Abstract
Background
Salivary duct carcinoma (SDC) is a highly aggressive disease which often metastasizes to distant sites, and there is no established standard therapy for this systemic disease. Given that SDC is biologically similar to breast and prostate cancer, anti-androgenic receptor (AR) and anti-human epidermal growth factor receptor 2 (HER2) therapies have the potential to exert effects, not only on patients with breast and prostate cancer but also on those with SDC.
Methods
The expression levels of HER2, epidermal growth factor receptor (EGFR), Ki-67, and AR were assessed in 32 patients with SDC, and their correlations with overall survival (OS) and disease-free survival (DFS) were analyzed retrospectively. SDC was classified into five subtypes using a method similar to that used for breast cancer.
Results
Anti-AR, HER2, and EGFR were positive in 23 (71.9 %), 14 (43.8 %), and 26 (81.3 %) cases, respectively. One or more of these 3 factors were positive in 30 (93.8 %) cases. The Ki-67 labeling index was greater than 15 % in all cases. While molecular status did not correlate with OS, EGFR and AR positivity were significantly associated with DFS in univariate analysis. Multivariate analysis revealed that EGFR was the only independent predictor of DFS.
Conclusions
The statuses of some molecules are useful to predict DFS in patients with SDC. Ki-67 overexpression suggests that cytotoxic agents are effective for SDC. Since the majority of SDCs express AR, HER2, and/or EGFR, assessing and targeting these molecules are promising strategies to improve the prognosis of unresectable, metastatic or recurrent SDC, and a classification system according to the molecular expression status may be useful to select appropriate therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salivary duct carcinoma (SDC) is a malignant disease which was first described in 1968 by Kleinsasser [1]. SDC was listed in the second edition of the World Health Organization (WHO) Classification of Salivary Tumors for the first time in 1991 [2] and described as “an aggressive adenocarcinoma which resembles high-grade breast ductal carcinoma” [3]. SDC has an extremely poor prognosis; 5-year survival rates of all patients, patients with T2 to T4 tumors, and patients with stage IV disease have been reported to be 20–30 % [4, 5], 0 % [6], and 23 % [7], respectively. The most frequent cause of death is distant metastasis, which occurs in 46–62 % of SDC patients. Thus, control of distant metastasis is the major concern when treating SDC [8]. Although the development of systemic therapies is required to improve the prognosis [9], prospective clinical trials cannot be performed because of the low prevalence of SDC. Some chemotherapeutic agents have been tried but nothing has had significant effectiveness thus far [10].
SDC is similar to breast cancer in that human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), Ki-67, and androgen receptor (AR) are overexpressed in many cases [11, 12]. Currently, breast cancer is classified into five molecular subtypes based on the expression profiles of estrogen receptor (ER), progesterone receptor (PgR), HER2, and Ki-67 labeling index (LI) as follows: luminal A, luminal B, luminal B HER2, HER2-enriched, and triple negative. This classification is mainly determined by immunohistochemical findings and offers a strong suggestion of which medical treatment should be selected [13]. Endocrine therapy is recommended for ER- or PgR-positive patients, and administration of anti-HER2 targeted agent is recommended for HER2-positive patients. Patients with high Ki-67 LI are additionally treated with cytotoxic agents. The efficacy of anti-EGFR therapy is under investigation for the basal-like subtype which is defined as breast cancer negative for ER, PgR, and HER2 (triple negative) but positive for CK5/6 and/or EGFR [14]. In SDC, ER and PgR are very rarely expressed while AR is often expressed [4, 5, 11, 15]. In prostate cancer, AR is involved in generation, progression, and relapse of the disease [16], and a treatment protocol targeting AR for AR-positive prostatic cancer is already established [17].
