Abstract
Background
The prophylactic effect of postoperative interferon on recurrence and distant metastasis in stage II or III renal cell carcinoma is unclear. In most studies, interferon has been administered for 6 months or less. Therefore, we performed a clinical study of the efficacy of 1-year postoperative administration of natural interferon α, which is generally used in Japan.
Methods
The subjects were patients diagnosed with stage II or III renal cell carcinoma who underwent radical nephrectomy. The subjects were randomly allocated to receive an intramuscular injection of natural interferon α (3 million to 6 million units) 3 times a week for 1 year or to receive follow-up observation until recurrence or metastasis occurred. Chest and abdominal CT were performed once yearly for all patients. The primary endpoint was progression-free survival.
Results
From September 2001 to August 2006, a total of 107 patients were registered, but 7 subsequently withdrew from the study. Therefore, 100 patients were included in the analysis. The primary endpoint of progression-free survival did not differ significantly between the groups that received natural interferon α or follow-up observation (p = 0.456, log-rank test). However, peak hazards of progression in the interferon group were delayed for about 6–10 months compared with the observation group.
Conclusion
Progression-free survival showed no improvement after administration of natural interferon α to patients with stage II or III renal cell carcinoma for 1 year after radical nephrectomy. The peak hazards of progression might be delayed by about 6 months by interferon administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The postoperative prognosis of stage II or III renal cell carcinoma is generally poor, even after radical nephrectomy, and control of recurrence and distant metastasis is required. In studies in Japan, natural interferon α has shown some efficacy for treatment of advanced renal cell carcinoma [1]. In the period from 2000 to 2001, when the current study was planned, molecular targeted drugs were not used in clinical practice; therefore, this study was performed to examine the effectiveness of postoperative adjuvant treatment with interferon. Also, the prophylactic effect of postoperative interferon as adjuvant treatment on recurrence and distant metastasis had not been examined in overseas trials at the time of planning of the current study. Subsequently, the results of multivariate analysis performed in an exploratory study by Pizzocaro et al. [2] suggested that interferon may prevent recurrence of renal cell carcinoma in patients with lymph node metastasis. However, administration of interferon was performed for 6 months or less in most overseas studies, and examination of the efficacy of postoperative adjuvant treatment with longer-term administration of interferon is required. Therefore, we planned a clinical study of the efficacy of 1-year postoperative administration of natural interferon α, which is the form of interferon that is generally used in Japan.
Subjects and methods
The subjects were patients diagnosed with stage II or III renal cell carcinoma who underwent resection of the primary tumor by radical nephrectomy, as described in the General Rules for Clinical and Pathological Studies of Renal Cell Carcinoma (3rd edition) [3], from September 2001 to August 2006. The subjects were randomly allocated to receive an intramuscular injection of natural interferon α (3 million to 6 million units) 3 times a week for 1 year or to receive follow-up observation until recurrence or metastasis occurred.
The inclusion criteria were: (1) a histopathological diagnosis of renal cell carcinoma; (2) resection of the primary tumor by nephrectomy, for which open or laparoscopic surgery could have been selected and lymph node dissection was possible; (3) no lung metastasis in chest computed tomography (CT), no hepatic metastasis or retroperitoneal node metastasis in abdominal CT, and no bone metastasis in CT (or magnetic resonance imaging) bone scintigraphy; (4) aged ≥20 and ≤75 years old; (5) a performance status (PS; 5-level Eastern Cooperative Oncology Group classification) of 0–2; (6) white blood cell count ≥3,500/mm3, platelets ≥100,000/mm3, aspartate transaminase (AST; serum glutamic oxaloacetic transaminase) and alanine transaminase (ALT; serum glutamic pyruvic transaminase) <2.5 times the upper limit of normal (ULN), serum creatinine ≤2.0 mg/dl, and serum total bilirubin <1.5 times the ULN (no jaundice); (7) written informed consent given by the patient; and (8) no administration of pyrimidine fluoride agents such as UFT, chemotherapeutic agents such as vinblastine, and cytokines such as glucocorticoids, interleukin-2 (IL-2), and interferon γ.
The exclusion criteria were: (1) active multiple cancer; (2) a histopathological diagnosis of Bellini duct carcinoma; (3) a complication of von Hippel–Lindau (VHL) disease; (4) women who were pregnant, nursing, or with an intention to become pregnant; (5) hypersensitivity to interferon agents and bovine-derived materials; (6) hypersensitivity to biological drugs such as vaccines; (7) current treatment with Shosaikoto, a herbal supplement widely used in Japan; (8) autoimmune hepatitis; and (9) other severe complications.
