotherapy or part of two-drug combination chemotherapy is preferable for recurrent adenocarcinoma (grade C1).
Chapter 7: Management of cervical cancer during pregnancy
CQ31. What treatments are recommended for stage 0 disease during pregnancy?
Recommendations (1) Cone biopsy may be delayed until after delivery as long as the diagnosis is stage 0 disease based on consistent cytology, colposcopy, or biopsy analysis results (grade C1). (2) If adenocarcinoma in situ is suspected, a cone biopsy should be performed to determine the diagnosis during pregnancy (grade C1).
CQ32. What treatments are recommended for stage IA disease during pregnancy?
Recommendations If stage IA or higher disease is suspected, a cervical cone biopsy should be considered to determine the diagnosis during pregnancy (grade C1).
CQ33. What treatments are recommended for invasive cancer during pregnancy?
Recommendations If the diagnosis made during the gestational period (usually during the 3rd trimester) indicates that the fetus can survive outside the uterus, standard treatment after delivery can be considered (grade C1).
Chapter 8: Surveillance after treatment for cervical cancer
CQ34. What intervals are recommended for post-treatment surveillance?
Recommendations The following intervals are recommended for standard surveillance (grade C1):
For the first 1–2 years: every 1–3 months
For the 3rd year: every 3–6 months
For the 4th and 5th years: every 6 months
From the 6th year: every 12 months
CQ35. What investigations and examinations should be performed during post-treatment surveillance?
Recommendations (1) A physical examination (including pelvic and rectal examination), cytological examination, chest radiography, measurement of tumor markers, and diagnostic imaging should be performed (grade C1). (2) Any complications associated with surgery, radiotherapy, or chemotherapy should be noted (grade C1).
References
Gynecologic cancer committee (2012) Gynecologic cancer committee report in 2011. Acta Obstetrica et Gynaecologica Japonica 64:2340–2388
Cancer mortality in Japan (1958–2012) Center for cancer control and information services, National Cancer Center, Japan. http://ganjoho.jp/professional/statistics/statistics.html. Accessed 15 Sep 2014
Nagase S, Inoue Y, Umesaki N et al (2010) Evidence-based guidelines for treatment of cervical cancer in Japan: Japan Society of Gynecologic Oncology (JSGO) 2007 edition. Int J Clin Oncol 15:117–124
Ariyoshi H (2002) Guideline for correct use of antineoplastic agents (draft). General theory. Gan To Kagaku Ryoho 29:970–977
Ochiai K, Okamoto A, Katsumata N (2002) Guidelines for proper use of antineoplastic agents. Gynecologic cancer. Gan To Kagaku Ryoho 29:1047–1054
Fukui T, Yoshida M, Yamaguchi N (2007) Minds Guidance of practice guidelines 2007. Igakushoin, Tokyo
Acknowledgments
We thank the Japan Society of Obstetrics and Gynecology, the Japan Association of Obstetricians and Gynecologists, the Japanese Gynecologic Oncology Group, the Japan Society of Clinical Oncology, and the Japanese Society for Therapeutic Radiology and Oncology for their comments and contributions throughout the project. We also acknowledge support by grants from the Ministry of Health, Labour and Welfare; H24-Clinical Cancer Research-001 (chief researcher, Koichi Hirata).
Conflicts of interest
None of the members of the committee in charge of the preparation of these guidelines has any conflict of interest with entities such as a specific profit or nonprofit organization. The board of the Society Conflict of Interest Committee confirmed the self-reported absence of any conflicts of interest by the Guideline Committee members.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article originally appeared in Japanese as Shikyu keigan chiryo gaidorain 2011 nen ban, published by Kanehara, Tokyo, 2011.
Appendices
Members of guidelines formulation committee (alphabetical order)
Yoichi Aoki, Yasuhiko Ebina, Takayuki Enomoto, Hisaya Fujiwara, Toru Hachisuga, Yasuyuki Hasuo, Atsushi Hongo, Seiryu Kamoi, Takahiro Kasamatsu, Hidetaka Katabuchi, Naoki Kawamura, Hiroaki Kobayashi, Yoichi Kobayashi, Takeshi Kodaira, Junichi Kodama, Kaneyuki Kubushiro, Masaki Mandai, Mikio Mikami, Yoshiki Mikami, Etsuko Miyagi, Toshinari Muramatsu, Tetsuro Nagasaka, Satoru Nagase, Toru Nakanishi, Kaei Nasu, Hideyuki Ohtake, Tsuyoshi Saito, Toshiaki Saito, Masaru Sakamoto, Muneaki Shimada, Nao Suzuki, Tsutomu Tabata, Masashi Takano, Nobuhiro Takeshima, Takafumi Toita, Yasuhiro Udagawa, Takashi Uno, Yoh Watanabe, Nobuo Yaegashi, Masanori Yasuda, Yoshihito Yokoyama
Members of guidelines evaluation committee (alphabetical order)
Daisuke Aoki, Masaki Fujimura, Keiichi Fujiwara, Masayuki Hatae, Masamichi Hiura, Yoshiki Inoue, Kiyoshi Ito, Hisao Ito, Tsuyoshi Iwasaka, Toshiko Jobo, Tsunehisa Kaku, Toshiharu Kamura, Noriyuki Katsumata, Junzo Kigawa, Eizo Kimura, Tsunekazu Kita, Hiroyuki Kuramoto, Yoshinari Matsumoto, Takashi Nishida, Kazunori Ochiai, Yujiro Ota, Aikou Okamoto, Isao Otsuka, Satoru Sagae, Noriaki Sakuragi, Mayumi Sugiura, Toru Sugiyama, Mitsuaki Suzuki, Hironori Tashiro, Hiroshi Tsuda, Masatsugu Ueda, Naohiko Umesaki, Masato Yamasaki, Hiroyuki Yoshikawa
About this article
Cite this article
Ebina, Y., Yaegashi, N., Katabuchi, H. et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 20, 240–248 (2015). https://doi.org/10.1007/s10147-015-0806-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0806-7