Given that SDC is similar in its histopathological and immunohistochemical findings to breast and prostate cancer, treatment strategies used for breast and prostate cancer have the potential to exert antitumor effects on SDC. In this study, we have examined the expression levels of AR, HER2, EGFR, and Ki-67, and classified SDCs using a novel method similar to the classification of breast cancer. The relationship between prognosis and the expression levels of AR, HER2, EGFR, and Ki-67 was also analyzed, thereby assessing the feasibility of a customized systemic treatment using cytotoxic drugs and/or molecularly targeted agents appropriate to the biological characteristics of SDC.
Patients and methods
Eligible patients
Tissue samples were obtained from patients diagnosed with SDC and other salivary gland carcinomas which are similar to SDC in histopathological findings, including adenocarcinoma, not otherwise specified, acinic cell carcinoma, squamous cell carcinoma, anaplastic carcinoma, high grade mucoepidermoid carcinoma, and carcinoma ex pleomorphic adenoma [18], at International University of Health and Welfare Mita Hospital from March 2005 to March 2012. All samples were fixed in 10 % formaldehyde solution and embedded in paraffin. Retrospective histological review was performed according to WHO classification criteria [3] by two pathologists (T.N. and S.M.) [18–20], and patients whose diagnoses were confirmed as SDC were eligible for this study. While SDCs of parotid gland, submandibular gland, sublingual gland, minor salivary glands in the oral cavity, oropharynx, and parapharyngeal space were included, the glandular tumors of hypopharynx, paranasal sinuses, larynx, and trachea were excluded from this study. Most patients were treated surgically and postoperative irradiation and/or chemotherapy were applied if necessary as the first-line treatment (Table 1). If recurrent tumor was detected, salvage surgery, radiotherapy, or chemotherapy were applied for treatable patients. Treatment plans were not changed according to the molecular markers. The study protocol was approved by the ethical board in our institution and written consent was obtained from each patient.
Immunohistochemical (IHC) analysis
Tumor tissue sections (4-μm, formalin-fixed, paraffin-embedded) were immunohistochemically assessed using the following primary antibodies: anti-HER2 (PATHWAY anti-HER-2/neu [4B5], Roche Diagnostics, Penzberg, Germany), anti-EGFR (CONFIRM EGFR, Roche Diagnostics), anti-AR (clone AR441, Dako, Glostrup, Denmark), and anti-Ki-67 (clone MIB-1, Dako). Heat-mediated antigen retrieval was conducted in 1 mmol/L ethylenediaminetetraacetic acid solution (pH 8.0) for 30 min. A polymer-based detection system with diaminobenzidine was used to detect antigen–antibody reactions. The immunohistochemical assessment was performed by two pathologists (Y.O. and S.M.). Appropriate positive and negative controls were employed for all conditions. AR positivity was evaluated in a manner similar to ER and PgR according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines for evaluation of breast cancer predictive factors [21, 22]; if ≥1 % of tumor cell nuclei are immunoreactive, the tumor is considered to be positive for AR. HER2 and EGFR positivity was scored 0–3+ based on the percentage of positive tumor cells and intensity as follows: 0, no staining or weak staining in fewer than 10 % of the tumor cells; 1+, weak staining in part of the membrane in 10 % or more of the tumor cells; 2+, complete staining of the membrane with weak or moderate intensity in 10 % or more of the tumor cells; 3+, strong and complete staining in 30 % or more of the tumor cells [23]. For EGFR, according to the criteria for evaluating responsiveness of colorectal carcinoma to anti-EGFR treatment, score 0 was considered as EGFR negative and scores 1+ to 3+ were considered as EGFR positive [24]. Ki-67 LI was the percentage of Ki-67-positive cells determined by counting the number of immunoreactive nuclei in at least 1,000 tumor cells. According to the criteria of the Breast Cancer Working Group, tumors with 15 % or more Ki-67 LI were considered to be Ki-67 high [25].