A subject registration center was established in the University Medical Information Network (UMIN) to register patients using a central registration system. Dynamic random allocation was performed using the minimization method: the factors used for the allocation included (1) institution; (2) pT factor (T1–2, T3a, and T3b–c); (3) N factor (pN0 with removal, pN1 with removal, N0 with no removal, and N1 with no removal); and (4) sex (male and female). Each participating hospital registered the patients after obtaining approval from the institutional review board of the hospital.
For subjects allocated to the interferon group, administration of interferon α began within 4 weeks after surgery. Administration was performed after confirming a PS ≤2, the absence of fever ≥38°C and infection, and the absence of hemorrhage. Administration was suspended upon occurrence of a severe adverse event, deterioration of a complication, or recurrence or metastasis, and appropriate treatment was provided. Subjects in the observation group did not receive postoperative adjuvant treatment, but regular examinations were performed for detection of recurrence and metastasis.
Chest and abdominal CT were performed once yearly for patients in both groups in consideration of feasibility and exposure to radioactivity. Laboratory tests for complete blood count, total protein, total bilirubin, C-reactive protein, AST, ALT, alkaline phosphatase, lactate dehydrogenase, neutral fat, blood glucose, blood urea nitrogen, creatinine, serum Na, K, Cl, and Ca were performed every 3 months in the first year of the study, and then once every 6 months.
Statistical analysis
The primary endpoint was progression-free survival, which was defined as the period from nephrectomy until an event of local redevelopment or distant metastasis was shown by imaging, or until the day of death for a patient who died before the onset of such an event. The secondary endpoints were overall survival, time to treatment failure, cause-specific survival, and safety.
We assumed that the survival rate in patients with no progression for 5 years in the follow-up observation group would be 60%, and that the risk of progression would be reduced by two-thirds by administration of interferon α. With the registration period and follow-up period set at 2 and 3 years, respectively, the number of subjects required for one group was 270 with an α error of 0.05 and a β error of 0.2. Thus, the sample size was determined to be 540 (270 for each group).
For calculation of the survival period, the day of nephrectomy was considered to be the starting point, and recurrence, metastasis, or death was considered to be an event. The survival rate was estimated using the Kaplan–Meier method [4], and comparison of the survival rate between the 2 groups was performed using a log-rank test. For the primary endpoint, a subset analysis was performed based on the stratification of stage (II and III) at registration. Smoothed hazards of progression were estimated using a kernel function method [5, 6].
The details of adverse events were recorded in both groups, and the incidence of these events were compared between the groups using a chi-squares test, Fisher’s exact test, or Mantel–Haenszel test. All tests were two-sided and the significance level was set at 0.05.
This study is registered at UMIN-CTR (http://www.umin.ac.jp/ctr/index.htm), which registry is accepted by the International Committee of Medical Journal Editors. The study number is C000000256.
Results
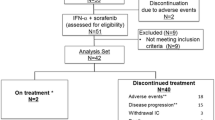
Registration was started in September 2001. In July 2003 and December 2005, the progress of the study was discussed by the study steering committee, and the registration period was extended until August 2006, at which time the total number of institutions was 266. A total of 107 patients were registered from 53 institutions, but 7 (4 in the interferon group and 3 in the observation group) subsequently withdrew from the study before commencement of treatment. Therefore, 100 patients were included in the analysis. However, since data for 1 subject could not be obtained after registration, prognostic data were analyzed for 50 subjects in the interferon group and 49 in the observation group (Fig. 1). The distribution of background factors in the subjects included in the analysis is shown in Table 1. There was no significant difference in any background factor between the interferon and observation groups.
The final investigation of prognosis was performed at 2 years and 4 months after completion of registration. The median observation period was 4.6 years. The primary endpoint of progression-free survival did not differ significantly between the two groups (p = 0.456, log-rank test). The survival curves are shown in Fig. 2. Thirteen subjects died, including 10 from cancer (6 in the interferon group and 4 in the observation group) and 3 from other causes (1 from myocardial infarction and 1 from pancreatic cancer in the interferon group, and 1 of unknown cause in the observation group).
There was also no significant difference in the secondary endpoint of overall survival between the two groups (p = 0.150, log-rank test). The curves for overall survival are shown in Fig. 3. Since there were only 5 N1 subjects with lymph node metastasis, subset analysis was performed only with T factors, without use of N factors. For the 40 T1 or T2 subjects, progression-free survival in the observation group was higher than that in the interferon group, but with no significant difference (p = 0.180, log-rank test, Fig. 4). For the 59 T3 subjects, progression-free survival was higher in the interferon group over 3 years, but again with no significant difference between the groups (p = 0.957, log-rank test, Fig. 5). The hazards of progression are shown graphically using the smoothing method in Fig. 6. Peak hazards of progression in the observation group were found at 6 and 28 months after nephrectomy, and hazard peaks in the interferon group were delayed for about 6–10 months compared to the observation group.
No death was caused by treatment toxicity, but various adverse events were observed. Interstitial pneumonia developed in 1 patient in the interferon group and was improved by administration of steroids. Other treatment-related adverse events were resolved or improved by discontinuation of the treatment. Adverse events in the observation group were examined after progression or at the start of interferon administration (see below). One subject in the observation group had a head injury due to a fall, but this was judged to be unrelated to the study. Other adverse events in the observation group were found to have improved or remitted in the final investigation of prognosis. All adverse events are shown in Table 2.
In the interferon group, treatment was suspended in 44 subjects (including for cases prescribed in the protocol): in 15 after 1 year, 1 after 5 years, 7 due to self-withdrawal, 4 with general malaise, 4 with no continuous self-injection, 2 with hepatic dysfunction, 2 with redevelopment, 2 with depression, 1 due to a change of address, 1 with interstitial pneumonia, 1 due to required treatment for angina, 1 due to family circumstances, 1 due to required orthopedic surgery, 1 with severe arthralgia, and 1 with aggravation of dementia. In the observation group, interferon was administered to 13 subjects: in 10 due to redevelopment of renal cell carcinoma and in 3 due to a request for interferon by the patient.
Discussion
The development of molecular targeted drugs in the 2000s has changed the treatment options for advanced renal cell carcinoma [7]. Immunotherapy with interferon and IL-2 currently has an important role [8], especially in Asia due to healthcare economics and the social system. Therefore, it is important to evaluate the efficacy and safety of immunotherapy including interferon used as postoperative adjuvant treatment. The results of this study did not show a significant improvement of progression-free or overall survival with interferon. Similarly, adjuvant regimens of immunotherapy have not shown significant benefits in previous studies. In patients with high-risk renal cell carcinoma, including M1 patients with no evidence of disease after resection, Clark et al. [9] found limited efficacy of adjuvant high-dose bolus IL-2 monotherapy. In a phase III study of interferon α-NL as adjuvant treatment for resectable renal cell carcinoma, Messing et al. [10] obtained negative results with a regimen of up to 12 cycles of interferon α-NL administered daily for 5 days every 3 weeks. The short period of administration (a maximum of 36 weeks) may have caused the negative results in this study. Atzpodien et al. [11] performed a randomized trial of adjuvant treatment with IL-2 and interferon α2a-based chemoimmunotherapy after radical nephrectomy for complete resection of a relapsed tumor or solitary metastasis. The regimen of adjuvant therapy was 8-week treatment with interferon α2a, IL-2 and intravenous 5-fluorouracil. However, in patients with such a high risk of progression, it is unlikely that an 8-week regimen would be sufficient to control the disease.
The efficacy of postoperative interferon in patients with lymph node metastasis has been suggested in a previous study [2]. In the current study, we abandoned a subset analysis with the N classification because the number of N1 subjects in both groups was small (3 in the interferon group and 2 in the observation group). Progression-free survival was evaluated in groups of T1/T2 and T3 subjects, since the T classification also reflects progression of the primary tumor. We found no significant difference between the interferon and observation groups for the T3 subjects, although progression-free survival in the interferon group was consistently higher in the 3 years after nephrectomy. This indicates a tendency for efficacy of postoperative adjuvant treatment with interferon in T3 subjects, who have a comparatively high risk among stage II or III subjects, as also seen in the effects of N factors in a previous study. Other factors (age, sex, and grade) showed no relationship with the efficacy of interferon.
Smoothing of hazards was estimated to examine the timing of the risk of progression in the postoperative period. Initial peaks for the risk of progression were observed at 6 and 12 months after surgery in the observation and interferon groups, respectively; thus, the peak in the interferon group was delayed for about 6 months. A second peak was observed at 2–3 and 3–4 years after surgery in the respective groups, again suggesting a delay of the peak hazards in the interferon group. Since the weights of individual events were calculated equally regardless of the time in the log-rank test used to examine the difference in progression-free survival, a difference in efficacy could not be detected when the number of events was almost the same at the end of follow-up of prognosis. However, based on the smoothing of hazards, interferon has an effect in delaying progression. Therefore, we suggest that postoperative administration of interferon for 1 year or longer might be considered as a treatment option after surgery for renal cell carcinoma, with careful observation of possible adverse events caused by interferon administration.
One of the limitations of this study is the small number of subjects. The sample size in the protocol design of the study was 540 patients (270 in each group), but an increase in the maximum diameter of the tumor to 7 cm in the criteria for T1 resulted in a smaller number of stage II or III patients than expected in developing the study protocol. Thus, the number of participating institutes was increased and the registration period was extended twice from the initial period of 2–5 years in total; however, the total number of patients was only 100. Therefore, the efficacy of interferon as postoperative adjuvant treatment could not be judged with certainty due to the insufficient power of the statistical tests. Due to the small number of subjects, we limited the analysis to factors that might affect prognosis in a multivariate analysis. For the same reason, we were unable to perform a detailed evaluation of the smoothed hazards peak at 4 years after nephrectomy, since the estimated values at this time point were unstable because of the smaller number of patients at risk.