Fluorescence in situ hybridization (FISH) for HER2
A 5-μm paraffin section from each block was placed on a glass slide and subjected to FISH. HER2 amplification was analyzed using FISH HER2 PharmDx (Dako), which contains both fluorescently-labeled HER2/neu gene and chromosome enumeration probe 17 (CEP17). FISH analyses were performed according to the manufacturer’s instructions. In each case, 100 non-overlapped, intact interphase tumor nuclei identified by DAPI staining were evaluated, and gene (red signal) and CEP17 (green signal) copy numbers in each nucleus were assessed. Samples were considered to be amplified when the average copy number ratio, HER2/CEP17, was ≥2.0 in all nuclei evaluated, or when the HER2 signals formed a tight gene cluster. For HER2, positive was defined as either IHC 3+ or FISH positive according to the ASCO/CAP guideline for evaluating breast cancer [23].
Classification of SDC subtypes
A novel classification of SDC into five molecular subtypes using a method similar to that of breast cancer was implemented. SDCs were classified based on expression levels of HER2, EGFR, Ki-67, and AR instead of ER and PgR into following subtypes: “luminal A” [AR (+)/HER2 (−)/Ki-67 (low)], “luminal B” [AR (+)/HER2 (−)/Ki-67 (high)], “luminal B HER2” [AR (+)/HER2 (+)/Ki-67 (any)], “HER2-enriched” [AR (−)/HER2 (+)/Ki-67 (any)], and “double negative” (instead of triple negative in breast cancer) [AR (–)/HER2 (–)/Ki-67 (any)].
Statistical analysis
The primary endpoint of this study was overall survival (OS). Among patients who achieved complete response (CR) after definitive treatment, disease-free survival (DFS) was measured as a secondary endpoint, and was defined as the number of days from the beginning of treatment to the date of relapse, which was evaluated and recorded by each physician. The associations between HER2, EGFR, AR, Ki-67 status and OS or DFS were evaluated by the Kaplan–Meier product-limit method and univariate and multivariate Cox proportional hazard models adjusted by age, sex, primary tumor site, and TNM stage. The measurement of association was the hazard ratio (HR) along with a 95 % confidence interval (CI). All statistical analyses were performed using the software STATA ver. 10 (StataCorp, College Station, TX, USA). All tests were two-sided, and P values <0.05 were considered to be statistically significant.
Results
Patient characteristics and survival
During the study period, 130 patients with salivary gland carcinoma were treated in our hospital and 32 patients (24.6 %) were diagnosed with SDC by histopathological review. The median age was 59 years (range 26–90 years) and the median follow-up period was 1.8 years (range 0.2–6.8 years). Male patients (84 %) were predominant. The primary tumor site was the parotid gland in 25 (78 %), submandibular gland in 4 (13 %), sublingual gland in 1 (3 %), and oral cavity in 2 cases (6 %). Regarding tumor and nodal stage, T2, T4a, N0 and N2b were predominant. Surgery was performed for 30 patients (94 %) including 2 cases (6 %) with metastatic disease as palliative surgery, and postoperative irradiation and/or chemotherapy were applied for 11 (34 %) and 8 (25 %) cases, respectively. Other than surgery, 1 case was treated by irradiation due to advanced age and 1 case with metastatic disease was treated with chemotherapy (Table 1). After the first-line treatment, CR was achieved in 28 patients and 4 patients attained a partial response. During the follow up period, 17 cases (53 %) had recurrent disease. Three cases underwent salvage surgery, 4 cases received irradiation, and 7 cases had chemotherapy after recurrence. Best supportive care was given to the remaining 3 patients. Two-year OS in all patients was 73.2 % (95 % CI 51.3–86.4) and 2-year DFS in patients who achieved CR was 50.9 % (95 % CI 29.3–68.9).
IHC and FISH findings
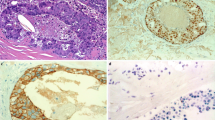
Anti-human epidermal growth factor receptor 2 IHC score was 0 in 4 cases (12.5 %), 1+ in 9 cases (28.1 %), 2+ in 5 cases (15.6 %), and 3+ in 14 cases (43.8 %) (Fig. 1a–d). HER2 FISH was positive in 13 cases (41.9 %) and negative in 18 cases (58.1 %) (Fig. 1e, f). All HER2 IHC scores of 0, 1+ and 2+ tumors were FISH negative, and 13 of 14 HER2 IHC 3+ tumors were positive for FISH. Finally, 14 (43.8 %) of 32 SDCs were HER2 positive.