Planning and performance of a future clinical study on postoperative adjuvant treatment with interferon may be difficult because several molecular targeted drugs have now been approved for treatment of renal cell carcinoma in Japan. Thus, we believe that the results of the current study are important as basic data for the efficacy of postoperative adjuvant treatment in Japan. In the future, it will be important to examine how immunotherapy with interferon should be used as a treatment option with molecular agents; to identify the patient population in which interferon may be effective; and to determine the timing of use, combination with other drugs, and the administration schedule for multiple agents.
Conclusion
Progression-free survival showed no improvement after administration of natural interferon α to patients with stage II or III renal cell carcinoma for 1 year after radical nephrectomy. All adverse events were well known, with incidences similar to those found in previous studies. An evaluation of postoperative hazards indicated that the peak hazards of progression might be delayed by about 6 months by interferon administration.
References
Aso Y, Homma Y, Arai Y et al (1995) The efficacy of alfa-interferon in renal cell carcinoma with special reference to adjuvant therapy. Hinyoki-Geka 8:333–334
Pizzocaro G, Piva L, Colavita M et al (2001) Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol 19:425–431
Japanese Urological Association, The Japanese Society of Pathology, Japan Radiological Society (1999) General rule for clinical and pathological studies on renal cell carcinoma, 3rd edn. Kanehara, Tokyo (in Japanese)
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457
Hinotsu S, Akaza H, Ohashi Y et al (1999) Intravesical chemotherapy for maximum prophylaxis of new early phase superficial bladder carcinoma treated by transurethral resection: a combined analysis of trials by the Japanese Urological Cancer Research Group using smoothed hazard function. Cancer 86:1818–1826
Gray RJ (1990) Some diagnostic methods for Cox regression models through hazard smoothing. Biometrics 46:93–102
Di Lorenzo G, Buonerba C, Biglietto M et al (2010) The therapy of kidney cancer with biomolecular drugs. Cancer Treat Rev 36(Suppl 3):S16–S20
Akaza H, Tsukamoto T, Onishi T et al (2006) A low-dose combination therapy of interleukin-2 and interferon-alpha is effective for lung metastasis of renal cell carcinoma: a multicenter open study. Int J Clin Oncol 11:434–440
Clark JI, Atkins MB, Urba WJ et al (2003) Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a Cytokine Working Group Randomized trial. J Clin Oncol 21:3133–3140
Messing EM, Manola J, Wilding G et al (2003) Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol 21:1214–1222
Atzpodien J, Schmitt E, Gertenbach U et al (2005) Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Br J Cancer 92:843–846
Acknowledgments
Subjects were enrolled in the institutions listed below. We sincerely appreciate the cooperation of urologists in these institutions. Oji General Hospital, Iwate Medical University, Kuji Prefectural Hospital, Akita University, Tohoku University Hospital, University of Tsukuba, The Prefecture West General Hospital, The Jikei University, The Jikei University Aoto Hospital, The Jikei University Daisan Hospital, The University of Tokyo, Tokyo Teishin Hospital, Tokyo Metropolitan Police Hospital, Kitasato University, Kitasato Institute, Tokyo Women’s Medical University, Kanazawa University, University of Yamanashi, Hamamatsu University School of Medicine, Seirei Hamamatsu General Hospital, Shinsiro Municipal Hospital, Osaka Medical Center for Cancer and Cardiovascular Diseases, Kobe University, Hyogo Cancer Center, Nara Medical University, Wakayama Medical University, Rinku General Medical Center, Tottori University, Shimane University Faculty of Medicine, Okayama University, Mitoyo General Hospital, Onomichi Municipal Hospital, Yamaguchi University, Ube Industries, LTD. Central Hospital, Kokura Memorial Hospital, The University of Tokushima, Yashima General Hospital, Takamatsu Hospital, Kyushu University, National Kyushu Medical Center, Harasanshin Hospital, Kitakyushu Municipal Medical Center, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital, Kitakyushu Municipal Wakamatsu Hospital, Saga Prefectural Hospital Koseikan, Kurume University, Nagasaki University, The Japanese Red Cross Nagasaki Genbaku Hospital, St. Francis Hospital, National Hospital Organization Nagasaki Medical Center, Kumamoto University, National Hospital Organization Kumamoto Medical Center, Oita University Faculty of Medicine Graduate School of Medicine.
Conflict of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hinotsu, S., Kawai, K., Ozono, S. et al. Randomized controlled study of natural interferon α as adjuvant treatment for stage II or III renal cell carcinoma. Int J Clin Oncol 18, 68–74 (2013). https://doi.org/10.1007/s10147-011-0345-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0345-9