IHC findings of HER2, AR, EGFR, and Ki-67 and FISH for HER2 compared with CEP17. a HER2 IHC 3+, b HER2 IHC 2+, c HER2 IHC 1+, d HER2 IHC 0, e HER2 FISH-positive (HER2/CEP17 ratio = 6.0), f HER2 FISH-negative (HER2/CEP17 ratio = 1.1), g AR IHC-positive, h AR IHC-negative, i EGFR IHC-positive, j EGFR IHC-negative, k Ki-67 high (LI 80 %). Scale bars, 100 μm (black a–d and g–k), 5 μm (white e, f)
Anti-androgenic receptor was positive in 24 cases (75.0 %). Among 27 male patients, 22 cases (81.5 %) were positive for AR and 2 cases (40.0 %) were positive for AR among 5 female patients (Fig. 1g, h). EGFR was positive in 26 cases (81.3 %) [1+, 7 cases (21.9 %); 2+, 14 cases (43.8 %); 3+, 5 cases (15.6 %)] and negative in 6 cases (18.7 %) (Fig. 1i, j). The median Ki-67 LI was 40 % (range 20–90 %), and all patients were assessed as Ki-67 high (Fig. 1k).
Subtypes of SDCs
There were no cases (0 %) presenting an immunoexpression pattern of luminal A. Twelve cases (37.5 %) of luminal B, 12 cases (37.5 %) of luminal B HER2, 2 cases (6.2 %) of HER2-enriched, and 6 cases (18.8 %) of double negative were observed. Among the double negative SDCs, 4 of 6 cases were EGFR positive. Thus, 30 of 32 (93.8 %) cases were positive for one or more of AR, HER2, and EGFR. There were no significant differences in OS or DFS among the five subtypes (data not shown).
Impact of molecular marker status on clinical outcomes
Anti-human epidermal growth factor receptor 2 status had no significant impact on either OS or DFS. Two-year OS of HER2-negative and HER2-positive cases were 68.4 % (95 % CI 39.5–85.6) and 80.8 % (95 % CI 41.0–95.0), respectively (P = 0.273), and 2-year DFS rates were 44.6 % (95 % CI 18.3–68.0) and 58.9 % (95 % CI 23.1–82.7; P = 0.185), respectively (Fig. 2). While there was no significant difference in 2-year OS between EGFR-negative and EGFR-positive cases [83.3 % (95 % CI 27.3–97.5) vs. 72.8 % (95 % CI 48.7–86.9), P = 0.972], 2-year DFS was significantly worse in EGFR-negative cases than EGFR-positive cases [0.0 % (95 % CI not evaluable) vs. 57.4 % (95 % CI 33.5–75.5), P = 0.020] (Fig. 3). Similarly, no significant difference in 2-year OS was observed between AR-negative and AR-positive cases [72.9 % (95 % CI 27.6–92.5) vs. 73.5 % (95 % CI 46.9–88.3), P = 0.923], whereas AR-negative cases had significantly worse 2-year DFS than AR-positive cases [28.6 % (95 % CI 4.1–61.2) vs. 59.0 % (95 % CI 32.0–78.3), P = 0.011] (Fig. 4). All Ki-67 LI in tumor tissue exceeded 15 % in this study. When the cutoff value of Ki-67 LI was set to 30 %, 2-year OS was 100.0 % (95 % CI not evaluable) in low Ki-67 cases and 64.9 % (95 % CI 39.6–81.7) in high Ki-67 cases, respectively (P = 0.069), and 2-year DFS was 58.3 % (95 % CI 15.7–85.5) in low Ki-67 cases and 47.8 % (95 % CI 23.2–68.9) in high Ki-67 cases (P = 0.520) (Fig. 5). Moreover, when 20, 40, and 60 % were used as arbitrary cutoff values of Ki-67 LI, there were also no significant correlations between Ki-67 LI and prognosis (data not shown). Univariate and multivariate analyses revealed that the expression levels of all molecules assessed in this study had no significant predictive value for OS (Table 2). However, when univariate analysis was performed with DFS as an endpoint, EGFR- and AR-positivity were the significant predictors of increased DFS rate (HR 0.21, 95 % CI 0.05–0.91, P = 0.036, and HR 0.27, 95 % CI 0.09–0.80, P = 0.018, respectively). Multivariate analysis of DFS indicated that only EGFR-positivity was an independent prognostic factor of better DFS (HR 0.02, 95 % CI < 0.01–0.29, P = 0.005), while HER2, AR, and Ki-67 status did not have prognostic value (Table 3).
Kaplan–Meier survival curves of OS and DFS in patients with HER2-positive and negative SDC. Two-year overall survival was 68.4 % (95 % CI 39.5–85.6) in HER2-negative and 80.8 % (95 % CI 41.0–95.0) in HER2-positive cases (logrank test, P = 0.273). Two-year DFS in patients who achieved CR after definitive treatment was 44.6 % (95 % CI 18.3–68.0) in HER2-negative and 58.9 % (95 % CI 23.1–82.7) in HER2-positive cases (logrank test, P = 0.185)
Kaplan–Meier survival curves of OS and DFS in patients with EGFR-positive and negative SDC. Two-year overall survival was 83.3 % (95 % CI 27.3–97.5) in EGFR-negative and 72.8 % (95 % CI 48.7–86.9) in EGFR-positive cases (logrank test, P = 0.972). Two-year DFS in patients who achieved CR after definitive treatment was 0.0 % (95 % CI not evaluable) in EGFR-negative and 57.4 % (95 % CI 33.5–75.5) in EGFR-positive cases (logrank test, P = 0.020)
Kaplan–Meier survival curves of overall and DFS in patients with AR-positive and negative SDC. Two-year OS was 72.9 % (95 % CI 27.6–92.5) in AR-negative and 73.5 % (95 % CI 46.9–88.3) in AR-positive cases (logrank test, P = 0.923). Two-year DFS in patients who achieved CR after definitive treatment was 28.6 % (95 % CI 4.1–61.2) in AR-negative and 59.0 % (32.0–78.3) in AR-positive cases (logrank test, P = 0.011)
Kaplan–Meier survival curves of OS and DFS in patients with SDC based on Ki-67 expression status. Two-year OS was 100.0 % (95 % CI not evaluable) in Ki-67 ≤30 % and 64.9 % (95 % CI 39.6–81.7) in Ki-67 >30 % cases (logrank test, P = 0.069). Two-year DFS among patients who achieved CR after definitive treatment was 58.3 % (95 % CI 15.7–85.5) in Ki-67 ≤30 % and 47.8 % (95 % CI 23.2–68.9) in Ki-67 >30 % cases (logrank test, P = 0.520)
Discussion
Salivary duct carcinoma is a comparatively rare carcinoma accounting for approximately 10 % of salivary gland malignancies [3]. However, in this cohort, SDC accounted for 24.6 % which supports the opinion of Nagao [18] and Batsakis [20] that SDC is not as rare as once thought. To develop customized systemic therapy for SDC, we examined the clinical significance of expression levels of HER2, EGFR, Ki-67, and AR instead of ER and PgR, with reference to the 2011 St. Gallen recommendations/guidelines for breast cancer [13]. As a result, it was found that EGFR- or AR-positive status in univariate analysis and EGFR-positive status in multivariate analysis are prognostic factors favorably affecting 2-year DFS. These molecular factors had no significant correlations with 2-year OS in both univariate and multivariate analyses. The reason for the discrepancy between OS and DFS is thought to be that there were many cases who have responded to secondary treatment after relapse, and further study is required to clarify the impact of these molecular factors on OS.
The expression levels of hormonal receptors such as AR in prostate [26] and breast cancer [12], and ER and PgR in breast cancer [22] have been reported to be associated with favorable prognosis. Hoang et al. [15] have reported that AR expression is associated with lower proliferative activity in SDC. Williams et al. [5] have reported that patients with AR−/ERβ− tumors have decreased survival compared with patients with AR+/ERβ+ tumors or AR+/ERβ− tumors. The present study is the first report to show a correlation between AR expression and favorable prognosis independent of ERβ status in SDC patients, although the prognostic value was not significant in multivariate analysis. AR is expressed in normal prostate, testis, mammary glands, sebaceous glands, and sweat glands, and is now known to be involved with intracellular signaling at all carcinoma stages, including generation, progression, and relapse in prostate cancer. On the other hand, the role of AR in generation or progression of SDC is still unclear, although AR is undetectable in normal salivary glands [27]. As a treatment target, AR plays a key role in androgen deprivation treatment (ADT) for prostate cancer. The efficacy and safety of ADT have been well described [17]. ADT has been reported to be effective for AR-positive salivary gland carcinoma including SDC [28–31]. Further study is warranted to assess whether AR-targeted therapies could improve the prognosis of unresectable and AR-positive SDC cases.
It has been reported that expression of EGFR is a poor prognostic factor in head and neck squamous cell carcinoma [32], prostate cancer [33], and breast cancer [22]. In SDC, there were no previous reports which showed significant correlation between EGFR expression and OS or DFS. In this study, the DFS rate at 2 years was significantly higher in EGFR-positive than EGFR-negative cases in univariate and multivariate analysis, although Williams et al. [9] reported that EGFR overexpression correlated with local recurrence with marginal significance (P = 0.046). Further study is needed as both sets of data were obtained from small cohorts. EGFR serves as a target for anticancer therapy in head and neck squamous cell carcinoma and colorectal carcinoma. There are some reports in which various histological types of salivary gland carcinoma have been treated with cetuximab and/or gefitinib [34–36], but none of these trials included SDC. KRAS mutation was detected in none of 18 studied patients (data not shown), suggesting that anti-EGFR drugs such as cetuximab and panitumumab may be effective for SDC if EGFR is positive.
HER2 gene amplification and protein overexpression was reported to be a significant predictor of poor prognosis in breast cancer [37], and trastuzumab, anti-HER2 monoclonal antibody, was first introduced into clinical practice as a molecularly targeted therapy [38]. Although HER2 gene amplification and protein overexpression is not a prognostic factor in gastric cancer, addition of trastuzumab to chemotherapy significantly improves OS [39]. While it is still unclear whether HER2 gene amplification and/or protein overexpression are predictors of poor prognosis in carcinomas other than breast cancer, a recent report indicates that HER2 is not a prognostic factor in SDC [9]. In this study, HER2 gene amplification and protein overexpression had no effect on OS or DFS. Although various kinds of HER2-positive carcinomas have been treated with trastuzumab combined with chemotherapy, addition of trastuzumab to chemotherapy shows no efficacy in patients with lung, prostate, ovary, pancreas, or urothelial cancer [40–43]. On the other hand, trastuzumab plus taxanes prolong DFS in some cases of metastatic or recurrent salivary gland carcinoma including SDC [44–48]. In this study, approximately 40 % of SDCs were HER2 positive, which could be a good indication for treatment with trastuzumab combined with taxanes.
Expression of Ki-67, an intranuclear protein expressed in all phases of the cell cycle other than the G0 phase, is regarded as an indicator of cell proliferation. Although the impact of Ki-67 LI on prognosis of breast cancer has been evaluated several times, its prognostic value remains controversial [49, 50]. A report, which includes only 9 cases, indicates that high Ki-67 LI is a predictor of poor prognosis in SDC [51]. In this cohort, while 20, 30, 40 and 60 % were used as arbitrary Ki-67 LI cutoff values, Ki-67 LI had no correlation with prognosis. Since there were no cases with less than 15 % Ki-67 LI, SDC is considered to have high proliferative activity. Thus, the use of cytotoxic drugs may be reasonable when systemic treatment for patients with SDC is planned.
As with the classification system of breast cancer [32, 52], in this study SDCs were classified into five subtypes based on the expression levels of HER2, EGFR, Ki-67, and AR. Although the usefulness of this classification has yet to be evaluated, the fact that 30 of 32 (93.8 %) cases were positive for one or more of AR, HER2, and EGFR indicates that anti-AR, anti-HER2, and/or anti-EGFR targeted therapies may have efficacy in many cases of SDC. Since whole exome sequencing has been performed to detect recurrent somatic mutations and translocations by which the efficacy of novel drugs has been assessed in previous studies of breast cancer [53], the same approach will be required when introducing new drugs to the treatment strategy for SDC.
In conclusion, AR-positivity and EGFR-positivity were associated with better DFS in patients with SDC in univariate analysis, and EGFR-positivity was an independent predictive factor in multivariate analysis. When anti-AR, anti-HER2, and/or anti-EGFR targeted therapies, which have the potential to exert efficacy in 90 % or more cases with SDC, and/or conventional cytotoxic therapy for high Ki-67 LI cases are planned, a classification system of SDC based on the expression levels of these molecular factors may be useful to select adequate treatment. Further studies are required to evaluate the usefulness of this classification system and of a customized treatment strategy for SDC in the clinical setting.
References
Kleinsasser O, Klein HJ, Hübner G (1968) Salivary duct carcinomas: a group of salivary gland tumors analogous to ductal carcinoma of the breast. Arch Klin Exp Ohren Nasen Kehlkopfheilkd 192:100–105
Seifert G, Batsakis JG, Brocheriou C et al (eds) (1991) World Health Organisation. Histological typing of salivary gland tumors, 2nd edn. Springer, New York
Barnes L, Eveson W, Reichart P et al (eds) (2005) World Health Organization classification of tumours, pathology and genetics of head and neck tumours. IARC Press Lyon, France
Lewis JE, McKinney BC, Weiland LH et al (1996) Salivary duct carcinoma. Clinicopathologic and immunohistochemical review of 26 cases. Cancer 77:223–230
Williams MD, Roberts D, Blumenschein GR Jr et al (2007) Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol 31:1645–1652
Guzzo M, Di Palma S, Grandi C et al (1997) Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck 19:126–133
Jaehne M, Roeser K, Jaekel T et al (2005) Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer 103:2526–2533
Adelstein DJ, Koyfman SA, El-Naggar AK et al (2012) Biology and management of salivary gland cancers. Semin Radiat Oncol 22:245–253
Williams MD, Roberts DB, Kies MS et al (2010) Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res 16:2266–2274
Lagha A, Chraiet N, Ayadi M et al (2012) Systemic therapy in the management of metastatic or advanced salivary gland cancers. Head Neck Oncol 4:4–19
Nagao T, Sato E, Inoue R et al (2012) Immunohistochemical analysis of salivary gland tumors: application for surgical pathology practice. Acta Histochem Cytochem 45:269–282
Castellano I, Allia E, Accortanzo V et al (2010) Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat 124:607–617
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes––dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22:1736–1747
Hudis CA, Gianni L (2011) Triple-negative breast cancer: an unmet medical need. Oncologist 16(Suppl 1):1–11
Hoang MP, Callender DL, Sola Gallego JJ et al (2001) Molecular and biomarker analyses of salivary duct carcinomas: comparison with mammary duct carcinoma. Int J Oncol 19:865–871
Russell PJ, Bennett S, Stricker P (1998) Growth factor involvement in progression of prostate cancer. Clin Chem 44:705–723
Prostate Cancer Trialists’ Collaborative Group (2000) Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355:1491–1498
Nagao T, Gaffey TA, Visscher DW et al (2004) Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol 28:319–326
Lewis JE, Olsen KD, Sebo TJ (2001) Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol 32:596–604
Batsakis JG, El-Naggar AK, Luna MA (1992) Adenocarcinoma, not otherwise specified: a diminishing group of salivary carcinomas. Ann Otol Rhinol Laryngol 101:102–104
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Dowsett M, Nielsen TO, A’Hern R et al (2011) Assessment of Ki-67 in breast cancer: recommendations from the International Ki-67 in Breast Cancer working group. J Natl Cancer Inst 103:1656–1664
Takeda H, Akakura K, Masai M et al (1996) Androgen receptor content of prostate carcinoma cells estimated by immunohistochemistry is related to prognosis of patients with stage D2 prostate carcinoma. Cancer 77:934–940
Ruizeveld de Winter JA, Trapman J, Vermey M et al (1991) Androgen receptor expression in human tissues: an immunohistochemical study. J Histochem Cytochem 39:927–936
van der Hulst RW, van Krieken JH, van der Kwast TH et al (1994) Partial remission of parotid gland carcinoma after goserelin. Lancet 344:817
Locati LD, Bossi B, Rinaldi GR et al (2003) Anti-androgen therapy in recurrent and/or metastatic salivary glands carcinoma (RSGC). Ann Oncol 14(Suppl 4):126
Locati LD, Quattrone P, Bossi P et al (2003) A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol 14:1327–1328
Jaspers HC, Verbist BM, Schoffelen R et al (2011) Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol 29(16):e473–e476
Kumar B, Cordell KG, Lee JS et al (2008) EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol 26:3128–3137
Schlomm T, Kirstein P, Iwers L et al (2007) Clinical significance of epidermal growth factor receptor protein overexpression and gene copy number gains in prostate cancer. Clin Cancer Res 13:6579–6584
Locati LD, Bossi P, Perrone F et al (2009) Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol 45:574–578
De Dosso S, Mazzucchelli L, Ghielmini M et al (2009) Response to oxaliplatin with cetuximab in minor salivary gland adenoid cystic carcinoma. Tumori 95:378–381
Glisson BS, Blumenschein G, Francisco M et al (2005) Phase II trial of gefitinib in patients with incurable salivary gland cancer. J Clin Oncol, ASCO Annual Meeting Proceedings, 23(16S), Part I of II (June 1 Supplement):5532
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Gatzemeier U, Groth G, Butts C et al (2004) Randomized phase II trial of gemcitabine–cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 15:19–27
Tuefferd M, Couturier J, Penault-Llorca F et al (2007) HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS ONE 2:e1138
Safran H, Iannitti D, Ramanathan R et al (2004) Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest 22:706–712
Hussain MH, MacVicar GR, Petrylak DP et al (2007) Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 25:2218–2224
Nabili V, Tan JW, Bhuta S et al (2007) Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck 29:907–912
Nashed M, Casasola RJ (2009) Biological therapy of salivary duct carcinoma. J Laryngol Otol 123:250–252
Prat A, Parera M, Reyes V et al (2008) Successful treatment of pulmonary metastatic salivary ductal carcinoma with trastuzumab-based therapy. Head Neck 30:680–683
Sharon E, Kelly RJ, Szabo E (2010) Sustained response of carcinoma ex pleomorphic adenoma treated with trastuzumab and capecitabine. Head Neck Oncol 26:2–12
Kaidar-Person O, Billan S, Kuten A (2011) Targeted therapy with trastuzumab for advanced salivary ductal carcinoma: case report and literature review. Med Oncol 29:704–706
Yerushalmi R, Woods R, Ravdin PM et al (2010) Ki-67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11:174–183
Tanei T, Shimomura A, Shimazu K et al (2011) Prognostic significance of Ki-67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol 37:155–161
Martinez-Barba E, Cortes-Guardiola JA, Minguela-Puras A et al (1997) Salivary duct carcinoma: clinicopathological and immunohistochemical studies. Am J Clin Pathol 109:75–84
Sørlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Banerji S, Cibulskis K, Rangel-Escareno C et al (2012) Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486:405–409
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Masubuchi, T., Tada, Y., Maruya, Si. et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol 20, 35–44 (2015). https://doi.org/10.1007/s10147-014-0674-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0674-6